 Research Article
Research Article
Opportunistic Virus BKV: Racial Disparities in Prostate Cancer
Shilpi Bhatia and Liesl Jeffers Francis*
Department of Biology, North Carolina A&T State University, Greensboro, NC, USA
Liesl Jeffers Francis Assistant Professor, Biology Department, College of Science and Technology, North Carolina A&T State University, 1601 E. Market Street, Greensboro, NC 27411, USA, 336-285-2506, ljeffers@ncat.edu.
Received Date: May 03, 2022; Published Date: June 29, 2022
Abstract
Prostate cancer (PCa) is the most common malignancy and the second most leading cause of cancer death among men in the United States. More specifically, African American (AA) men are at 1.6 times higher risk of being diagnosed and 2 times higher risk of death from PCa, compared to European-American (EA) men or other ethnicities. BKV is an emerging opportunistic pathogen that infects 90% of the human population and is known to reactivate in immune-compromised hosts and has been implicated as a cofactor in early stages of PCa. Reactivated BKV may be a more virulent strain that emerges in the absence of host immune response. Therefore, it is plausible that BKV genetic diversity within immune-compromised cancer hosts may allow more virulent strains to reactivate to opportunistically cause disease.BKV replication is orchestrated by a regulatory protein large T antigen (LTag) which binds products of tumor suppressor genes (pRb family, p53) and interferes with the strategic checkpoints of the cell cycle of infected cells, thereby resulting in transformation and cancer progression. The association of viruses and their evolutionary dynamics in human disease is critical for advancement in tumor biology therapeutics and clinical diagnoses. This review article describes to better understand racially derived differences in PCa and a potential role for BKV as a cofactor in PCa progression..
Abbreviations:BKV: BK polyomavirus; PCa: Prostate cancer; JCV: JC virus; SV40: Simian Virus 40; CMV: Cytomegalovirus; HSV: Herpes Simplex Virus; HHV: Human Herpes Virus; HPV: Human Pappilloma virus; EBV: Epstein Barr Virus; XMRV: Xenotropic murine leukemia virus-related virus; TAg: Major T antigen; tAg: Minor T antigen; NCCR: non-coding control region; CRPC: Castration Resistant Prostate Cancer; SEER: Surveillance, Epidemiology and End Results Program; PCA3: Prostate Cancer gene 3; PIA: Proliferative Inflammatory Atrophy PIN: Prostatic Intraepithelial Neoplasia; BPH: Benign Hyperplasia; TMPRSS2-ERG: Transmembrane protease, serine 2; MALAT-1: Metastasis-associated lung adenocarcinoma transcript-1; HI: Hemagglutination-inhibition; IF: indirect immunofluorescence RIA: radioimmunoassay; ELISA: enzyme-linked immunoassay
Introduction to BKV
BK polyomavirus (BKPyV) is a small circular, double-stranded (~5kb), non-enveloped virus with an icosahedral capsid and a member of the Betapolyomavirus genus in the Polyomaviridae family [1]. It was isolated from the urine of a Sudanese kidney transplant recipient with ureteric stenosis, with initials BK [2]. Other common polyomaviruses that affect humans include JC virus (JCV), Simian virus 40 (SV40), and Merckle cell virus, to name a few. While BKV and JCV are ubiquitous in humans, SV40 is believed to be introduced in the human population by contaminated polio vaccines produced in SV 40- infected monkey cells. The DNA sequence identity shared between BKV and JCV is 72%, while 69% between BKV and SV40 and 68% between JCV and SV40 [3]. It has been suggested that BKV co-evolved with humans and remains latent in healthy individuals for most of their lives. Studies have reported that 90% of the human population is infected with BKV. Typically, 10-30% infants and 65- 90% individuals become infected with BKV at a young age (~5-10 years old), however, the virus remains asymptomatic or mildly symptomatic in immunocompetent individuals and persists in the kidney, peripheral-blood leukocytes, and the brain [4-6].
BKV Pathogenesis
BKV has been implicated in multiple diseases such as hemorrhagic cystitis, ureteric stenosis, vasculopathy, pneumonitis, encephalitis, retinitis, autoimmune diseases, and cancer [1]. However, due to the lack of many studies in understanding the role and mechanism of BKV replication, the exact role of BKV in these diseases is still obscure. Unlike most common viruses, information on BKV diversity is limited and the understanding of its evolution and variability is based on analogous studies of other viruses such as HIV, HPV or SV40. In 2012, the International Agency for Research on Cancer, a part of the World Health Organization, classified BK polyomavirus as a group 2B- “potentially carcinogenic to humans” [7].
The detection of BKV, JCV and SV40 sequences have been found in peripheral blood mononuclear cells (PBMC), suggesting a possible route of entry for the virus to spread to other tissues of the infected host [8&9]. In case of BKV, the transmission routes are not fully recognized, and it is assumed that the infection is transmitted via the respiratory tract, fecal-oral tract, blood, or through organ transplant [10]. The most common high throughput techniques, such as quantitative real-time PCR, DNA Microarray, Fluorescent in situ hybridization (FISH) have been employed in determining BKV DNA detection in tissues of cancer patients. Out of these, qRTPCR remains the best gene assay method for BKV DNA detection in tissues. Other methods such as Hemagglutination-inhibition (HI), complement fixation, indirect immunofluorescence (IIF), RIA, ELISA, and western blot are also widely employed assays [11]. Therefore, while the sensitivity of each test may vary, the modalities of virus transmission in humans are still presently not clear. Although the virus is known to infect many different cell types, the renal tubular and urinary tract epithelium are most commonly infected. Upon reactivation, the immune system controls the replication of BKV and allows it to remain latent. During immunosuppression, the virus enters its lytic phase in kidney cells, which results in detachment from the basement membrane, and the cells get infected with the virus that may appear in urine as decoy cells, leading to viruria.
Subsequently, the virions can egress capillaries, causing viremia. All these events can result in necrosis and lytic destruction of the renal epithelium layer followed by inflammation, a common occurrence in kidney transplant recipients or in hemorrhagic cystitis [4].
BKV DNA has been detected in brain tumors, neuroblastoma, bone, insulinoma, prostate and Kaposi sarcoma [8,12-14]. Flagstead et al. [15] confirmed the presence of BKV in brain cancers via PCR that detected the DNA in 17 out of 18 patients with neuroblastoma along with T-Ag expression in them. Arthur et al. [16] refuted the study indicating a lack of association of BKV DNA to brain cancers. BKV DNA has also been detected in high-grade squamous intraepithelial cervical lesions [17]. In addition, the risk for invasive bladder cancer in kidney transplant recipients and prostate cancer is strongly correlated with BKV infection [18-23]. Several studies have reported the manifestation of kidney or bladder cancer due to BKV infection- related nephropathy. Narayanan et al, [24] confirmed the presence of BK virus in kidney transplantation and cancer, although no infection in the donor’s healthy kidney was found. Similarly, Geetha et al. [25] reported the presence of BKV DNA in a patient with bladder cancer and BKV nephropathy, confirming the role of the virus as a causal transforming agent confirmed via the differential expression of BKV large T antigen in the malignant cells except for stromal cells or nondysplastic urothelium.
Limited studies have also reported the role of BKV, JCV and SV40 prevalence in colorectal cancer [26&27]. Drop et al. [28] reported a significant correlation among different viruses upon co-infection such as HPV, EBV and BKV in polish patients with oral, oropharyngeal, and laryngeal squamous cell carcinomas. Several studies have reported a connection between BKV and the oral cavity [29-31]. Jeffers and Cyriaque [31] suggested a role of BKV transmission via the oral route as it was reported in HIV-SGD (salivary gland disease).
BKV Structure and Genome
The site of entry, mechanism of dissemination and mode of transmission modulating BKV infection mechanisms are still not clearly known. It has been reported that the initial binding of capsid protein to host cellular receptors such as N-linked glycoprotein with 2,3- linked sialic acids and ganglioside receptors Gt1b and GD1b; initiates the entry of the virus into the host [32]. Subsequently, the virus gains entry into the host cell via caveolin- dependent endocytosis [33]. Once delivered to the endoplasmic reticulum (ER), the capsid disassembly initiates [34,35]. The translocation from ER into the host cell cytosol and finally, to the nucleus is orchestrated by the nuclear localization signal (NLS) domains within the capsid minor structural proteins and the host nuclear pore complex components, thereby facilitating its nuclear import [36], wherein the cell transcription machinery initiates viral gene expression [37]. Upon viral replication initiation, the late genes are transcribed from the opposite strand in an opposite direction from the early genes. The translocation of capsid proteins after being synthesized in the cytoplasm is followed by their assembly with the viral genome into the virion particles in the nucleus. The viral genome is also packed with histones H2A, H2B, H3 and H4. Virions are finally released by lysis-dependent/ independent mechanisms. In case of abortive infections, the viral genome is integrated into the host cells as an episome and remains latent [38].
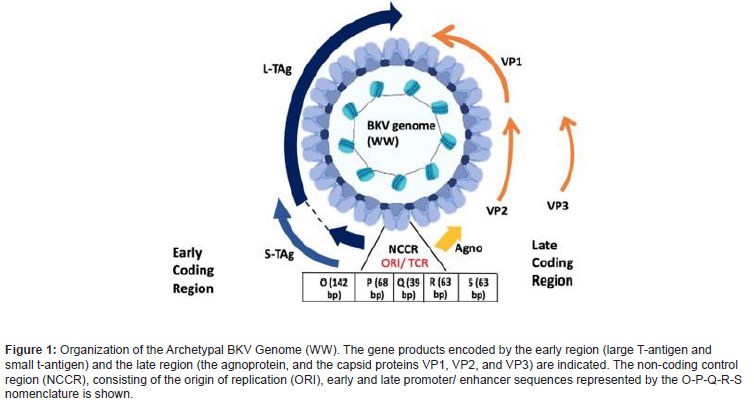
The polyomavirus genome organization consists of three functional regions: the non- coding control region (NCCR) and two coding regions: early and late. The non-coding region is the site of replication origin (ORI) and transcription. The NCCR/TCR of the proposed archetypal BK strain WW is divided arbitrarily into five transcription factor binding sequence blocks, called O (142 bp), P (68 base-pairs), Q (39 base- pairs), R (63 base-pairs), and S (63 base-pairs). These binding sites entail the promoter/enhancer sequences. The early and late region code for proteins, two and four, respectively. The early proteins include the large tumor antigen (TAg), the small tumor antigen (tAg), and the recently discovered truncated tumor antigen (TruncTAg). The regulatory protein large tumor antigen (LTag), coded by the early protein, is primarily associated with BKV replication [39]. Expression of the large T antigen immortalizes human cells in culture, transforms rodent cells in culture, and induces tumors in transgenic mice. The Dna J domain in the N-terminal part of LTAg as well as the ATPase/ helicase activity, are necessary for efficient viral DNA replication. LTAg of most polyomaviruses binds repeats of the 5’-GRGGC-3’ motif. In higher quantities, the accumulation of LTag protein initiates DNA replication in the cell nucleus via DNA polymerase to the viral origin of replication, thereby shutting off early gene transcription and regulating expression of late proteins. This results in lysis of cells, eliciting an antibody response to VP1 in most healthy individuals. In the case of abortive infection, uncoupling of LTag from VP1 induces an oncogenic response, by binding to the products of tumor suppressor genes (pRb family, p53) and inactivating by sequestration in the cytoplasm, thus obstructing the strategic cell cycle checkpoints of infected cell and causing transformation and tumor progression. The late region codes for the capsid proteins, that facilitate the virion assembly. It consists of three capsid proteins (VP1, VP2, VP3) and agnoprotein. The capsid consists of 72 copies of homomeric VP1 pentamers cross-linked by intermolecular disulfide bonds to form a T=7d icosahedron structure [40]. The role of LTag and VP1 have been identified as therapeutic markers against anti-polyomavirus agents [38].
Based on the NCCR sequences, BKV can be classified into two main groups: the archetype and the rearranged. The archetype group is commonly found among healthy individuals and is difficult to culture in vitro, whereas the rearranged BKV is associated with diseases and can be easily propagated using cell culture methods. In addition, based on genetic heterogeneity in the VP1 sequence, there are four types of BKV viruses: Subtype I is most distributed worldwide (80%), followed by subtype IV (15%) that is prevalent in Asia and parts of Europe, while subtypes II and III are sister groups that are rarely detected [41-44]. [45] identified a 100 bp-region of the VP1 gene using phylogenetic algorithms for identification of the various BKV subtypes and classified it as the BK typing and grouping region (BKTGR). Another BKV variant, BKV-IR, was detected using Southern blot hybridization in human insulinoma [46]. The genome of BKV-IR contains an IS-like structure, a type of stem-loop transposable element that can integrate and excise from the host genome, thus promoting cell transformation by excision from viral DNA and insertion in the cell genome, functioning as a mutagen. However, the study failed to show the integrated viral sequences in the human tumor from which it was isolated although BKV-IR was able to infect monkey and human cells, transform rodent cells and induce tumors in hamsters. Therefore, more comprehensive research focusing on comparable analysis to determine the prevalence of different strains worldwide should delve into the viral oncogenic potential leading to progression of tumors. (Figure 1).
Prostate cancer and racial disparities
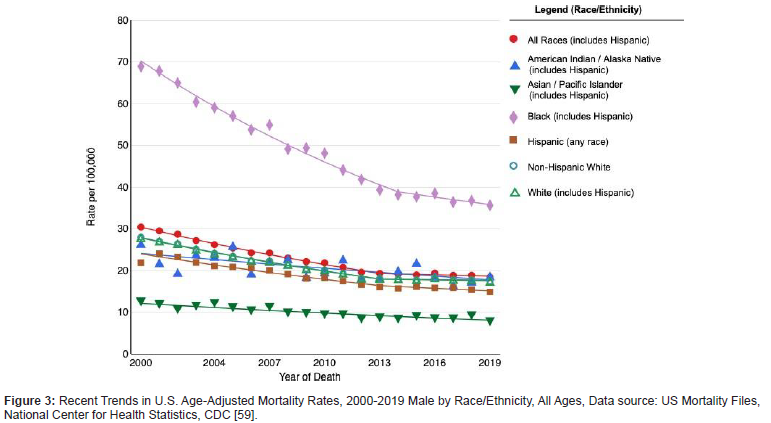
Prostate cancer (PCa) is the most common malignancy and the second leading cause of death among men in the United States [47]. The greatest incidence is witnessed in more developed countries such as North America, Western and Northern Europe, Australia, and New Zealand and in parts of the Caribbean and sub- Saharan Africa (Figure 2). Race/ethnicity is a crucial factor in the mortality rates of prostate cancer representing one of the largest cancer disparities within the US. The incidence of prostate cancer as well as mortality rate in African American (AA) men is higher as compared to Caucasian (CA) or European (EA) and Asian-American men. More specifically, AA men are at 1.6 times higher risk of being diagnosed and 2 times higher risk of death from PCa compared to Americans of European ancestries. Most African - American men are diagnosed at a younger age, with higher tumor grade and volume at surgery and with a greater potential for metastasis [48]. Overall, The Cancer Genome Atlas (TGCA) cohort indicates AAs have a lower median age at diagnosis with an average Gleason score of 8. According to the Surveillance Epidemiology and End Results Program (SEER) Statistics data, the five- year relative survival rate was 99.6% for European men and 95.9% for African- American men. The age-adjusted PCa mortality rate was also found to be two to three-fold higher in African- American men, compared to European men or men of other ethnicities (Figure 2-4). Both non-/ biological risk factors such as lack of access to care, diet, age, lifestyle, family history, hormones, socio-economic status have been implicated as causal factors to the difference in racial disparities of PCa incidence [48,49]. Moreover, the major challenges in different treatment regimens result in diminished survival rates following treatment in AA men as opposed to EA men. Besides socio-economic reasons, phenotypic and genomic heterogeneity can be attributed to racial disparity differences in tumor etiology [50], such as genetic polymorphism, gene mutations, epigenetic modifications, and miRNA alterations [49].
Prostate specific antigen (PSA) levels in serum of the patient serve as the most reliable physiological/pathological diagnostic indicator of PCa [48]. US Preventive Task Force (USPTF) reported a correlation between PSA screening linked to the potential benefit of decreasing the number of deaths in men with PCa aged 55-69, however the data for men from all races over 70 is less convincing [51]. Notably, significantly higher PSA levels were seen in African - American men, with or without prostate cancer when compared to European-American counterparts [52–54]. Another parameter in the diagnosis of PCa is the biomarker Prostate Cancer gene 3(PCA3); which is a non-coding mRNA detected in the urine of patients [55]. A strong indication of PCa is commonly depicted through higher ratios of PCA3 mRNA to PSA mRNA (PCA3/PSA). However, PCA3 is not solely a reliable predictor for prognosis in PCa patients as opposed to PSA. An androgen-regulated Transmembrane protease, serine 2 (TMPRSS2-ERG) fusion gene has also been found to be a promising urinary biomarker test; considered to be highly PCa specific [56,57]. In addition, certain DNA methylation biomarkers for PCa have also been reported. Although biomarker sensitivity and specificity play a crucial role in the initial diagnosis of PCa and have revolutionized the epidemiology and prognosis of PCa, additional investigation for more accurate specificity of other biomarkers is required (Figure 2,3) [58&59] Table 1.
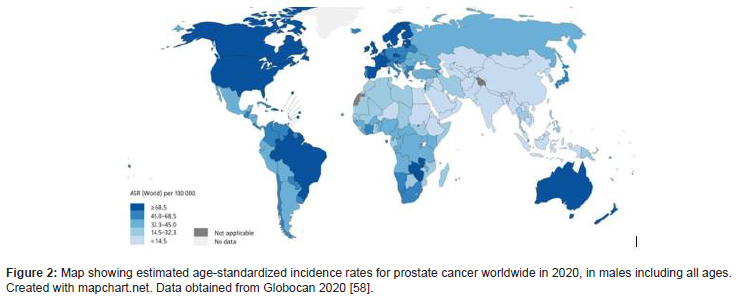
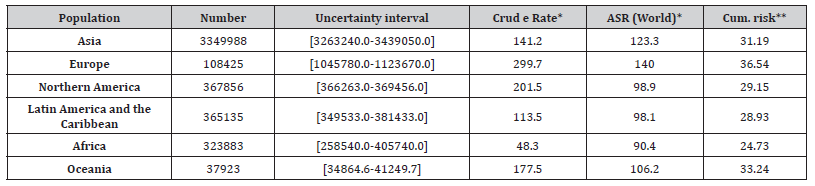
Table 1: Estimated Number of Incident Cases for Prostate Cancer, Males, All Ages [58].
Racial genetic susceptibility differences in PCa
Gene polymorphisms
The most prevalent alterations in prostate cancer genomes are fusions of androgen- regulated promoters with ERG and ETS family of transcription factors. 40-50% of prostate tumor foci translating to over 100,000 cases annually in the US amounts to TMPRSS2-ERG fusion type of molecular alteration [60,61]. Besides these, the most frequently mutated genes in primary prostate cancers are SPOP, TP53, FOXA1, and PTEN [62]. Among the several different subtypes of PCa reported, ERG subtype was found to be lower (27.3%) while the SPOP subtype was more (22.7%) prevalent among AA subpopulation than EAs. The common chromosome 8q24 variants associated with increased prostate cancer risk, has been found to be increased in African – American men [63-65]. Other genetic variations suppressing tumors such as EphB2 [66] or regulating cell apoptosis such as BCL2 [67] are found to be significantly higher in AA men. These genetic variations manifest a more aggressive form of the disease in AA men, although external factors such as disparities in lack of adequate screening and access to healthcare cannot be neglected.
Genomic and transcriptomic dysregulation such as SPOP mutations, TMPRSS2-ERG fusions, PTEN deletions/losses, immune signaling, and expression of non-coding RNAs have been reported to be significantly different in AA men than EA men [68]. Genomewide association studies (GWAS) have identified novel susceptibility loci in African men [64,69,70]. Jaratlerdsiri et al. [71] reported the use of deep whole genome sequencing (WGS) for profiling genomic variation in somatic variants in men with PCa from Africa, particularly South Africa and found an overall 1.8-fold increase in somatic variants in African tumors as opposed to those of European ancestry, although genomic rearrangements were found to be less frequent. Other genomic rearrangements reported in African men were in accordance with those reported in African - American men. Androgen receptor (AR) is an intracellular hormone receptor and a transcription factor and the androgen receptor (AR) signaling is a critical parameter in PCa pathogenesis [72]. Exon encoding aminoterminal transcriptional domain of AR consists of two polymorphic repeats of high frequency, CAG and GGN. The length of CAG repeats is reported to be different in AA (average 19-20) and EA (average 21-22) races. Additionally, CAG repeats polymorphism has been found higher in AA men, increasing the risk of PCa in them [73&74].
p53 polymorphisms
The transcription factor p53, encoded by TP53 gene, is also known as the guardian of the genome and plays a major role in regulation of the cell cycle, DNA repair, apoptosis and as a tumor suppressor [75]. Somatic mutations in p53 have been suggested in ~50% of cancers although relatively low in PCa (76,77). Missense mutations are the most common type of mutations reported for p53 in cancers. In the TGCA cohort, a whole genome sequencing of 333 samples from men with localized prostate cancer detected a mutation rate of 8% in TP53 [78]. Whereas, in castrationnaïve metastatic prostate cancer, TP53 mutations were found to be significantly higher ranging between 28% to 36% than those in primary prostate cancer but lower than those found in Castration-resistant prostate cancer (CRPC) [79-81]. Therefore, the marked differences in TP53 across different grades of prostate cancer indicate towards the immunological target mutant p53 [82]. However, cytoplasmic localization of p53 and wild type nuclear localization signal/sequence (NLS) have been reported in association with BKV LTag in PCa, rendering the possibility of mutant p53 as an immunological target [20&22]. Cytosolic p53 expression has been shown to be associated with poor outcome and progression to CRPC (83). A study demonstrated that combined therapy of Pmp53 (a plasmid harboring both mdm2 siRNA (Si-MDM2) and the wtp53) with zinc inhibited tumor growth in mouse xenograft models, while retaining wild-type p53 conformation, thereby enhancing the downstream transcriptional regulation of p21 and bax gene expression. The in-vivo findings in this study were supplemented with an in vitro culture of PCa cell lines analysis, showing a similar positive effect on cell cycle arrest and apoptosis [84]. Another study demonstrated restoration of the native p53 function in PCa by knockdown of G3BP2, an AR target gene that associates with SUMO E3 ligase RanBP2 and promotes p53 nuclear export, thus increasing p53 nuclear accumulation and reduced tumor growth in-vivo, indicative of G3BP2 as a plausible therapeutic target to restore native p53 function in PCa [83]. Moreover, while Suzuki et al. [85] reported that the Pro/Pro genotype of p53 codon72 was associated with a risk of PCa only in patients with a family history of Japanese population. A metaanalysis suggested a weak association of p53 codon Pro72Arg polymorphism with prostate cancer risk [86].
As for racial differences owing to p53 polymorphisms, population- dependent allele frequencies have been found to be less prevalent in Europeans compared to Africans with respect to p53 P72R SNP and Pro (C allele). The p53 Pro allele role has been indicated to be significantly variable in some major ethnic groups such as Caucasians and Taiwanese populations, whereas a contributing risk factor in men of African descent. Ricks-Santi et al. [87] in a case-controlled study demonstrated a marginal prevalence with respect to p53 Pro72Arg SNP polymorphism, the G allele coding for Arg allele associated with the prevalence of PCa in men of African - American descent./
Differential expression of miRNAs in PCan
MicroRNAs (miRNAs) are small, non-coding, 19-25 nts in length RNA that regulate gene expression post-transcriptionally. They do so either by degradation of target mRNA or inhibiting translation by complementarity targeting mRNA [88]. Differential expression of miRNAs has been reported in prostate cancer. Calin and Croce, [89] reported five miRNAs differentially expressed in prostate tumors among AA and EA populations, which are, miR- 301, miR- 219, miR-26a, miR-1b-1 and miR-30c-1. These were at least three times differentially expressed in AA vs EA origins. Theodore et al. [90], also investigated the expression of miR-26a in non-malignant, malignant, and metastatic PCa cell lines of AA and EA populations. They reported increased (2.2 to 3.3 folds) expression of miR-26a in AA prostate cancer cells as compared to EA, examined under similar clinical stage and grade of tumor. However, an increase in miR-26a expression levels in more aggressive prostate cancer cells of both AA and EA origins was also seen. Later, the same group observed significantly lower expression levels of another miRNA; miR-152 in AA populations as compared to EA patients. The ectopic expression of miR-152 in PCa cell lines down- regulated DNA (cytosine-5)- methyltransferase 1 (DNMT1) through direct binding in the 3’UTR. Thus, leading to a possible conclusion that miR-152/DNMT1 may contribute to tumor aggressiveness, specifically in AA PCa patients [91]. Wang et al. [92] identified 22 miRNA signatures in AA and 18 in EA populations. They also reported novel AA-specific enriched miRNA- mRNA pairs critical in driving oncogenesis, including miR- 133a/MCL1, miR-513c/STAT1, miR-96/FOXO3A, miR-145/ITPR2, and miR-34a/PPP2R2A. These deregulated miRNA-mRNA pairs targeted the EGFR-PI3K-AKT signaling, driving PCa aggressiveness in the AA populations. Therefore, the use of miRNAs can serve as potential therapeutic targets for PCa treatment and unravel the underlying mechanisms associated with racial heterogeneity in tumor aggressiveness.
Overexpression of LncRNAs in PCa progression
Besides miRNA, long non-coding RNAs (lncRNA) as the name suggests are long non-coding RNAs (>200 nts in length) have been reported to be overexpressed in PCa [93]. Lnc- SNHG17, was found to be highly expressed in castration resistant prostate cancer (CRPC), serving as a competing endogenous RNA (ceRNA), inhibiting miR-144, which in turn, upregulated CD51, amplifying CRPC cell proliferation and invasion [94]. Another lncRNA, LncRNA HOXD-AS1 promotes castration via WDR5 mediated histone 3 lysine 4 tri- methylation and regulation of other downstream genes, such as PLK 1, AURKA and CDC25C [95]. Two LncRNAs; Lnc- HOTAIR and Lnc- LBCS, have been reported to interact with the AR and result in CRPC progression. The former prevents the ubiquitination and degradation of the AR while the latter inhibits translation of the AR mRNA via interaction with hnRNPK [96].
Metastasis-associated lung adenocarcinoma transcript-1 (MALAT-1), approximately 8000 nt long non-coding RNA, originally overexpressed in non-small cell lung cancer metastasis, has been suggested to play a role in CRPC progression both in vivo and in vitro. Silencing of MALAT1 was further reported to inhibit CRPC cell proliferation by arresting the CRPC cell in the G0/G1 cycle, corroborating its role in PCa progression, aggression, and proliferation of prostate cancer cells [97]. In addition, MALAT-1 knockdown was shown to inhibit proliferation and migration, facilitating apoptosis by upregulating the expression of miR-1 (a tumor suppressor miRNA reported to be downregulated in PC tissues and cells) and downregulating KRAS (a target of miR-1) in androgen–receptor negative PCa cells [98]. Thus, these studies established the role of MALAT-1 as a potential/ therapeutic target for suppressing castration- resistant PCa. Moreover, Jeffers et al. [99] found a substantial upregulation of MALAT-1 in the presence of both polyoma (BKV) and papilloma (HPV) oncoproteins, which disrupt p53 expression. Thus, considering MALAT-1 as a biomarker for p53 deregulation and therapeutic target for PCa, it can be hypothesized that BKV- mediated upregulation of MALAT-1 may play a role in PCa progression.
BKV: a cofactor in etiology of prostate cancer
Viruses, as infectious agents have an association with ~20% of human cancers worldwide. Viral infections could act as cofactors in etiology of prostate cancer progression. Many viruses are known to interact with host proteins and induce changes in their genetic, immunological and inflammatory pathways leading to initiation or progression of tumors [100]. Viral products, such as, Large T antigen of polyomaviruses, or E6/E7 proteins of HPV, can transform PCa cells and interfere with the interferon (IFN) signaling pathways [101]. The complexities of host genetics additionally favor or impede control of viral infections, enabling the virus to exert its cytopathic effects and transform normal prostate cells. A detailed literature of host genetics and viral infection interplay in prostate carcinogenesis is reviewed in [102].
Viruses such as the human papillomavirus (HPV), herpesviruses including cytomegalovirus (CMV), human herpes simplex virus type 2 (HSV2), human herpesvirus type 8 (HHV8) and Epstein-Barr virus (EBV), and xenotropic murine leukemia related virus (XMRV), have been found in the prostate. However, a direct association of these viruses with prostate tumorigenesis has not yet been established [103]. Not much evidence has indicated the role of viral infections in the etiology of PCa, possibly due to small sample size, inability to test non-persistent infections and inadequate tissue sampling or sensitivity of current viral detection methods [103]. Briefly, a link between HPV and Mediterranean and Asian population for HPV subtypes 16 and 18 with PCa was reported [104-106]. In addition, a Finnish cohort study demonstrated the seropositivity for HPV-18 and HPV-16 associated with PCa [107&108]. However, a meta-analysis showed a weak association between HPV-16 and PCa but not for HPV-18 [109]. CMV, HSV-1, HSV-2, HHV-8 and EBV infections have also been investigated in PCa, although a significant association between these sexually transmitted pathogens and PCa has not been established [110]. RNASEL/HPC, a candidate gene for PCa, also involved in antiviral and antiproliferative role of IFNs and its variant R462Q is also known to affect the suppression of viral infection, as reported for XMRV in PCa [100]. Two proteins of HPV; E6 and E7 are known to interact with p53 and pRb respectively resulting in their degradation or inactivation [111]. Increased titer of antibody to L-Tag was found to be associated with an increase in expression of IL-10 as opposed to IFN- γ in patients with PCa and benign prostate hyperplasia.
BKV has been reported to cause proliferative inflammatory atrophy (PIA) and prostatic intraepithelial neoplasia (PIN) in early- stages of prostate cancer. High frequency of BKV and JC virus DNA have been reported in prostate cancer patients. Delbue et al. [21] reported BKV prevalence of 16.5% (95% CI 13.8%-19.2%) among the tissues from PCa patients, and 7.0% among the control tissues from Benign Hyperplasia (BPH) patients (95% CI 5.2%- 8.8%; p<0.0001). Highest BKV infection rates of about 28% in PCa and 15% in BPH samples were also reported in a study from Iran [112]. Among the spectrum of human cancers, prostate tumors have a relatively low frequency of mutations with respect to p53 and RBI genes, however, the presence of oncoproteins LTag and small antigen in polyomaviruses are capable of transformation of normal cells (19,20). A case-controlled study among cancerous prostate cancer patients in Sudan detected higher frequency of BKV LTag as compared to patients with BPH prostates. The study also showed aging and geographical affiliation as important cofactors in progression of PCa [22]. BKV infection in different cell types was shown to upregulate genes associated with cell proliferation and IFN-signaling pathway, indicating the possible dissemination of the virus to various sites and lodge a persistent infection [113]. Elevated levels of CXCL10 and ZBP1, released by leukocytes in response to BKV infection has also been reported, facilitating BKV’s ability to trigger an innate immune response [114]. The mechanistic basis of the viral-host interaction was not explained in these studies and thus needs more insight. Due to the high prevalence and frequent reactivation of BKV, it has been difficult to define its role in cancer and is still considered controversial. Until now, the exact underlying molecular mechanism orchestrating the viral oncogenic activity in the tumor microenvironment is not known.
Additionally, a comparative analysis among racial profiles of men with PCa and a causative link with the prevalence of BKV in them has not been demonstrated so far. A thorough investigation of comparable analysis for viral infections driving tumor evolution and associated heterogeneity between racial groups would be interesting to look at. Therefore, underpinning the genomic signatures/biomarkers determining racial disparities within a particular geographical location should advance our understanding of the virus oncogenic potential.
Thus far, the only plausible explanation for BKV’s co-factorial role in PCa progression has been explained by the ‘hit and run hypotheses. Reeves and Dong [115&116] explained it as the binding of a viral antigen to a self/autoantigen; triggers an immune response that subsequently can be perpetuated by self-antigen. In other words, it can be described as an autocrine-paracrine effect, involving secretion of growth factors by cells expressing polyomavirus Tag, that may be responsible for recruiting to proliferate Tag-negative cells in polyomavirus-associated human tumors. This explains the ability of polyomaviruses to interfere with the cell cycle during oncogenic transformation. Subsequently, the cell cycle is manipulated and the sequestration of p53 via LTag, results in accumulation of gene mutations in the infected cells causing the transformation without the actual support of viral components [19&20]. In conclusion, it may be interesting to decipher the role of other genes and non-coding RNAs such as miRNA or LnCRNAs (described in the previous sections) differentially expressed in PCa, in conjunction to their association with p53 upon LTag binding. The model described below depicts one such connection between BKVmediated upregulation of MALAT-1 expression among AA and EA populations with PCa as MALAT-1 might represent a biomarker for p53 deregulation and modulate those differences.
However, it cannot be underestimated that more work is required to unravel the molecular mechanism underlying the viral oncogenic activity and understand the basis of oncogenic virus interference within the tumor microenvironment, thereby driving tumor progression (Figure 4) [49].
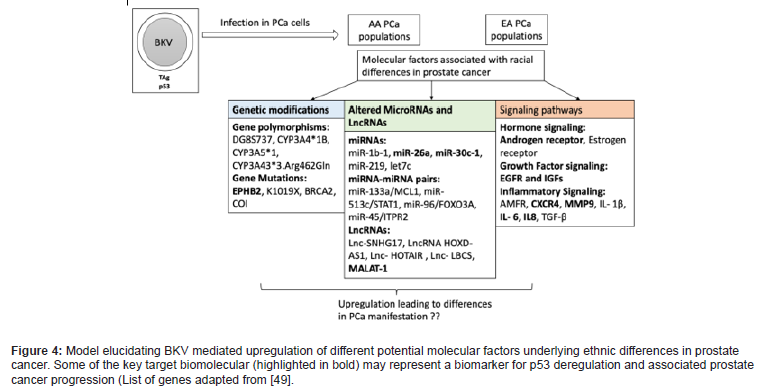
Conclusions- Racial disparities in PCa and way forward
The racial heterogeneity representing the African diaspora comprises of west African descendants who had migrated to the US, and thus classified as African- American. The geographic ancestry varies among African, European, and Native American groups.
Factoring in an individual’s race/ethnicity are instrumental for understanding the underlying mechanistic racial profile differences in cancer and other diseases. Differences in prostate cancer risk could be attributed to tumor gene expression profiles that in turn could be largely associated to heritable factors as well as other nonbiological factors.
Important developments pertaining to racial heterogenetic profiles can leverage the impact of difference in treatment regimens due to systemic racism on tumor biology. Thereby, resulting in profound impacts on health policy and disease prevention to improve and mitigate racial disparities in healthcare. Albeit, a significant proportion of research has been conducted to address these disparities, more evaluative future studies require the inclusion of diverse patient populations with a focus on marginalized communities.
Genomic and molecular investigations of PCa owing to racial heterogeneity have identified molecular signatures that may serve as therapeutic targets, paving a way for development of targeted cancer therapies. In addition, tumor virology as a field is still not greatly established and needs more attention to determine the role of viruses in etiology of cancer progression. The classical Koch’s postulates, formulated to demonstrate the etiologic role of microorganisms in infectious diseases, cannot be applied to prove the viral etiology of human tumors directly. Among various reasons, the ubiquitous nature of viruses in human tumors, which produce a persistent or latent infection in human tissues or eventual dilution of viral episome due to lack of replication. Therefore, detection and prevalence of viruses in human tumors does not establish causation. More direct efforts should be considered in order to evaluate the oncogenic potential of viruses in human cancers. Lastly, designing studies to fully characterize viral oncogenic differences in tumor heterogeneity across racial/ethnic groups and identifying key biomarkers for treatment that are effective across all PCa patients can revolutionize the field of tumor virology.
Acknowledgment
None.
Conflict of Interest
No conflict of Interest.
References
- Hirsch HH, Steiger J (2003) Polyomavirus BK. Lancet Infect Dis 3(10): 611-623.
- Gardner Sylvia D, Field Anne M, Coleman Dulcie V, Hulme B (1971) New Human Papovavirus (BK Isolated from Urine After Renal Transplantation. The Lancet 297(7712): 1253-1257.
- Knowles WA (2006) Discovery and Epidemiology of the Human Polyomaviruses BK Virus (BKV) and JC Virus (JCV). Adv Exp Med Biol 577: 19-45.
- Barraclough KA, Isbel NM, Staatz CE, Johnson DW (2011) BK Virus in Kidney Transplant Recipients: The Influence of Immunosuppression. J Transplant pp. 1-9.
- Fioriti D, Videtta M, Mischitelli M, Degener AM, Russo G (2005) The human polyomavirus BK: Potential role in cancer. J Cell Physiol 204(2): 402-406.
- Walker DL, Padgett BL (1983) The epidemiology of human polyomaviruses. Prog Clin Biol Res 105: 99-106.
- World Health Organization, International Agency for Research on Cancer (Eds.) Malaria and some polyomaviruses (SV40, BK, JC, and Merkel cell viruses). Lyon: IARC Press; 2014. (IARC monographs on the evaluation of carcinogenic risks to humans).
- Barbanti Brodano G, Sabbioni S, Martini F (200-2013) BK and JC Virus and Simian Virus 40 Infection in Humans, and Association with Human Tumors. In: Madame Curie Bioscience Database.
- Barbanti-Brodano G, Sabbioni S, Martini F, Negrini M, Corallini A, et al. (2006) BK Virus, JC Virus and Simian Virus 40 Infection in Humans, and Association with Human Tumors. Adv Exp Med Biol 577: 319-341.
- Pasternak J, Kliszczewska E, Polz-Dacewicz M (2018) BK Virus in Cancer Development. Curr Issues Pharm Med Sci 31(2): 65-68.
- Abend JR, Jiang M, Imperiale MJ (2009) BK virus and human cancer: Innocent until proven guilty. Semin Cancer Biol 19(4): 252-260.
- Imperiale MJ (2000) The Human Polyomaviruses, BKV and JCV: Molecular Pathogenesis of Acute Disease and Potential Role in Cancer. Virology 267(1): 1-7.
- Lee W, Langhoff E (2006) Polyomavirus in Human Cancer Development. Adv Exp Med Biol 577: 310-318.
- Tognon M, Corallini A, Martini F, Negrini M, Barbanti-Brodano G (2003) Oncogenic transformation by BK virus and association with human tumors. Oncogene 22(33): 5192-5200.
- Flaegstad T, Andresen PA, Johnsen JI, Asomani SK, Jørgensen GE, et al. (1999) A possible contributory role of BK virus infection in neuroblastoma development. Cancer Res 59(5): 1160-1163.
- Arthur RR, Shah KV (1989) Occurrence and significance of papovaviruses BK and JC in the urine. Prog Med Virol 36: 42-61.
- Comar M, Bonifacio D, Zanconati F, Di Napoli M, Isidoro E, Martini F, et al. (2011) High prevalence of BK Polyomavirus Sequences in Human Papillomavirus-16-Positive Precancerous Cervical Lesions. J Med Virol 83(10): 1770-1776.
- Balis V, Sourvinos G, Soulitzis N, Giannikaki E, Sofras F, et al. (2007) Prevalence of BK virus and human papillomavirus in human prostate cancer. Int J Biol Markers 22(4): 245-51.
- Das D, Shah RB, Imperiale MJ (2004) Detection and expression of human BK virus sequences in neoplastic prostate tissues. Oncogene 23(42): 7031-7046.
- Das D, Wojno K, Imperiale MJ (2008) BK Virus as a Cofactor in the Etiology of Prostate Cancer in Its Early Stages. J Virol 82(6): 2705-2714.
- Das D, Wojno K, Imperiale MJ (2008) BK Virus as a Cofactor in the Etiology of Prostate Cancer in Its Early Stages. J Virol 82(6): 2705-2714.
- Delbue S, Ferrante P, Provenzano M (2014) Polyomavirus BK and prostate cancer: an unworthy scientific effort? Oncoscience 1(4): 296-303.
- Gorish BMT, Ournasseir MEH, Shammat IM (2019) A correlation study of BK Polyoma Virus infection and prostate Cancer among Sudanese patients - immunofluorescence and molecular based case-control study. Infect Agent Cancer 14(1): 25.
- Lau SK, Lacey SF, Chen YY, Chen W G, Weiss LM (2007) Low frequency of BK virus in prostatic adenocarcinomas. APMIS Acta Pathol Microbiol Immunol Scand 115(6): 743-749.
- Narayanan M, Szymanski J, Slavcheva E, Rao A, Kelly A, et al. (2007) BK Virus Associated Renal Cell Carcinoma: Case Presentation with Optimized PCR and Other Diagnostic Tests. Am J Transplant 7(6): 1666-1671.
- Geetha D, Tong BC, Racusen L, Markowitz JS, Westra WH (2002) Bladder carcinoma in a transplant recipient: evidence to implicate the BK human polyomavirus as a causal transforming agent: Transplantation 73(12): 1933-1936.
- Campello C, Comar M, Zanotta N, Minicozzi A, Rodella L, et al. (2010) Detection of SV40 in colon cancer: a molecular case-control study from northeast Italy. J Med Virol 82(7): 1197-1200.
- Lundstig A, Stattin P, Persson K, Sasnauskas K, Viscidi RP, et al. (2007) No excess risk for colorectal cancer among subjects seropositive for the JC polyomavirus. Int J Cancer 121(5): 1098-1102.
- Drop B, Strycharz-Dudziak M, Kliszczewska E, Polz-Dacewicz M (2017) Coinfection with Epstein–Barr Virus (EBV), Human Papilloma Virus (HPV) and Polyoma BK Virus (BKPyV) in Laryngeal, Oropharyngeal and Oral Cavity Cancer. Int J Mol Sci 18(12): 2752.
- Burger-Calderon R, Ramsey KJ, Dolittle-Hall JM, Seaman WT, Jeffers-Francis LK, et al. (2016) Distinct BK polyomavirus non-coding control region (NCCR) variants in oral fluids of HIV- associated Salivary Gland Disease patients. Virology 493: 255-266.
- Jeffers LK, Madden V, Webster-Cyriaque J (2009) BK virus has tropism for human salivary gland cells in vitro: Implications for transmission. Virology 394(2): 183-193.
- Jeffers LK, Webster-Cyriaque J (2010) HIV-associated salivary gland disease: a role for BK birus. Infect Agent Cancer 5(S1):
- Low JA, Magnuson B, Tsai B, Imperiale MJ (2006) Identification of Gangliosides GD1b and GT1b as Receptors for BK Virus. J Virol 80(3): 1361-1366.
- Eash S, Querbes W, Atwood WJ (2004) Infection of Vero Cells by BK Virus Is Dependent on Caveolae. J Virol 78(21): 11583-11590.
- Jiang M, Abend JR, Tsai B, Imperiale MJ (2009) Early Events during BK Virus Entry and Disassembly. J Virol 83(3): 1350-1358.
- Moriyama T, Sorokin A (2008) Intracellular trafficking pathway of BK Virus in human renal proximal tubular epithelial cells. Virology 371(2): 336-349.
- Bennett SM, Zhao L, Bosard C, Imperiale MJ (2015) Role of a nuclear localization signal on the minor capsid Proteins VP2 and VP3 in BKPyV nuclear entry. Virology 474: 110-116.
- Helle F, Brochot E, Handala L, Martin E, Castelain S, et al. (2017) Biology of the BKPyV: An Update. Viruses 9(11): 327.
- Kane JR, Fong S, Shaul J, Frommlet A, Frank AO, et al. (2020) A polyomavirus peptide binds to the capsid VP1 pore and has potent antiviral activity against BK and JC polyomaviruses. eLife 9: e50722.
- Johannessen M, Myhre MR, Dragset M, Tümmler C, Moens U (2008) Phosphorylation of human polyomavirus BK agnoprotein at Ser-11 is mediated by PKC and has an important regulative function. Virology 379(1): 97-109.
- Nilsson J, Miyazaki N, Xing L, Wu B, Hammar L, et al. (2005) Structure and Assembly of a T =1 Virus-Like Particle in BK Polyomavirus. J Virol 79(9): 5337-5345.
- Ikegaya H, Saukko PJ, Tertti R, Metsärinne KP, Carr MJ, et al. (2006) Identification of a genomic subgroup of BK polyomavirus spread in European populations. J Gen Virol 87(11): 3201-3208.
- Knowles WA (2001) Propagation and assay of BK virus. Methods. Mol Biol Clifton NJ 165: 19-31.
- Krumbholz A, Bininda-Emonds ORP, Wutzler P, Zell R (2008) Evolution of four BK virus subtypes. Infect Genet Evol 8(5): 632-643.
- Vaezjalali M, Azimi H, Hosseini SM, Taghavi A, Goudarzi H (2017) Different Strains of BK Polyomavirus: VP1 Sequences in a Group of Iranian Prostate Cancer Patients. Urol J [Internet] 15(2): 44-48.
- Morel V, Martin E, François C, Helle F, Faucher, Jet al. (2017) A Simple and Reliable Strategy for BK Virus Subtyping and Subgrouping. J Clin Microbiol 55(4): 1177-1185.
- Pagnani M, Negrini M, Reschiglian P, Corallini A, Balboni PG, et al. (1986) Molecular and biological properties of BK virus-IR, a BK virus variant isolated from a human tumor. J Virol 59(2): 500-505.
- Chornokur G, Dalton K, Borysova ME, Kumar NB (2011) Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer: Prostate Cancer Disparities in African American Men. The Prostate 71(9): 985-997.
- Rawla P (2019) Epidemiology of Prostate Cancer. World J Oncol 10(2): 63-89.
- Bhardwaj A, Sanjeev K Srivastava, Mohammad Aslam Khan, Vijay K Prajapati, Seema Singh et al. (2017) Racial disparities in prostate cancer a molecular perspective Front. Biosci 22(5): 772-782.
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, et al. (2005) Recurrent Fusion of TMPRSS2 and ETS Transcription Factor Genes in Prostate Cancer. Science 28 310(5748): 644-648.
- Tomlins SA, Bjartell A, Chinnaiyan AM, Jenster G, Nam RK, et al. (2009) ETS Gene Fusions in Prostate Cancer from Discovery to Daily Clinical Practice. Eur Urol 56(2): 275-286.
- Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, et al. (2012) Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet 44(6): 685-689.
- Freedman ML, Haiman CA, Patterson N, McDonald GJ, Tandon A, et al. (2006) Admixture mapping identifies 8q24 as a prostate cancer risk locus in African American men. Proc Natl Acad Sci 103(38): 14068-14073.
- Haiman CA, Chen GK, Blot WJ, Strom SS, Berndt SI, et al. (2011) Genome-wide association study of prostate cancer in men of African ancestry identifies a susceptibility locus at 17q21. Nat Genet 43(6): 570-573.
- Okobia MN, Zmuda JM, Ferrell RE, Patrick AL, Bunker CH, et al. (2011) Chromosome 8q24 variants are associated with prostate cancer risk in a high-risk population of African ancestry Chromosome 8q24 Variants and Prostate Cancer Risk. The Prostate 71(10): 1054-1063.
- Robbins CM, Hooker S, Kittles RA, Carpten JD (2011) EphB2 SNPs and Sporadic Prostate Cancer Risk in African American Men. Chi J-TA editor PLoS ONE 6(5): e19494.
- Karakas C, Wang C, Deng F, Huang H, Wang D, et al. (2017) Molecular mechanisms involving prostate cancer racial disparity. Am J Clin Exp Urol 5(3): 34-48.
- Yuan J, Kensler KH, Hu Z, Zhang Y, Zhang T, et al. (2020) Integrative comparison of the genomic and transcriptomic landscape between prostate cancer patients of predominantly African or European genetic ancestry. McKinnon P editor PLOS Genet 16(2): e1008641.
- Conti DV, Wang K, Sheng X, Bensen JT, Hazelett DJ, et al. (2017) Two Novel Susceptibility Loci for Prostate Cancer in Men of African Ancestry. J Natl Cancer Inst: 109.
- Cook MB, Wang Z, Yeboah ED, Tettey Y, Biritwum RB, et al. (2014) A genome-wide association study of prostate cancer in West African men. Hum Genet133(5): 509-521.
- Jaratlerdsiri W, Chan EKF, Gong T, Petersen DC, Kalsbeek AMF, et al. (2018) Whole-Genome Sequencing Reveals Elevated Tumor Mutational Burden and Initiating Driver Mutations in African Men with Treatment-Naïve, High-Risk Prostate Cancer. Cancer Res 78(24): 6736-6746.
- Watson PA, Arora VK, Sawyers CL (2015) Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer 15(12): 701-711.
- Farrell J, Petrovics G, McLeod DG, Srivastava S (2013) Genetic and molecular differences in prostate carcinogenesis between African American and Caucasian American men. Int J Mol Sci 14(8): 15510-15531.
- Lange EM, Sarma AV, Ray A, Wang Y, Ho LA, et al. (2008) The androgen receptor CAG and GGN repeat polymorphisms and prostate cancer susceptibility in African American men results from the Flint Men’s Health Study. J Hum Genet 53(3): 220-226.
- Jin S, Levine AJ. (2001) The p53 functional circuit. J Cell Sci 114(Pt 23): 4139-4140.
- Muller PAJ, Vousden KH (2014) Mutant p53 in cancer new functions and therapeutic opportunities. Cancer Cell 25(3): 304-317.
- Grasso CS, Wu Y-M, Robinson DR, Cao X, Dhanasekaran SM, et al. (2012) The mutational landscape of lethal castration-resistant prostate cancer. Nature 487(7406): 239-243.
- Abeshouse A, Ahn J, Akbani R, Ally A, Amin S, et al. (2015) The Molecular Taxonomy of Primary Prostate Cancer. Cell 163(4): 1011-1025.
- Abida W, Armenia J, Gopalan A, Brennan R, Walsh M, et al. (2017) Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis Oncol: 1-16.
- Faisal FA, Sundi D, Tosoian JJ, Choeurng V, Alshalalfa M et al. (2016) Racial Variations in Prostate Cancer Molecular Subtypes and Androgen Receptor Signaling Reflect Anatomic Tumor Location. Eur Urol 70(1): 14-17.
- Negoita S, Feuer EJ, Mariotto A, Cronin KA, Petkov VI, et al. (2018) Annual Report to the Nation on the Status of Cancer, part II: Recent changes in prostate cancer trends and disease characteristics: Recent Changes in Prostate Cancer Trends. Cancer 124(13): 2801-2814.
- Morgan TO, Jacobsen SJ, McCarthy WF, Jacobson DJ, McLeod DG, et al. (1996) Age-Specific Reference Ranges for Serum Prostate-Specific Antigen in Black Men. N Engl J Med 335(5): 304-310.
- Moul JW, Sesterhenn IA, Connelly RR, Douglas T, Srivastava S et al. (1995) Prostate-specific antigen values at the time of prostate cancer diagnosis in African American men. JAMA 274(16): 1277-1281.
- Moul JW, Connelly RR, Mooneyhan RM, Zhang W, Sesterhenn IA, et al. (1999) Racial differences in tumor volume and prostate specific antigen among radical prostatectomy patients. J Urol 162(2): 394-397.
- Merola R, Tomao L, Antenucci A, Sperduti I, Sentinelli S, et al. (2015) PCA3 in prostate cancer and tumor aggressiveness detection on 407 high-risk patients: a National Cancer Institute experience. J Exp Clin Cancer Res 34(1): 15.
- Hermans KG, Boormans JL, Gasi D, van Leenders GJHL, Jenster G, et al. (2009) Overexpression of Prostate-Specific TMPRSS2(exon 0)-ERG Fusion Transcripts Corresponds with Favorable Prognosis of Prostate Cancer. Clin Cancer Res 115(20): 6398-6403.
- Yang Z, Yu L, Wang Z (2016) PCA3 and TMPRSS2-ERG gene fusions as diagnostic biomarkers for prostate cancer. Chin J Cancer Res 28(1): 65-71.
- Ferlay JEM, Lam F, Colombet M, Mery L, Pineros M, et al. (2020) Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer. Created with mapchart.net. Data obtained from Globocan.
- National Center for Health Statistics (2017) Compressed Mortality File, 1999-2016 (machine readable data file and documentation, CD‑ROM Series 20, No. 2V) as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. Hyattsville, Maryland.
- Nientiedt C, Endris V, Jenzer M, Mansour J, Sedehi NTP, et al. (2020) High prevalence of DNA damage repair gene defects and TP53 alterations in men with treatment-naïve metastatic prostate cancer –Results from a prospective pilot study using a 37 gene panel. Urol Oncol Semin Orig Investig 38(7): 637.e17-637.e27.
- Teroerde M, Nientiedt C, Duensing A, Hohenfellner M, Stenzinger A, et al. (2022) Revisiting the Role of p53 in Prostate Cancer. In: Urology Department, Frimley Park Hospital, Portsmouth Rd, Frimley, Camberley GU16 7UJ, UK, Bott SR, Lim Ng K, editors. Prostate Cancer [Internet]. Exon Publications; 2021. pp. 113-24.
- Ashikari D, Takayama K, Tanaka T, Suzuki Y, Obinata D, et al. (2017) Androgen induces G3BP2 and SUMO-mediated p53 nuclear export in prostate cancer. Oncogene 36(45): 6272-6281.
- Gu J, Wang B, Liu Y, Zhong L, Tang Y, et al. (2014) Murine Double Minute 2 siRNA and wild type p53 gene therapy interacts positively with zinc on prostate tumours in vitro and in vivo. Eur J Cancer 50(6): 1184-1194.
- Suzuki K, Matsui H, Ohtake N, Nakata S, Takei T, et al. (2003) A p53 codon 72 polymorphism associated with prostate cancer development and progression in Japanese. J Biomed Sci 10(4): 430-435.
- Zhang L, Shao N, Yu Q, Hua L, Mi Y, et al. (2012) Association between p53 Pro72Arg polymorphism and prostate cancer risk: a meta-analysis. J Biomed Res 25(1): 25-32.
- Ricks Santi L, Mason T, Apprey V, Ahaghotu C, McLauchlin A, et al. (2010) p53 Pro72Arg polymorphism and prostate cancer in men of African descent: p53 Pro72Arg Polymorphism and Prostate Cancer. The Prostate 70(16): 1739-1745.
- Bhardwaj A, Singh S, Singh AP (2010) MicroRNA-based Cancer Therapeutics: Big Hope from Small RNAs. Mol Cell Pharmacol. 2(5): 213-219.
- Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6(11): 857-866.
- Theodore SC, Rhim JS, Turner T, Yates C (2010) MiRNA 26a expression in a novel panel of African American prostate cancer cell lines. Ethn Dis 20(1 Suppl 1): S1-96-100.
- Theodore SC, Davis M, Zhao F, Wang H, Chen D, et al. (2014) MicroRNA profiling of novel African American and Caucasian Prostate Cancer cell lines reveals a reciprocal regulatory relationship of miR- 152 and DNA methyltranferase 1. Oncotarget. 5(11): 3512-3525.
- Wang BD, Ceniccola K, Yang Q, Andrawis R, Patel V, et al. (2015) Identification and Functional Validation of Reciprocal microRNA–mRNA Pairings in African American Prostate Cancer Disparities. Clin Cancer Res 21(21): 4970-4984.
- Hua JT, Chen S, He HH (2019) Landscape of Noncoding RNA in Prostate Cancer. Trends Genet. 35(11): 840-851.
- Bai M, Lei Y, Wang M, Ma J, Yang P, et al. (2020) Long Non-coding RNA SNHG17 Promotes Cell Proliferation and Invasion in Castration-Resistant Prostate Cancer by Targeting the miR-144/CD51 Axis. Front Genet 11: 274.
- Gu P, Chen X, Xie R, Han J, Xie W, et al. (2017) lncRNA HOXD-AS1 Regulates Proliferation and Chemo- Resistance of Castration-Resistant Prostate Cancer via Recruiting WDR5. Mol Ther 25(8): 1959-1973.
- Zhang A, Zhao JC, Kim J, Fong K, Yang YA, et al. (1015) LncRNA HOTAIR Enhances the Androgen- Receptor-Mediated Transcriptional Program and Drives Castration-Resistant Prostate Cancer. Cell Rep 13(1): 209-221.
- Ren S, Liu Y, Xu W, Sun Y, Lu J, et al. (2013) Long Noncoding RNA MALAT-1 is a New Potential Therapeutic Target for Castration Resistant Prostate Cancer. J Urol 190(6): 2278-2287.
- Chang J, Xu W, Du X, Hou J (2018) MALAT1 silencing suppresses prostate cancer progression by upregulating miR-1 and downregulating KRAS. OncoTargets Ther 11: 3461-3473.
- Jeffers LK, Duan K, Ellies LG, Seaman WT, Burger-Calderon RA, et al. (2013) Correlation of Transcription of MALAT-1, a Novel Noncoding RNA, with Deregulated Expression of Tumor Suppressor p53 in Small DNA Tumor Virus Models. J Cancer Ther 04(03): 774-786.
- Martinez Fierro ML, Leach RJ, Gomez Guerra LS, Garza Guajardo R, Johnson Pais T, et al. (2010) Identification of viral infections in the prostate and evaluation of their association with cancer. BMC Cancer 10: 326.
- Zheng ZM (2010) Viral Oncogenes, Noncoding RNAs, and RNA Splicing in Human Tumor Viruses. Int J Biol Sci pp. 730-755.
- Abidi SH, Bilwani F, Ghias K, Abbas F (2018) Viral etiology of prostate cancer: Genetic alterations and immune response. A literature review. Int J Surg 52: 136-140.
- De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, et al. (2007) Inflammation in prostate carcinogenesis. Nat Rev Cancer 7(4): 256-269.
- Michopoulou V, Derdas SP, Symvoulakis E, Mourmouras N, Nomikos A, et al. (2014) Detection of human papillomavirus (HPV) DNA prevalence and p53 codon 72 (Arg72Pro) polymorphism in prostate cancer in a Greek group of patients. Tumor Biol 35(12): 12765-12773.
- Singh N, Hussain S, Kakkar N, Singh SK, Sobti RC, et al. (2015) Implication of high-risk Human papillomavirus HR-HPV infection in prostate cancer in Indian population- A pioneering case-control analysis. Sci Rep 5(1): 7822.
- Whitaker NJ, Glenn WK, Sahrudin A, Orde MM, Delprado W, et al. (2013) Human papillomavirus and Epstein Barr virus in prostate cancer: Koilocytes indicate potential oncogenic influences of human papillomavirus in prostate cancer: HPV and EBV in Prostate Cancer. The Prostate 73(3): 236-241.
- Al Moustafa AE (2008) Involvement of human papillomavirus infections in prostate cancer progression. Med Hypotheses 71(2): 209-211.
- Dillner J, Knekt P, Boman J, Lehtinen M, Af Geijersstam V, et al. (1998) Sero-epidemiological association between human-papillomavirus infection and risk of prostate cancer. Int J Cancer 75(4): 564-567.
- Lin Y, Mao Q, Zheng X, Yang K, Chen H, et al. (2011) Human papillomavirus 16 or 18 infection and prostate cancer risk: a meta-analysis. Ir J Med Sci 180(2): 497-503.
- Hrbacek J, Urban M, Hamsikova E, Tachezy R, Heracek J (2013) Thirty years of research on infection and prostate cancer: no conclusive evidence for a link. A systematic review. Urol Oncol 31(7): 951-965.
- Leiros GJ, Galliano SR, Sember ME, Kahn T, Schwarz E, et al. (2005) Detection of human papillomavirus DNA and p53 codon 72 polymorphism in prostate carcinomas of patients from Argentina. BMC Urol 5: 15.
- Taghavi A, Mohammadi Torbati P, Kashi AH, Rezaee H, et al. (2015) Polyomavirus Hominis 1(BK virus) Infection in Prostatic Tissues: Cancer versus Hyperplasia. Urol J 12(4): 2240-2244.
- Sais G, Wyler S, Hudolin T, Banzola I, Mengus C, et al. (2012) Differential Patterns of Large Tumor Antigen-Specific Immune Responsiveness in Patients with BK Polyomavirus-Positive Prostate Cancer or Benign Prostatic Hyperplasia. J Virol 86(16): 8461-8471.
- An P, Sáenz Robles MT, Duray AM, Cantalupo PG, Pipas JM (2019) Human polyomavirus BKV infection of endothelial cells results in interferon pathway induction and persistence. Imperiale MJ, editor. PLOS Pathog 15(1): e1007505.
- Dong X, Hamilton KJ, Satoh M, Wang J, Reeves WH (1994) Initiation of autoimmunity to the p53 tumor suppressor protein by complexes of p53 and SV40 large T antigen. J Exp Med 179(4): 1243-1252.
- Reeves WH, Dong X, Wang J, Hamilton K (1997) Initiation of Autoimmunity to Self-proteins Complexed with Viral Antigens. Ann N Y Acad Sci 815(1 B-Lymphocytes): 139-154.
-
Shilpi Bhatia, Liesl Jeffers Francis. Opportunistic Virus BKV: Racial Disparities in Prostate Cancer. Curr Tr Clin & Med Sci. 3(2): 2022. CTCMS.MS.ID.000558.
-
Betapolyomavirus genus, Polyomaviridae, JC virus, Peripheral blood leukocytes, Kidney, Symptomatic, Brain, Cancer, Epidemiology, Benign Hyperplasia, Health Organization, DNA Microarray, Fluorescent, Hybridization
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






