 Research Article
Research Article
Molecular Docking Studies for the Evaluation of Cannabinoids as Multi-Target Inhibitor for Type 2 Diabetes
Guy Roussel Takuissu1,2*, Dupon Bruno Ambamba3, Shabnor Iqbal1, Kolowole Olofinsan1, Cedric Fossi Tchinda1,2, Fils Armand Ella1,3, Corinne Raissa Ngnameko1,2, Dany Joel Ngoumen1, Matsabisa Gilbert Motlalepula1*
1Department of Pharmacology, University of the Free State, Bloemfontein, South Africa
2Institute for the Medical Research and Medicinal Plant Studies (IMPM), Ministry of Scientific Research and Innovation, Yaoundé, Cameroon
3Department of Biochemistry, University of Yaounde, Yaounde, Cameroon
Matsabisa Gabriel Motlalepula, Department of Pharmacology, University of the Free State, Bloemfontein, South Africa
Received Date:January 16, 2025; Published Date:February 18, 2025
Abstract
Type 2 Diabetes is due to dysregulation of glycemic control through multiple mechanisms. Cannabinoids from Cannabis sativa L. show promise as anti-diabetic agents, though currently their molecular mechanisms are unclear. This study carried out in silico molecular docking of cannabinoids (cannabidiol (CBD), tetrahydrocannabivarin (THCV), cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN), Δ9-tetrahydrocannabinol (THC)) against key diabetes drug targets namely: dipeptidyl peptidase-4 (DPP-4), α-glucosidase, α-amylase and invertase. Enzyme structures were obtained from the RCSB Protein Data Bank, while ligand structures were generated and optimized using ChemDraw. AutoDockTools-1.5.7 determined docking parameters, and Biovia Discovery Studio visualized interaction profiles. Normal mode analysis was performed for molecular dynamics simulations. Cannabinoids used in this study showed higher binding affinities (-5.02 to -7.85 kcal/mol) than reference drugs for the targets, with the exception of DPP-4.
For DPP-4, CBN (-7.85 kcal/mol) showed best affinity followed by THC (-6.64 kcal/mol). All cannabinoids interacted with catalytic residues except cannabichromene. For α-amylase, THC (-7.14 kcal/mol) was strongest, with residues Asp197, Glu233, Asp300 involved. Against invertase, THC (-7.27 kcal/mol) had highest affinity interacting with Glu230. For α-glucosidase, THC (-7.86 kcal/mol) again dominated via key residues. Normal mode analysis revealed THC binding modulated complex dynamics differently for each enzyme. This study provides insights into cannabinoids’ multitarget binding profiles against important T2D drug targets, supporting their potential as natural anti-diabetic agents requiring further research.
Keywords:Type 2 diabetes; cannabinoids; carbohydrate digestive enzymes; DPP-4
Introduction
Diabetes is a growing global health problem, with more than 537 million people affected worldwide [1]. Around 90% of them have type 2 diabetes mellitus (T2DM). Individuals with T2DM are predisposed to developing comorbidities, like cancer, renal impairment, retinopathy, and cardiovascular disease [2]. Current pharmacological therapies employ a multi-pronged approach including curbing hepatic gluconeogenesis, potentiating insulin secretion, amplifying insulin receptor sensitivity, and optimizing peripheral glucose uptake, all with the goal of stabilizing postprandial glucose levels [3]. One approach for maintaining postprandial glucose control involves inhibiting the enzymes α-glucosidase, α-amylase and invertase, which prevents the hydrolysis of carbohydrates and subsequent absorption of glucose. Another approach is preventing the breakdown of Glucagon Like Peptide-1 (GLP-1) through inhibition of DPP-4, thereby allowing GLP-1 to stimulate insulin secretion and suppress glucagon levels after meals.
Both mechanisms help regulate post-meal blood glucose spikes in patients with type 2 diabetes [4,5]. However, the available anti-diabetic drugs are still associated with undesirable side effects. Therefore, is an ongoing need to discover and develop safer and more effective anti-diabetic agents. Cannabinoids, the active constituents of Cannabis sativa, have emerged as potential alternatives or adjuncts for managing T2D. Several studies have shown that cannabinoids can suppress hyperglycemia and lower insulin resistance by modulating multiple targets implicated in glucose homeostasis [6-8]. However, the molecular mechanisms underlying their anti-diabetic actions remain unclear. Molecular docking is a computational technique that can provide insights into ligand-protein interactions at the atomic level and predict their binding affinity. In silico studies have demonstrated the alphaglucosidase inhibitory activities of CBD and THC [9,10], while one study found that CBD, CBG, CBN and THC exhibited DPP-4 inhibitory activities [11].
No study, currently, was done on the effects of cannabinoids on the activities of alpha-amylase and invertase enzymes, and while just some few cannabinoids were evaluated against alphaglucosidase and DPP-4 inhibition. In this study, we carried out in silico molecular docking analysis of major cannabinoids from Cannabis sativa (THC, CBD, THCV, CBC, CBG and CBN), with key enzymes involved in carbohydrate metabolism that are therapeutic targets for T2D management was performed. The target enzymes investigated were DPP-4, α-amylase, invertase, and α-glucosidase. The current study will provide new mechanistic insights at the molecular level about the potential multi-target inhibitory effects of cannabinoids against T2DM.
Methodology
Software Used
The following software Python 2.5, and 2.7, Molecular Graphics Lab Tools (MGL), AutoDockTools-1.5.7, Discovery Studio Visualizer 2.5.5, and ChemDraw were used. The Python 2.7 was downloaded from www.python.com, Cygwin (a data store) c:\program; and Python 2.5 were downloaded simultaneously from www.cygwin. com. Molecular Graphics Lab Tools (MGL) and AutoDockTools-1.5.7 were downloaded from www.scripps.edu; Discovery Studio Visualizer 2.5.5 was downloaded from www.accelerys.com; and ChemDraw was downloaded from www.clubic.com [12].
Preparation of The Ligand
The two-dimensional structures (2D) of the cannabinoids and the reference (Acarbose and Sitagliptin) compounds were drawn using ChemDraw software. Three dimensional structures (3D) were generated using ChemDraw 3D software. Molecular mechanics (MM2) energy minimization was performed using the MM2 forcefield in ChemDraw 3D. The 3D structures were saved in PDB format.
Preparation of The Target Enzyme
The crystal structure of α-amylase protein (ID: 1B2Y), DPP4 (ID: 1R9N), invertase (ID: 3KF3) and α-glucosidase (ID: 3W37) were downloaded from the Research Collaboratory for Structural Bioinformatics (RCSB) protein database. The preparation of the target proteins with the AutoDock tools involved the addition of hydrogen atoms to the macromolecule, a necessary step for the correct calculation of partial atomic charges. Three-dimensional affinity grids of α-amylase contained the amino acids of its active site [Asp 197, Glu 233, and Asp 300] [13] of size 40 × 40 × 44 Å with spacing of 0.375 Å, the macromolecule with X, Y and Z coordinates of 8.22, 47.41 and 19.66. Three-dimensional affinity grids of DDP-4 contained the Catalytic Triad [ Ser 630, Asp 708, His 740] [14] of size 40 × 40 × 40 Å with spacing of 0.375 Å, the macromolecule with X, Y and Z coordinates of 24.173, 0.944 and 16.655. Threedimensional affinity grids of invertase contained the Catalytic Triad [Arg 178, Glu 230, Tyr 293] [15] of size 48 × 48 × 40 Å with spacing of 0.375 Å, the macromolecule with X, Y and Z coordinates of 18.917, -5.028 and 20.750. Active sites of α-glucosidase were predicted with Computed Atlas of Surface Topography of proteins (CASTp) [16]. Three-dimensional affinity grids of α-glucosidase contained the amino acid predicted with CASTp of size 70 × 64 × 86 Å with spacing of 0.375 Å, the macromolecule with X, Y and Z coordinates of 22.972, -25.056 and -52.083.
Table 1:

Docking Simulations
The molecular docking was performed using
AutoDockTools-1.5.7 which uses a Lamarckian genetic algorithm.
The algorithm performed 2,500,000 energy evaluations for each
run over 10 separate docking runs. Binding free energy (ΔG) was
estimated using an empirical scoring function that considers
various molecular mechanics energy terms:
ΔG = ΔGvdw + ΔGhbond + ΔGelec + ΔGconform + ΔGtor + ΔGsol
Where ΔGvdw is the van der Waals interaction energy, ΔGHBond is the hydrogen bonding energy, ΔGElec is the electrostatic interaction energy, ΔGConform represents penalty due to unfair conformations, ΔGTor accounts for forbidden torsional states and ΔGSol represents solvation energy [17]. All other docking parameters were set to AutoDock defaults. Discovery Studio Visualizer software was used to visualize the protein-ligand interactions in 3D and to analyze the binding modes.
Table 2:Molecular docking scores of the compound against DPP-4.
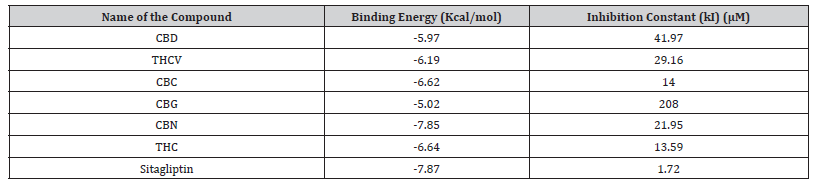
CBD: Cannabidiol; THCV: Tetrahydrocannabivarin; CBC: Cannabichromene; CBG: Cannabigerol; CBN: Cannabinol; THC: Δ-9-tetrahydrocannabinol.
Molecular Dynamics Simulation of the Ligand-Receptor Complex
The iMOD server was used to execute molecular dynamics simulations. The stability and flexibility of protein and ligand complex was assessed by iMODS server (http://imods.Chaconlab. org/) which carries out Normal Mode Analysis (NMA) in internal coordinates (dihedral) using an elastic network model [18]. The server illustrates the stability of the complex by providing deformability plots, covariance map, eigenvalues, and elastic network.
Results
The docking scores of the cannabinoids and their reference compounds on the therapeutic targets for T2D are presented in Tables 1, 3, 5 and 7 below. Compounds likely to bind to a target with the lowest binding energy (ΔG) are the ones with the strongest and most possible binding affinity. From this perspective, a binding energy is always the weaker than its affinity and its power.
Molecular docking of cannabinoids with DPP-4 Molecular Docking Scores with Cannabinoids
The binding affinity results of cannabinoids and the reference compound sitagliptin against DPP-4 are reported in Table 1. As shown, the docking scores for cannabinoids ranged from -5.97 kcal/ mol for CBD to -7.85 kcal/mol for CBN. A more negative binding energy results indicates a stronger predicted binding affinity. Among the cannabinoids, CBN (−7.85 kcal/mol) exhibited the best binding affinity for DPP-4, followed by THC (−6.64 kcal/mol) and CBC (−6.62 kcal/mol). All cannabinoids showed lower binding energies than the reference sitagliptin (-7.87 kcal/mol).
Profiles of Amino Acid Residues in the DPP-4 Interacting with Cannabinoids
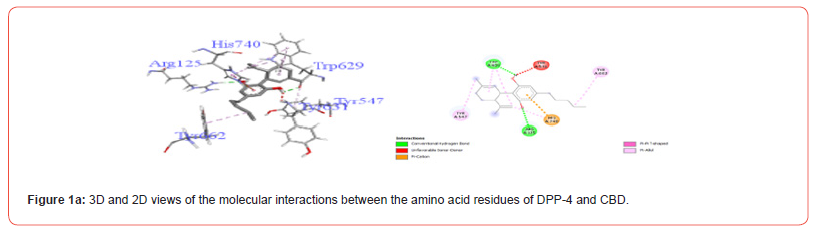
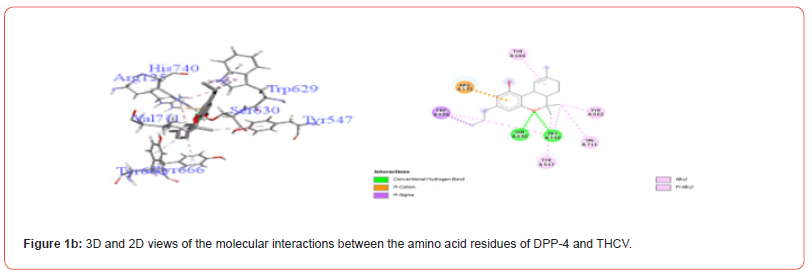
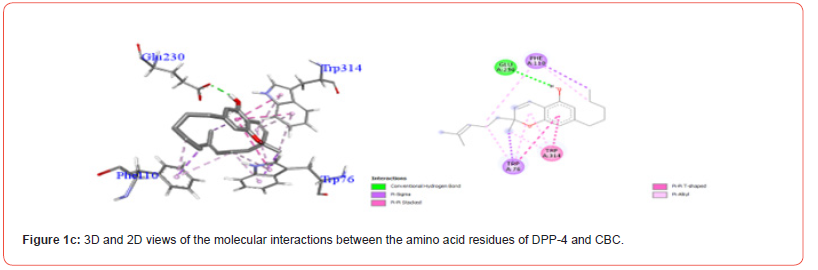
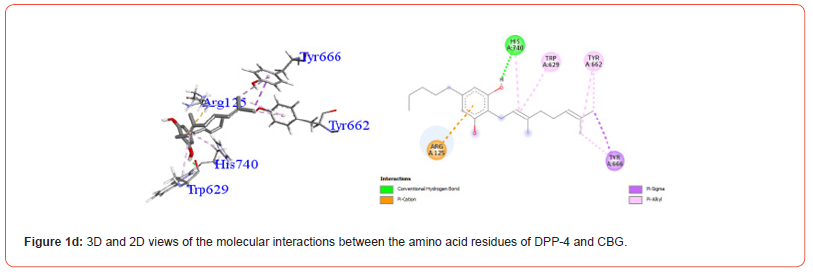
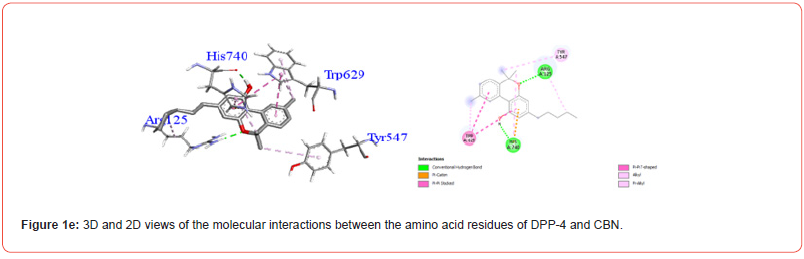
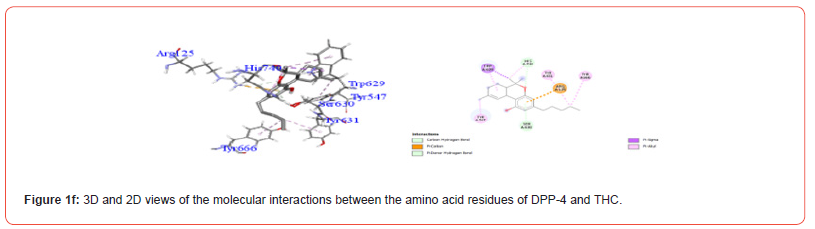
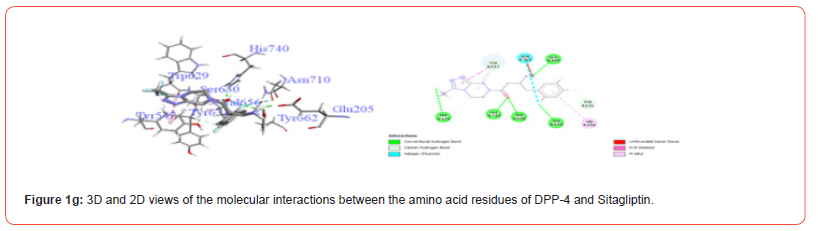
Table 3:Profiles of amino acid residues important for DPP-4 that interact with Cannabinoids.
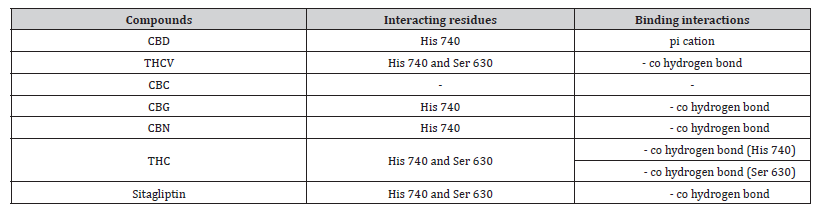
CBD: Cannabidiol; THCV: Tetrahydrocannabivarin; CBC: Cannabichromene; CBG: Cannabigerol; CBN: Cannabinol; THC: Δ-9-tetrahydrocannabinol; co: conventional; ca: carbon.
The amino acid residues in the active site of DPP-4 involved in interactions with each ligand are summarized in Table 2. Figures 1a-1g illustrate the 2D and 3D interactions between ligands and key residues Ser630, Asp708 and His740 within the DPP-4 active site. All cannabinoids were found to interact with at least one of these important residues (notably His740), except CBC which did not interact with any. Hydrogen bonding interactions were observed between Ser630, and THC and THCV.
Molecular Docking of Cannabinoids with α-amylase Molecular Docking Scores with Cannabinoids
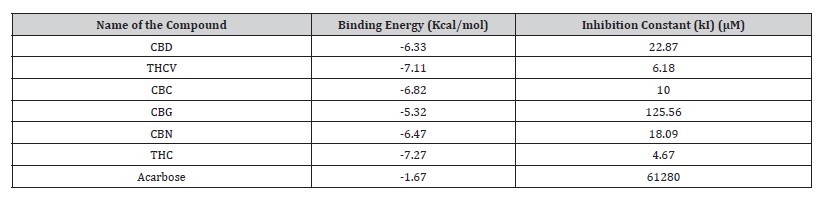
Molecular docking scores of cannabinoids and the reference compound acarbose against α-amylase are reported in Table 3. Cannabinoids showed better binding affinities (− 6.10 to −7.14 kcal/mol) than acarbose (−3.64 kcal/mol). The best binding was observed for THC (−7.14 kcal/mol) followed by CBC (−7.04 kcal/ mol).
Table 4:Molecular docking scores of the cannabinoid compounds against α-amylase.
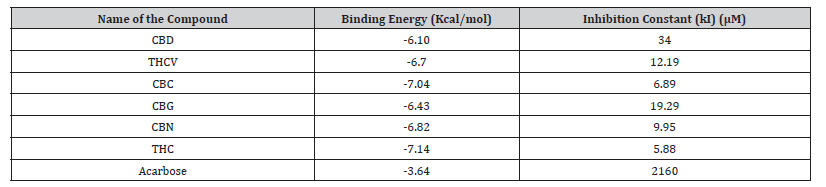
CBD: Cannabidiol; THCV: Tetrahydrocannabivarin; CBC: Cannabichromene; CBG: Cannabigerol; CBN: Cannabinol; THC: Δ -9-tetrahydrocannabinol.
Profiles of Amino Acid Residues in the α-amylase Interacting with Cannabinoids
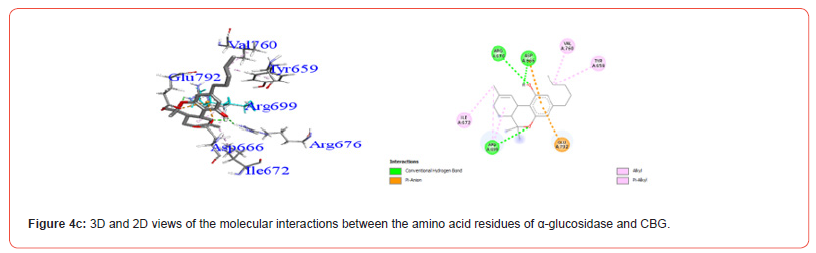
Interactions between ligands and key α-amylase residues Asp197, Glu233 and Asp300 are summarized in Table 4. As seen in Figures 2a-2g, all cannabinoids interacted with at least one of these residues, similar to Acarbose. CBG was the only ligand, along with acarbose, that interacted with all three key residues.
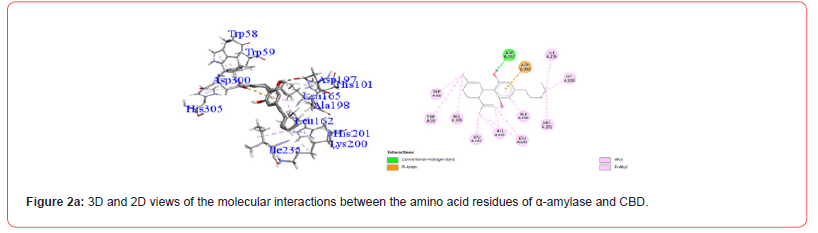
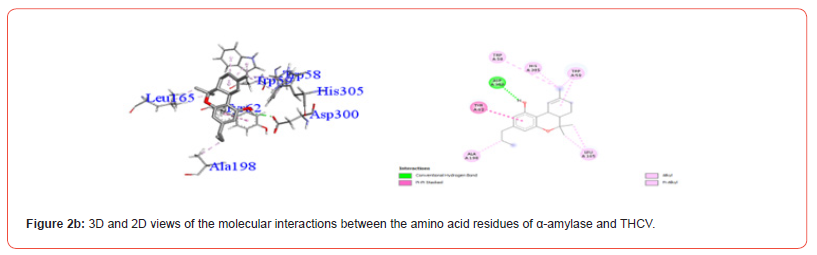
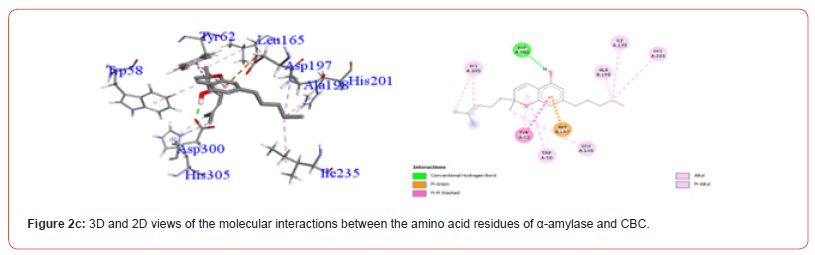
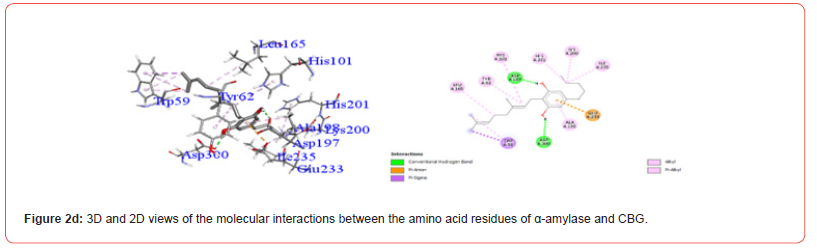
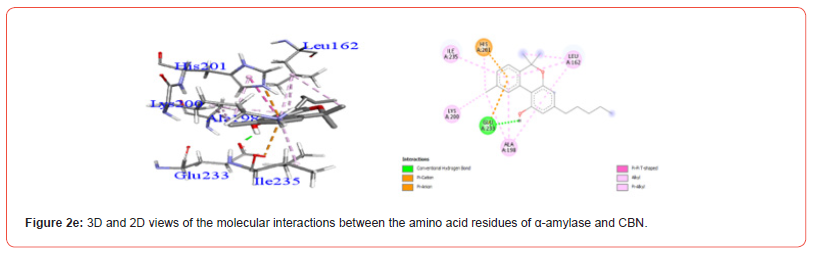
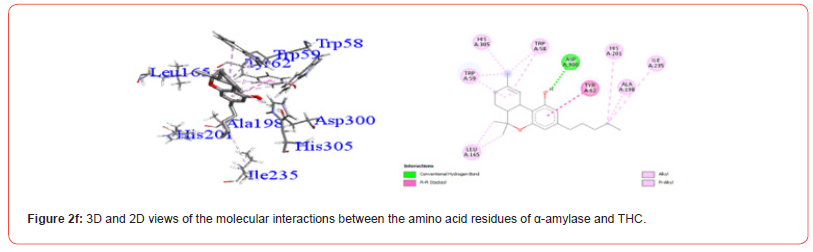
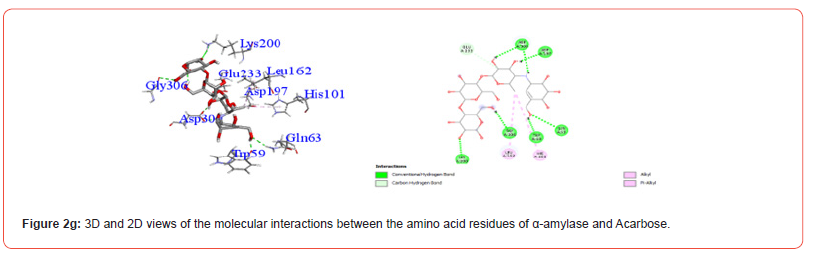
Table 5:Profiles of α-amylase amino acid residues interacting with Cannabinoids.
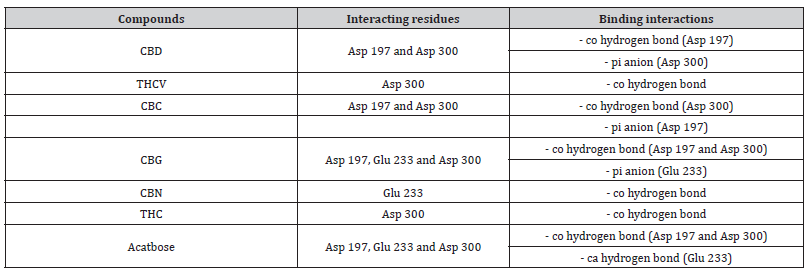
CBD: Cannabidiol; THCV: Tetrahydrocannabivarin; CBC: Cannabichromene; CBG: Cannabigerol; CBN: Cannabinol; THC: Δ -9-tetrahydrocannabinol; co: conventional; ca: carbon.
Molecular docking of cannabinoids with invertase Molecular docking scores with Cannabinoids
Binding affinities of cannabinoid compounds against invertase are reported in Table 5. Cannabinoids exhibited better binding (−5.32 to −7.27 kcal/mol) than the reference compound (−1.67 kcal/mol), with the strongest affinity shown by THC (−7.27 kcal/ mol).
Table 6:Molecular docking scores of the compound against invertase.
CBD: Cannabidiol; THCV: Tetrahydrocannabivarin; CBC: Cannabichromene; CBG: Cannabigerol; CBN: Cannabinol; THC: Δ -9-tetrahydrocannabinol.
Profiles of Amino Acid Residues in the Invertase Interacting with Cannabinoids
Table 6 summarizes interactions between ligands and important residues Arg178, Glu230 and Tyr293 within the invertase active site. As shown in Figures 3a-3g, all ligands interacted with Glu230 (pi-cation bonds), while only CBG formed an interaction with Arg178. Besides this, all other cannabinoids except THCV and THC have negative interactions with Tyr 293.
Table 7:Profiles of amino acid residues important for invertase that interact with Cannabinoids.
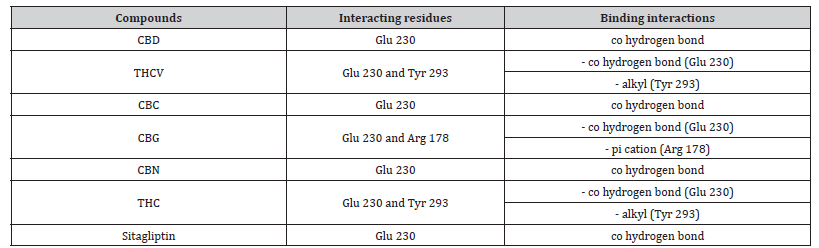
CBD: Cannabidiol; THCV: Tetrahydrocannabivarin; CBC: Cannabichromene; CBG: Cannabigerol; CBN: Cannabinol; THC: Δ -9-tetrahydrocannabinol; co: conventional; ca: carbon..
Molecular Docking of Cannabinoids with α-glucosidase Molecular Docking Scores with Cannabinoids
Binding scores of cannabinoids against α-glucosidase are presented in Table 7. Cannabinoids showed improved binding (−6.17 to −7.86 kcal/mol) over the reference acarbose (−4.12 kcal/ mol). THC exhibited the best inhibition potential (−7.86 kcal/mol).
Table 8:Molecular docking scores of the compound against α-glucosidase.
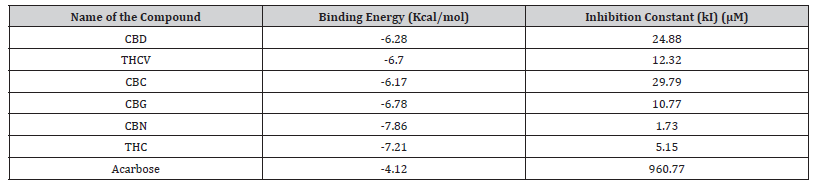
CBD: Cannabidiol; THCV: Tetrahydrocannabivarin; CBC: Cannabichromene; CBG: Cannabigerol; CBN: Cannabinol; THC: Δ-9-tetrahydrocannabinol.
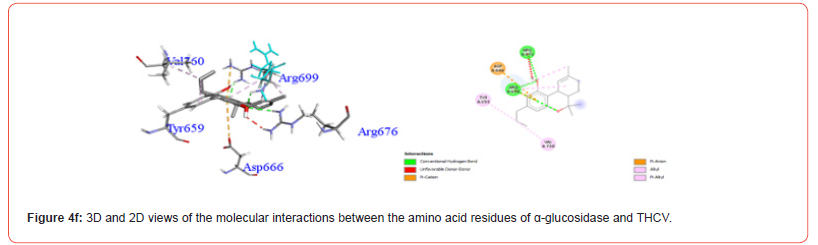
Profiles of α-glucosidase Amino Acid Residues Interacting with Cannabinoids
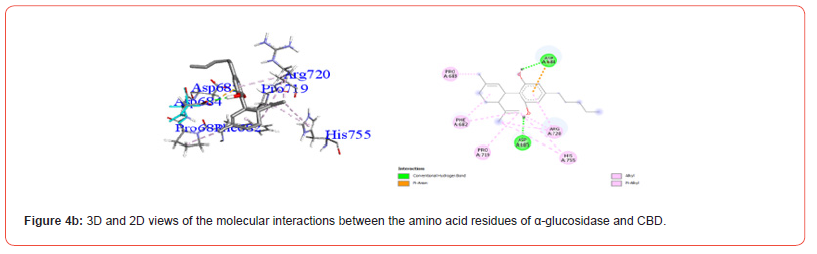
Figures 4a-4g show the various residues from the major sites from which the compounds of interest interact. The analysis of these interactions showed that all the compounds investigated were likely to establish conventional hydrogen and hydrophobic type interactions with the amino acid residues contained in the predicted active pocket. We found that THC generally showed the best activity on all of these enzymes and was selected for the molecular dynamic simulation.

Results of Molecular Dynamics Simulation of THC with the 4 Enzymes Molecular Dynamics Simulation of THC and DPP4
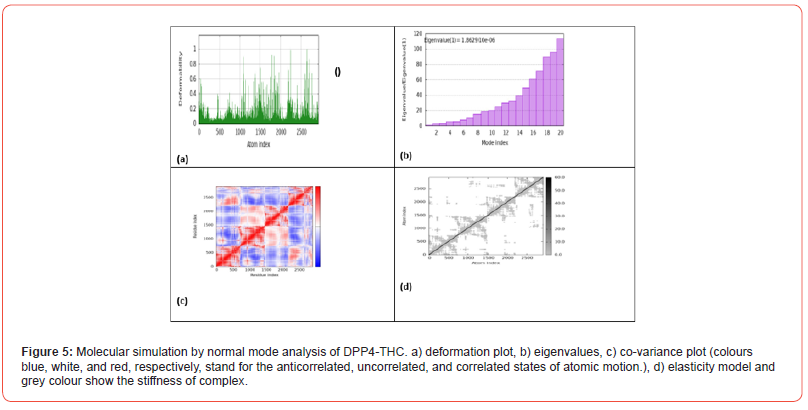
Figure 5 shows the results of molecular simulation by normal mode analysis of the DPP4-THC protein-ligand complex obtained from molecular docking. Figure 5a displays the deformation plot, which visualizes displacements along low frequency normal modes. This helps identify regions within the complex that are more flexible vs rigid upon THC binding to DPP4. Figure 5b shows the eigenvalue spectrum, with lower values corresponding to softer, more deformable motions. The pattern of peaks and valleys provides insight into how binding modifies variation in complex stiffness across different atomic motions. Figure 5c presents the co-variance map, where colors represent correlated, uncorrelated or anticorrelated atomic motions. This gives information on cooperative structural changes induced within the complex. Figure 5d depicts the elastic network model, where darker dots signify stiffer springs between atom pairs. This indicates how cannabinoid interaction alters intrinsic protein elasticity.
Molecular Dynamics Simulation of THC and Amylase
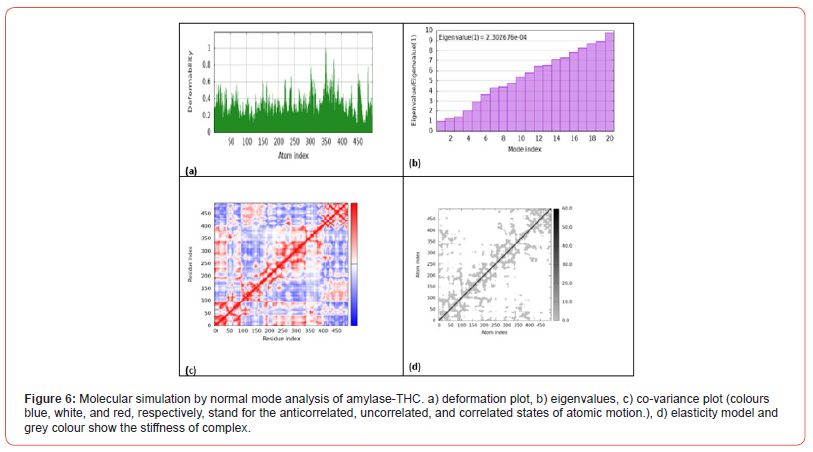
Figure 6 shows the results of molecular simulation by normal mode analysis of the amylase-THC protein-ligand complex obtained from molecular docking. Figure 6a displays the deformation plot, visualizing displacements along low frequency normal modes. This helps identify regions within the complex that are more/ less flexible upon THC binding to amylase. Figure 6b shows the eigenvalue spectrum, with lower values corresponding to softer, more deformable motions. The pattern provides insight into how binding modifies variation in complex stiffness across atomic motions. Figure 6c presents the co-variance map, where colors represent correlated, uncorrelated or anticorrelated atomic motions induced within the complex. Figure 6d depicts the elastic network model, indicating how THC interaction alters intrinsic protein elasticity between atom pairs.
Molecular Dynamics Simulation of THC and Invertase
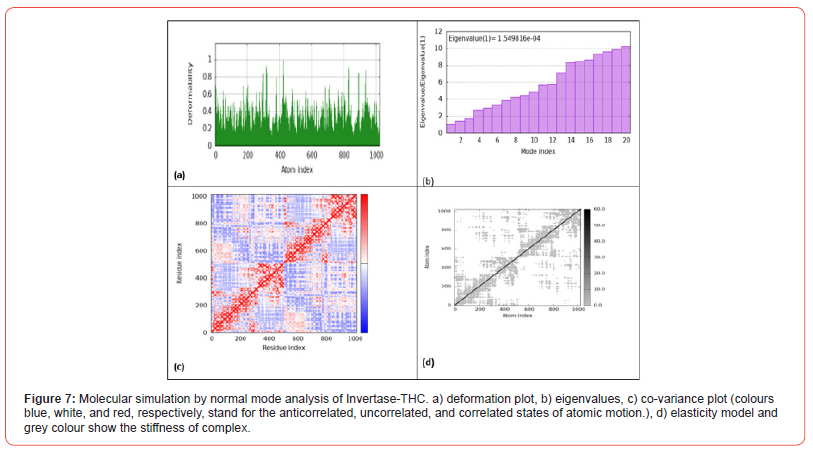
Figure 7 shows the results of molecular simulation by normal mode analysis of the invertase-THC protein-ligand complex obtained from molecular docking. Figure 7a displays the deformation plot, visualizing displacements along low frequency normal modes upon THC binding to invertase. This helps identify flexible/rigid regions within the complex. Figure 7b shows the eigenvalue spectrum, with lower values corresponding to softer, more deformable motions. The pattern provides insight into variation in complex stiffness across atomic motions induced by cannabinoid interaction. Figure 7c presents the co-variance map, where colors represent correlated, uncorrelated or anticorrelated atomic motions within the complex. Figure 7d depicts the elastic network model, indicating how THC interaction modifies intrinsic protein elasticity between atom pairs.
Molecular Dynamics Simulation of THC and Glucosidase
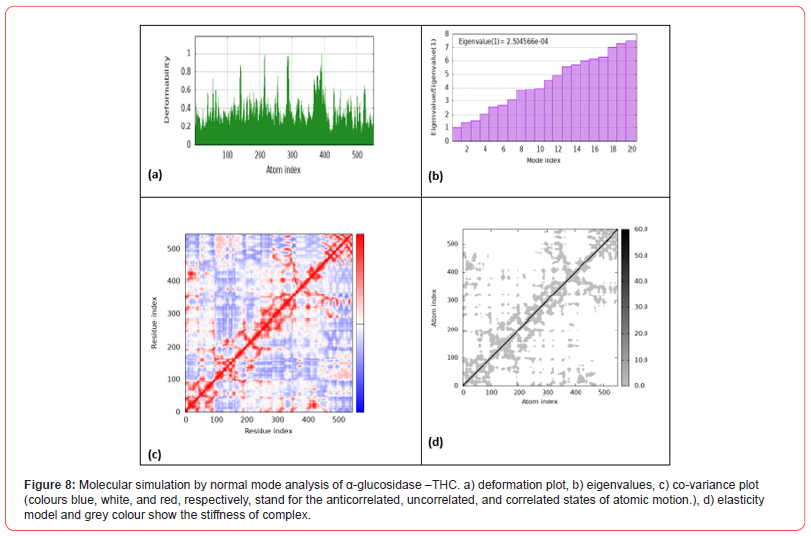
Figure 8 shows the results of molecular simulation by normal mode analysis of the α-glucosidase-THC protein-ligand complex obtained from molecular docking. Figure 8a displays the deformation plot, visualizing displacements along low frequency normal modes upon THC binding to α-glucosidase. This helps identify flexible and rigid regions within the complex. Figure 8b shows the eigenvalue spectrum, with lower values corresponding to softer, more deformable motions. The pattern provides insight into how cannabinoid interaction modifies variation in complex stiffness across atomic motions. Figure 8c presents the co-variance map, where differing colors represent correlated, uncorrelated or anticorrelated atomic motions induced within the complex. Figure 8d depicts the elastic network model, indicating how THC binding influences intrinsic protein elasticity between atom pairs.
Discussion
This study investigated the anti-diabetic potential of major cannabinoids from Cannabis sativa against key enzyme targets involved in glucose metabolism: DPP-4, α-amylase, invertase and α-glucosidase. α-glucosidase, α-amylase and invertase inhibition blocks the hydrolysis of carbohydrates in the digestive tract, reducing glucose absorption in the intestine [19,20]. DPP-4 inhibition would prevent breakdown of the incretin hormone GLP- 1, allowing it to stimulate insulin secretion and suppress glucagon levels after meals. This helps regulate post-prandial glucose spikes. Together, these multi-target effects would assist in controlling post-meal blood sugar surges in diabetics. Using in silico molecular docking, we evaluated the binding affinity and interactions of THC, CBD, THCV, CBC, CBG and CBN with the active sites of these enzymes. The results showed that all cannabinoids exhibited stronger binding than the reference drug, acarbose, suggesting that they could serve as promising compounds for the development of new anti-diabetic agents targeting multiple mechanisms.
These results corroborate previous studies reporting the anti-hyperglycemic effects of THC, CBN CBG and CBD [9-11]. Specifically, CBN demonstrated the best inhibition of DPP-4 while THC showed the strongest inhibition of α-amylase, invertase and α-glucosidase. The binding modes revealed interactions between the cannabinoids and important catalytic residues within each enzyme active pocket. At the molecular level, examination of amino acid interactions provided in Table 2 and illustrated in Figures 1a- 1g provides insight into how cannabinoids may inhibit DPP-4. Key residues in the DPP4 active site known to be important for substrate binding and catalysis include His740, Asp708 and Ser630 [21,22]. The observation that most cannabinoids interacted with His740 through hydrogen bonding correlations with this residue playing a central role in DPP-4 substrate recognition. THCV and THC were also found to form hydrogen bonds with Ser630, another residue critical for DPP-4 activity.
Interestingly, CBC did not show any interactions with important DPP-4 residues based on docking analysis. This could explain its relatively weaker binding affinity compared to other cannabinoids examined. The α-amylase residues Asp197, Glu233 and Asp300 in the catalytic domain are key determinants of substrate recognition and binding [23,24]. As seen in Table 4 and Figures 2a-2g, cannabinoids interacted with one or more of these critical residues, forming hydrogen bonds and ionic interactions. CBG was notable for interacting with all three residues, correlated with its favorable docking score. This correlates activity with specific interactions important for catalysis. THCV and THC showed selective interactions with Asp300 involves the optimized orientation of the substrate in the binding site [25]. Key residues Arg178, Glu230 and Tyr293 in the invertase active site mediate substrate binding and catalysis [15]. As depicted in Table 6 and Figures 3a-3g, cannabinoids interacted with Glu230 residues via hydrogen bonding and pication interactions, mimicking natural substrates.
Additionally, CBG interacted with Arg178, correlated with its strong docking score. Notably, THC and THCV formed pi-alkyl interactions with Tyr293. These site-specific interactions provide insight into how cannabinoids competitively inhibit invertase. While the specific amino acid interactions are not described, Figures 4a-4g provide visualization of cannabinoid binding poses within the α-glucosidase active site. All ligands seem to form conventional hydrogen bonding and hydrophobic contacts with residues lining the pocket. Based on their docking scores, one can infer that CBC, CBD, CBN and THCV likely interact strongly with crucial catalytic residues, similar to other α-glucosidase inhibitors. The favorable binding orientation of THC seen in Figure 4e correlated with its best docking score, indicating optimal interactions for inhibition. Finally, we performed molecular dynamics simulation to provide valuable insights into the ways by which THC binding alters the dynamics and flexibility of the protein-ligand complexes. The normal mode analysis results reveal that THC binding alters the dynamics and flexibility of all four protein-ligand complexes, albeit in different ways (Figures 5-8).
For DPP-4, THC binding stabilizes certain flexible segments within the active site pocket, which could promote inhibitory complex formation [26]. In contrast, THC binding induces increased flexibility in specific regions within the binding groove of amylase, potentially facilitating induced-fit binding. For invertase, THC induces both flexibility increases and decreases in different complex regions, introducing variation in stiffness that may impact catalytic domain dynamics. Finally, THC interaction alters flexibility profiles within the active site and substrate binding site of glucosidase, leading to changes in cooperative motions that influence enzyme allostery and function [27}.
Conclusion
This study provides compelling evidence that major cannabinoids exhibit promising multi-target inhibitory effects against key enzymes linked to glycemic control. Using a robust in silico molecular docking approach, we demonstrated that THC, CBD, THCV, CBC, CBG and CBN consistently bound the active sites of DPP-4, α-amylase, invertase and α-glucosidase more favorably than clinically used reference drugs. Notably, THC and CBN emerged as a pan-inhibitors, exhibiting the strongest affinity and interacting optimally within multiple enzyme active pockets, suggesting possible synergistic effects through multi-target mechanisms. This could represent a significant advancement over the single-target strategies of existing diabetes medications.
Author’s Contributions
GRT designed the study; GRT and DBA: wrote the manuscript; DBA and SI performed in silico analysis; FAE, CFT, CRN, KO and DJN reviewed and revised the manuscript; and MGM supervised the study and the manuscript writing. All authors have read and approved the final manuscript.
Competing Interests
The authors declare that they have no financial or non-financial interests that are directly or indirectly related to the work.
Acknowledgments
We acknowledge the researchers of the IK-based Technology Innovations at the Department of Science and Innovation, University of the Free State, Bloemfontein, South Africa.
References
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, et al. (2022) IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:
- Smoorenburg AN, Hertroijs DFL, Dekkers T, Elissen AMJ, Melles M (2019) Patients’ perspective on self-management: type 2 diabetes in daily life. BMC Health Serv Res 19(1): 605.
- Meneses MJ, Silva BM, Sousa M, Sá R, Oliveira PF, et al. (2015) Antidiabetic Drugs: Mechanisms of Action and Potential Outcomes on Cellular Metabolism. Curr Pharm Des 21(25): 3606-
- Elya B, Handayani R, Sauriasari R, Azizahwati, Hasyyati US, et al. (2015) Antidiabetic activity and phytochemical screening of extracts from indonesian plants by inhibition of alpha amylase, alpha glucosidase and dipeptidyl peptidase IV. Pakistan Journal of Biological Sciences 18(6): 273-
- Lim WXJ, Gammon CS, von Hurst P, Chepulis L, Page RA (2022) The Inhibitory Effects of New Zealand Pine Bark (Enzogenol®) on α-Amylase, α-Glucosidase, and Dipeptidyl Peptidase-4 (DPP-4) Enzymes. Nutrients 14(8):
- Ghasemi-Gojani E, Kovalchuk I, Kovalchuk O (2022) Cannabinoids and terpenes for diabetes mellitus and its complications: from mechanisms to new therapies. Trends Endocrinol Metab 33(12): 828-
- Wiciński M, Fajkiel-Madajczyk A, Kurant Z, Gryczka K, Kurant D, et al. (2023) The Use of Cannabidiol in Metabolic Syndrome—An Opportunity to Improve the Patient’s Health or Much Ado about Nothing? J Clin Med 12(14): 4620.
- Zhang J, Lin C, Jin S, Wang H, Wang Y, et al. (2023) The pharmacology and therapeutic role of cannabidiol in diabetes. Exploration (Beijing) 3(5):
- Ma H, Li H, Liu C, Seeram NP (2021) Evaluation of cannabidiol’s inhibitory effect on alpha-glucosidase and its stability in simulated gastric and intestinal fluids. J Cannabis Res 3(1):
- Suttithumsatid W, Shah MA, Bibi S, Panichayupakaranant P (2022) α-Glucosidase inhibitory activity of cannabidiol, tetrahydrocannabinol and standardized cannabinoid extracts from Cannabis sativa. Curr Res Food Sci 5: 1091-
- Mkabayi L, Viljoen Z, Krause RWM, Lobb KA, Pletschke BI, et al. (2024) Inhibitory effects of selected cannabinoids against dipeptidyl peptidase IV, an enzyme linked to type 2 diabetes. Heliyon 10(1):
- Kuppusamy A, Arumugam M, George S (2017) Combining in silico and in vitro approaches to evaluate the acetylcholinesterase inhibitory profile of some commercially available flavonoids in the management of Alzheimer’s disease. Int J Biol Macromol 95: 199-
- Brayer GD, Luo Y, Withers SG (1995) The structure of human pancreatic alpha-amylase at 1.8 A resolution and comparisons with related enzymes. Protein Sci 4(9): 1730-
- Röhrborn D, Wronkowitz, N, Eckel J (2015a) DPP4 in Diabetes. Front Immunol 6:
- Alvaro-Benito M, Polo A, González B, Fernández-Lobato M, Sanz-Aparicio J (2010) Structural and Kinetic Analysis of Schwanniomyces occidentalis Invertase Reveals a New Oligomerization Pattern and the Role of Its Supplementary Domain in Substrate Binding. J Biol Chem 285(18): 13930-
- Tian W, Chen C, Lei X, Zhao J, Liang J (2018) CASTp 3.0: computed atlas of surface topography of proteins. Nucleic Acids Research 46(W1): W363-
- Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, et al. (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. Journal of Computational Chemistry 19(14): 1639-
- López-Blanco JR, Aliaga JI, Quintana-Ortí ES, Chacón P (2014) iMODS: internal coordinates normal mode analysis server. Nucleic Acids Res 42: W271-276.
- Alvaro-Benito M, Polo A, González B, Fernández-Lobato M, Sanz-Aparicio J (2010) Structural and kinetic analysis of Schwanniomyces occidentalis invertase reveals a new oligomerization pattern and the role of its supplementary domain in substrate binding. J Biol Chem 285(18): 13930-
- Dona A, Pagès G, Gilbert R, Kuchel P (2010) Digestion of starch: In vivo and in vitro kinetic models used to characterise oligosaccharide or glucose release. Carbohydrate Polymers 80(3): 599-
- Röhrborn D, Wronkowitz N, Eckel J (2015b) DPP4 in Diabetes. Front Immunol 6:
- Tanwar O, Deora GS, Tanwar L, Kumar G, Janardhan S, et al. (2014) Novel hydrazine derivatives as selective DPP-IV inhibitors: findings from virtual screening and validation through molecular dynamics simulations. J Mol Model 20(4):
- Ponnusamy S, Ravindran R, Zinjarde S, Bhargava S, Ravi Kumar A (2011) Evaluation of traditional Indian antidiabetic medicinal plants for human pancreatic amylase inhibitory effect in vitro. Evid Based Complement Alternat Med 2011:
- Williams LK, Li C, Withers SG, Brayer GD (2012) Order and disorder: differential structural impacts of myricetin and ethyl caffeate on human amylase, an antidiabetic target. J Med Chem 55(22): 10177-
- Nada AA, Metwally AM, Asaad AM, Celik I, Ibrahim RS, et al. (2024) Synergistic effect of potential alpha-amylase inhibitors from Egyptian propolis with acarbose using in silico and in vitro combination analysis. BMC Complement Med Ther 24(1):
- Lambeir AM, Durinx C, Scharpé S, De Meester I (2003) Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci 40(3): 209-
- Yang LQ, Sang P, Tao Y, Fu YX, Zhang KQ, et al. (2014) Protein dynamics and motions in relation to their functions: several case studies and the underlying mechanisms. J Biomol Struct Dyn 32(3): 372-
-
Guy Roussel Takuissu*, Dupon Bruno Ambamba, Shabnor Iqbal, Kolowole Olofinsan, Cedric Fossi Tchinda, Fils Armand Ella, Corinne Raissa Ngnameko, Dany Joel Ngoumen, Matsabisa Gilbert Motlalepula*. Molecular Docking Studies for the Evaluation of Cannabinoids as Multi-Target Inhibitor for Type 2 Diabetes. Curr Tr Clin & Med Sci. 4(1): 2025. CTCMS.MS.ID.000577.
-
epidemiology, Enterobacteriaceae, infection pneumonia, urinary tract infection
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






