 Case Report
Case Report
Successful Resuscitation of an Elderly Diabetic Patient Having Life Threatening Acute Severe Hyperkalemia
Satyanand Sathi1*, Anil Kumar Garg1, Sudhanshu Shekhar2, Virendra Singh Saini1 and Arvind Trivedi1
1Department of Medicine, S.M.M.H. Medical College Saharanpur, Uttar Pradesh, India
2Department of Medicine, Shaksham Hospital Saharanpur, Uttar Pradesh, India
Satyanand Sathi (DM Nephrology), Assistant Professor, Department of Medicine, S.M.M.H. Medical College, Near Pilakhni, Saharanpur, Uttar Pradesh, India.
Received Date: May 12, 2020; Published Date: May 26, 2020
Abstract
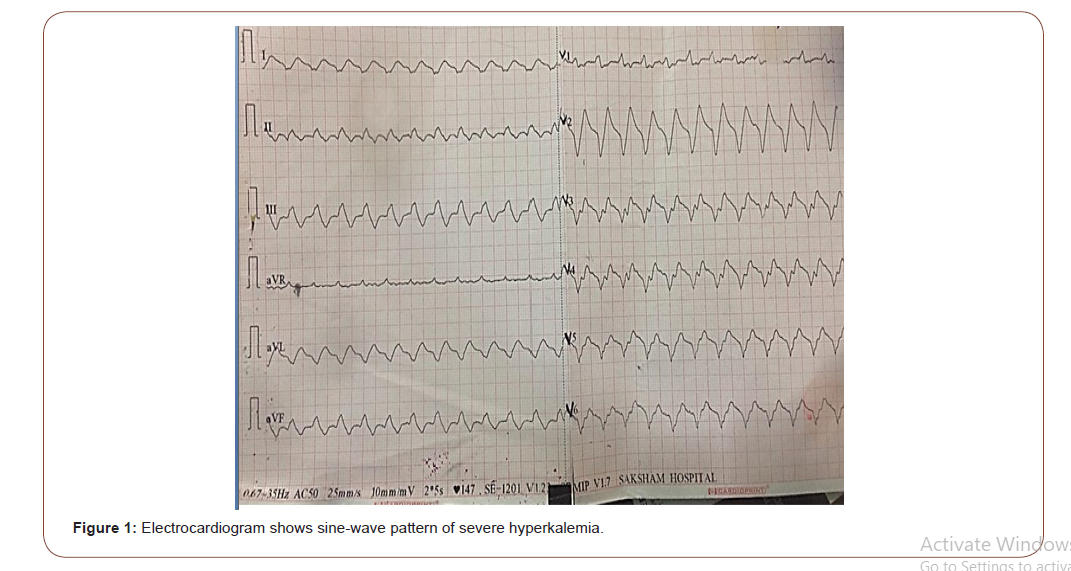
Hyperkalemia is a potentially lethal electrolyte disorder, encountered by nephrologists and intensivists in emergency department. Symptoms of hyperkalemia are often nonspecific and can ocassionally lead to life threatening cardiac arrhythmia. Here, we report the case of an 86 years old diabetic female who presented with acute kidney injury and severe hyperkalemia with serum potassium (9.3 mg/dl) that was out of proportion to fall in estimated glomerular filtration rate (23.5 ml/min/1.73m2 ). Additional analyses revealed high anion gap metabolic acidosis. The electrocardiogram showed sine-wave pattern of severe hyperkalemia. Echocardiography showed ischemic dilated cardiomyopathy with left ventricular ejection fraction 30%. The electrocardiogram did not normalize with the conservative medical treatment. Hemodialysis was initiated immediately and patient developed ventricular tachycardia during hemodialysis but patient was resuscitated successfully.
Keywords: Acute kidney injury; Elderly; Diabetes mellitus; Hyperkalemia; High anion gap acidosis
Introduction
Introduction
Among hospitalized patients, the prevalence of hyperkalemia has been approximated at 1% to 10% [1, 2]. Patients with diabetes mellitus, heart failure (HF), chronic kidney disease (CKD), and those using renin-angiotensin-aldosterone system inhibitors (RAASi) are at 2 to 3 times higher risk for hyperkalemia [3-5]. Hyperkalemia has become a more common concern as diabetic and HF patients usually take RAASi and mineral corticoid receptor antagonists (MRAs).The incidence of hyperkalemia is higher in diabetic patients as compare to the general population[6,7]. Normal ageing, particularly after the sixth decade, is associated with a decrease in renin production and may cause hyporeninemic hypoaldosteronism [8]. Shifting of potassium from the intracellular to the extracellular compartment can cause hyperkalemia and is called as shift hyperkalemia. The most common causative factor of chronic hyperkalemia in diabetics is the decreased tubular secretion of potassi um due to the syndrome of hyporeninemic hypoaldosteronism [9]. This syndrome is specified by mild to moderate renal insufficiency and patients usually present with asymptomatic hyperkalemia. Degree of severity of hyperkalemia is generally classified as mild (5.5- 6.5 mmol/l), moderate (6.5-7.5 mmol/l) and severe (>7.5 mmol/l) [10]. Hyperkalemia is further classified as acute or chronic [11]. Acute hyperkalemia occurs as a single event, over hours to days and usually requires emergency treatment. Chronic hyperkalemia develops over a period of weeks to months, may be persistent or develop periodically, and requires ongoing outpatient management [11].
Case Report
An 86-year-old female presented with a 2 day history of generalized weakness, fatigue, dizziness and inability to walk without any history of loss of consciousness, seizure, headache, sensory loss, or bowel and bladder involvement. She had 18 years history of diabetes mellitus, 4 years history of coronary artery disease (angioplasty 4 year before) and 4 years history of left ventricular dysfunction (left ventricular ejection fraction; 20 %) . She was on telmisartan 40 mg since last 4 years. There was no history of any intake of non steroidal anti inflammatory drugs (NSAIDS), beta blockers and indigenous medicine. On admission, she was awake. Physical examination showed that her body temperature was 97.6 degrees F, blood pressure was 80/60mmHg, pulse was feeble with rate146 beats/min, bilateral pitting type mild pedal edema was present. No remarkable findings were observed in her chest and abdomen. The patient’s laboratory profile was as follows: hemoglobin: 10.7 g/dl, total leukocyte count: 4900/mm3, platelet count: 1.8 × 105/ mm3, urinary protein: 2+, urinary sugar: 1+, urine microscopy: white blood cell count: 1–2/high-power field, red blood cell count: 0/high-power field, urinary pH: –6.5, 24-hour urinary protein: 2.2 g/day, serum albumin: 2.34 g/dl, HBsAg: negative, anti-HCV: negative, HIV I and II: negative, blood urea: 85mg/dl, serum creatinine: 1.9 mg/dl, estimated glomerular filtration rate (eGFR) by chronic kidney disease - improved prediction equations(CKD-EPI): 23.5 ml/min/1.73m2 ,random blood sugar: 224 mg/dl, serum sodium: 143 mEq/l, serum potassium: 9.34 mEq/l, serum chloride: 112 mEq/l, albumin corrected serum calcium:9.32 mg/dl, serum PO4: 4.2 mEq/l, arterial blood gas: pH 7.24, pCO2: 28 mm Hg, pO2: 104 mm Hg, HCO3: 12.9 mEq/l, anion gap: 18.1 mEq/l (normal range: 10–12). The electrocardiogram showed sine-wave pattern of severe hyperkalemia (fig.1). Ultrasonography abdomen showed bilateral normal size kidneys with increased bilateral renal cortical echogenicity. 2D-Echocardiography showed ischemic dilated cardiomyopathy with left ventricular ejection fraction (LVEF); 30 %. Fundus examination showed evidence of diabetic retinopathy. Thus, the diagnosis of type 2 diabetes mellitus with diabetic nephropathy with diabetic retinopathy with ischemic dilated cardiomyopathy with acute kidney injury with high anion gap metabolic acidosis with acute severe hyperkalemia was made.
Treatment
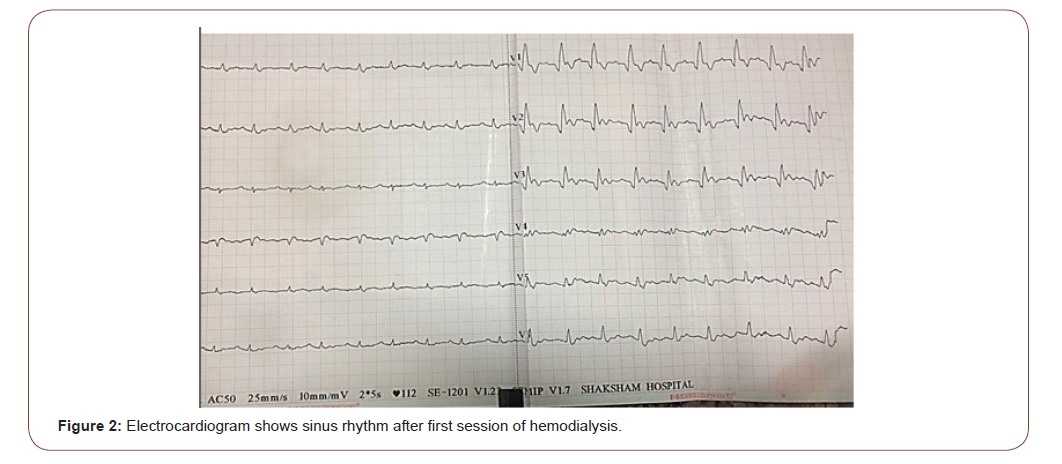
We initiated conservative medical treatment in the form of intravenous 4.65 mEq of calcium gluconate, intravenous bolus of 10 units of regular insulin with 25 grams of dextrose and nebulization with 20 mg albuterol (5mg/ml) but her electrocardiogram did not normalize. Patient was put on inotropic support to maintain normal blood pressure. Repeat serum potassium was 8.2 mEq/L. Hemodialysis was initiated in the view of severe hyperkalemia and metabolic acidosis. After 30 minutes of initiation of hemodialysis she developed ventricular tachycardia. Successful cardioversion was done by delivering 200 joules of direct current (DC) shock and hemodialysis was completed. After first session of hemodialysis, her potassium was 5.8 mEq/Land changes of severe hyperkalemia in electrocardiogram were normalized (fig. 2). Next day serum potassium was 6.8 mEq/L and arterial blood gas analysis showed metabolic acidosis. Second session of hemodialysis was done. After second hemodialysis, serum potassium was 5.7 mEq/L. Fludrocortisones .1 mg was added in the view of hyporeninemic hypoaldosteronism. On fourth day of admission her serum potassium was 4.8mEq/L and serum creatinine was 1.6mg%. After one month of follow up, her serum potassium was 3.8mEq/L and serum creatinine was 1.0 mg% .
Discussion
Hyperkalemia can be caused by either decreased renal excretion or excessive leakage of potassium from the intracellular space (cell shifts). Besides it acute and chronic renal failure, hypoaldosteronism and rhabdomyolysis, are typical examples leading to hyperkalemia [10]. In our case patient hyperkalemia was acute and severe with serum potassium level 9.3 mEq/L. In the setting of normal renal function adaptive response includes an intact cortical collecting duct, normal mineral corticoid levels and adequate distal delivery of sodium [12].Renal adaptive mechanisms allow the kidneys to maintain potassium homeostasis until the glomerular filtration rate (GFR) drops to less than 15 ml/min/1.73 m2 [10]. But in our case patient eGFR calculated by CKD-EPI was 23.5 ml/ min/1.73m2. Acute hyperkalemia in our case patient was out of proportion to fall in eGFR .In patients with diabetes, decreased mineral corticoid activity is often an early finding due to hyporeninemic hypoaldosteronism. This is the reason, hyperkalemia in diabetic patient usually develops even after mild or moderate decrease in the GFR. Elderly patients may have decreased renal function even without significant increase in serum creatinine levels (< 1.2 mg/ dL) [16]. It is studied that after the age of about 30 years, the glomerular filtration rate (GFR) begins to decline at an average rate of 1 ml per year [17].The older age (especially after sixth decade) is independent factor, responsible for high prevalence of hyperkalemia [8, 16]. It is due to age-related declines in plasma renin activity and aldosterone, as well as aldosterone resistance at the level of the renal tubule [8]. That’s why elderly subjects are at risk to develop hyperkalemia, when the patients are on drugs that block the renin-angiotensin-aldosterone axis or interfere with distal tubular potassium secretion. Our case patient was 86 years old female and had history of telmisartan intake that might be additional risk factor for hyperkalemia. As compare to acute kidney injury, in predialysis CKD patients, loss of nephron mass is equilibrated by compensatory increase in the secretory rate of potassium in remaining nephrons, such that fractional excretion of potassium is increased [13]. However, in heart failure, increased aldosterone causes increased absorption of sodium in proximal tubules, resulting in its decreased delivery to the distal nephrons, which in turn, results in decreased potassium excretion [14]. In heart failure patients with renal failure, the prevalence of hyperkalemia can be up to 20% and is associated with an increased risk of morbidity and cardiovascular mortality [14,15]. Our case patient had four years history of left ventricular dysfunction and on echocardiography her LVEF was 30%. Shift hyperkalemia can cause hyperkalemia with no net increase in total body potassium. Example of shift hyperkalemia in diabetes mellitus is metabolic acidosis [16]. For each 0.1 fall in pH , there is increase in potassium by approximately 0.4 mmol/L [16]. Arterial blood gas analysis of our case patient showed pH 7.24, pCO2: 28 mm Hg, HCO3: 12.9 mEq/l and anion gap was 18.1 mEq/l. Our case patient had high anion gap metabolic acidosis. 86 years of age, diabetes mellitus, high anion gap metabolic acidosis of renal failure, use of the renin-angiotensin-aldosterone axis blocker and heart failure were collectively responsible for acute severe hyperkalemia in our case patient. Chronic hyperkalemia is caused by impaired renal potassium excretion and not by the cell shift. The electrocardiogram in a hyperkalemic subject can progress from normal to ventricular tachycardia and asystole in a precipitous manner [18]. Serum creatinine of our case patient was 1.9 mg/dl at the time of admission and serum potassium was 9.3 mg/dl. The electrocardiogram showed classical sine-wave pattern of severe hyperkalemia. Medical management for hyperkalemia did not normalize the electrocardiogram in our case patient and patient developed ventricular tachycardia during hemodialysis. But after completion of first session of hemodialysis, electrocardiogram showed sinus rhythm.
Conclusion
Acute hyperkalemia occurs due to cell shifts and chronic hyperkalemia occurs due to decreased renal potassium excretion. Acute severe hyperkalemia in elderly diabetic patients may occur out of proportion to fall in eGFR. If electrocardiogram does not normalize with the conservative medical treatment, hemodialysis should be initiated immediately for hyperkalemia irrespective of serum creatinine level.
Statement of Ethics
The authors followed the guidelines for human studies and the research was conducted ethically. Information revealing the subject’s identity was avoided. Guardians have given their written informed consent to publish this case, including publication of images.
Acknowledgement
The authors express their gratitude to the patient and her guardians for full cooperation during prolonged hospital stay and providing medical records for preparing this manuscript. We express sincere thanks to patient’s family.
Conflict of Interest
No conflict of interest.
References
- An JN, Lee JP, Jeon HJ, Kim DH, Oh YK, et al. (2012) Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care 16(6): R225.
- Acker CG, Johnson JP, Palevsky PM, Greenberg A (1998) Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Arch Intern Med 158(8): 917-924.
- Jain N, Kotla S, Little BB, Weideman RA, Brilakis ES, et al. (2012) Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol 109(10): 1510-1513.
- McMurray JJV, Adamopoulos S, Anker SD (2012) ESC Committee for Practice Guidelines ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur J Heart Fail 14: 803-869.
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, et al. (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62(16): e147-239.
- Palmer BF (2004) Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med 351(6): 585-592.
- Uribarri J, Oh MS, Carroll HJ (1990) Hyperkalemia in diabetes mellitus. J Diabet Complications 4(1): 3-7.
- Michelis MF (1990) Hyperkalemia in the elderly. Am J Kidney Dis 16: 296-299.
- DeFronzo RA (1980) Hyperkalemia and hyporeninemic hypoaldosteronism. Kidney Int 17(1): 118-134.
- Lehnhardt A, Kemper MJ (2011) Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. Mar 26(3): 377-384.
- Kraft MD, Btaiche IF, Sacks GS, Kudsk KA (2005) Treatment of electrolyte disorders in adult patients in the intensive care unit. Am J Health Syst Pharm 62(16): 1663-1682.
- Palmer BF (2010) A physiologic-based approach to the evaluation of a patient with hyperkalemia. Am J Kidney Dis 56(2): 387-393.
- Liamis G, Liberopoulos E, Barkas F, Elisaf M (2014) Diabetes mellitus and electrolyte disorders. World J Clin Cases 2(10): 488-496.
- Zhou XJ, Rakheja D, Yu X, Saxena R, Vaziri ND, et al. (2008) The aging kidney. Kidney Int 74(6): 710-720.
- Palmer BF (2012) Hyperkalemia in predialysis patients. Clin J Am Soc Nephrol 7(8): 1201-1202.
- Sarwar CM, Papadimitriou L, Pitt B, (2016) Hyperkalemia in Heart Failure. J Am Coll Cardiol 68(14): 1575-1589.
- Luo J, Brunelli SM, Jensen DE, Yang A (2016) Association between Serum Potassium and Outcomes in Patients with Reduced Kidney Function. Clin J Am Soc Nephrol 11(1): 90-100.
- Montague BT, Ouellette JR, Buller GK (2008) Retrospective review of the frequency of ECG changes in hyperkalemia. Clin J Am Soc Nephrol 3(2): 324-330.
-
Satyanand Sathi, Anil Kumar Garg, Sudhanshu Shekhar. Successful Resuscitation of an Elderly Diabetic Patient Having Life Threatening Acute Severe Hyperkalemia. Annal Urol & Nephrol. 2(1): 2020. AUN.MS.ID.000527.
-
Acute kidney injury, Elderly, Diabetes mellitus, Hyperkalemia, High anion gap acidosis, Chronic kidney disease, Renin-angiotensin-aldosterone system inhibitors, Heart failure, Mineral corticoid receptor antagonists
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






