 Research Article
Research Article
Oral Desmopressin in The Treatment of Nocturia in Aging Population: A Pilot Study
Mustafa Alwani1,4, Aksam Yassin1,2*, Bassam Albaba2, Raidh Talib1, Aftab Mohammad Azad3 and Mohamed M. Arous4
1Surgical Research Section, Department of Surgery, Hamad Medical Corporation, Doha, Qatar
2Center of Medicine and Health Sciences, Dresden International university, Germany
3Department of Emergency Medicine, Hamad Medical Corporation and College of Medicine, Qatar University
Center for Neurosurgery and Pain Management, Hamburg, Germany
Aksam Yassin, Department of Surgery, Hamad Medical Corporation, Surgical Research Section, Hamburg, Germany.
Received Date: September 21, 2022; Published Date: October 11,2022
Abstract
Nocturia is common in the elderly. Nocturnal polyuria, caused by insufficient anti-diuretic hormone (ADH), is one of the main causes of nocturia associated with aging. We investigated the effects of desmopressin (synthetic ADH analogue) on nocturia and sleep quality in elderly patients. Forty elderly (mean age 61 years) patients (22 females) were recruited following screening for nocturia (>2 nocturnal voids) and nocturnal polyuria (nocturnal urine volume/functional bladder capacity >1). An initial open-label dose-titration period established optimal desmopressin dosage of 0.2mg/day. Following a 1-week washout period patients were randomized to treatment or placebo group for 4 weeks. Nocturia was reduced by ≥55% in 60% of patients receiving desmopressin compared to 10% of patients receiving placebo. Urine osmolality was increased following treatment (810 mOsmol/kg H2O) compared to placebo (630mOsmol/kg H2O). Nocturnal urine production was reduced by 44.3% (1.51mL/min to 0.84mL/min) in treated versus 4.1% in placebo patients (1.44mL/min to 1.38mL/min). Sleep parameters improved following desmopressin treatment with 60% of patients having >5hr unbroken sleep compared to 6% in the placebo group, and mean sleep duration 180 min versus 42 min respectively. Oral desmopressin tend to be an effective treatment for nocturia and improves sleep quality in an aging population independent of gender.
Keywords: Nocturia; Anti-diuretic hormone; Vasopressin; Desmopressin; Sleep; Aging
Introduction
Nocturia is a condition in which a person frequently wakes up at night to pass urine. The disruption of sleep associated with nocturia has a severe negative impact on the quality of life of the patients [1], and is associated with daytime fatigue [2, 3], increased susceptibility to diseases [4] and poorer mental health [5]. Awakening at night to void also increases risk of falls [6] and hip fractures [7] in the elderly and is independently associated with increased mortality [8].
Nocturia is multifactorial but has a higher prevalence in older patients of both genders indicating age as a primary risk factor. It has been reported that up to 59% men and 62% women over 70 experience nocturia (more than 2 voids per night), compared to 16% to 18% respectively, for younger men and women (aged 20 to 40 years)[9]. Other underlying causes vary from non-urological conditions such as diabetes insipidus, hypoalbuminemia or chronic heart failure; and urological issues including bladder cancer [10]. Nocturia could be caused by a combination of these pathophysiologies or via other underlying conditions such as reduced nocturnal bladder capacity, global polyuria or nocturnal polyuria [11].
Nocturnal polyuria, or the over-production of urine at night, is the most common underlying cause for nocturia, with studies reporting its prevalence to be up to 76% in patients with nocturia [12,13]. Nocturnal polyuria is frequently secondary to the dysregulation in the levels of anti-diuretic hormone (ADH) [14]. ADH, also known as D-arginine vasopressin (DAVP), acts on the V2 receptors in the renal collecting duct to promote reabsorption of water from the nephron via aquaporins back into systemic circulation to maintain serum osmolality and volume [15]. ADH analogues (in the form of 1-desamino-8D-arginine vasopressin, DDAVP) have traditionally been used to treat central diabetes insipidus, bleeding disorders such as von Willebrand disease, and primary nocturnal enuresis in pediatrics. However, recent interest in its role for treating nocturia has spawned a growing body of literature examining the role and effect of desmopressin in its treatment. In healthy individuals, the levels of ADH have a circadian rhythm and are elevated nocturnally to control urine production, and this diurnal variation is often impaired in the elderly leading to increased diuresis [16].
Desmopressin is a synthetic analogue of ADH, but with a more potent antidiuresis effect due to its selective affinity for V2 receptors, unlike endogenous ADH that binds to both V1 and V2 receptors; and has a prolonged half-life [17,18]. Indeed, several studies have provided support for the use of desmopressin for the management of nocturia [19-21]. However, it has been recognized that the optimal dose of desmopressin differs among patients, especially associated with gender [22,23], and further research needs to be undertaken to support existing evidence on its use for the treatment of nocturia. In this two-phased study, which includes an open-labelled dose titration period, we provide further evidence for the effective use of desmopressin in nocturia in an elderly population.
Patient and Method
An intention to treat (ITT) population of 211 patients were identified with nocturnal diuresis. Patients were included in the study when nocturia was indicated as voiding more than twice per night without urological pathologies. The main exclusion criteria were the presence of comorbidities including uncontrolled disease such as diabetes insipidus, cardiac disease, hypertension, use of diuretics, severe kidney diseases or diseases which influences medulla of kidney such as medullary cystic of kidney diseases, multiple sclerosis, urge incontinence, had undergone surgical treatment for BPH in last 6 months and known functional disease in urinary system for example neurogenic bladder. Following initial screening, an initial open-labelled period over 4 weeks for dose titration, followed by 1-week of washout was undertaken whereby patients received an increasing oral dose of Desmopressin starting at 0.1mg/day, till they responded to the treatment indicated by a >20% decrease in nocturnal diuresis. The effective dose was optimized from 0.1-0.2mg/day and only patients that obtained the 20% reduction in nocturnal diuresis were included from the initial 211 Intend-To-Treat (ITT) subjects.
A total of forty patients (22 females, 18 males) were recruited into the study with an average age of 61 and further screened for nocturia, defined as at least two voids per night and nocturnal polyuria - a nocturia index score of >1 (nocturnal urine volume/ functional bladder capacity; the latter being the largest single volume of urine measured at any time). The study took a randomized double-blind placebo-controlled approach (Figure 1). Following the 1-week washout period, patients were randomized to placebo or active treatment groups and received optimized dosage of oral Desmopressin or placebo tablets. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori review and approval by Ajman, United Arab Emirates and the institutional ethical committee at the Gulf Medical College and University hospital. Written informed consent was obtained from each patient included in the study and for receiving their treatment protocol.
The primary efficacy endpoint of this research was the proportion of patients who had a reduction by more than 50% in the mean number of nocturnal voids after treatment compared with baseline. Several secondary endpoints were also assessed which included the number of nocturnal voids, elevation of urine osmolality (according to the recommendations of Mount Sinai, New York on Urine Specific Gravity and Osmolality) and prolonged duration of the sleep period. A micturition diary was used to record the primary and secondary endpoints.
Statistical Analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) v.11 for Windows software package (SPSS Inc., Chicago, USA). Data are expressed as mean group values with standard deviations at each time point. Clinical parameters were compared between groups across the treatment periods using mixed-effects, repeated-measures model with period, group and their interaction as fixed effects. Analysis of variance (ANOVA) was used to compare continuous variables. A value of p<0.05 was considered significant.
Result
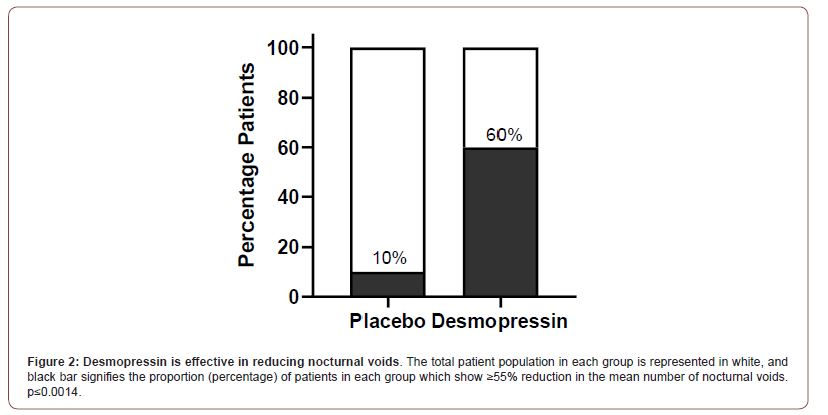
Patient demography and baseline characteristics were similar for both the desmopressin treated and placebo group for bladder capacity, nocturnal void number, nocturnal urine volume, mean sleep duration and female/male patient numbers. No adverse effects were demonstrated in either desmopressin or placebo treated groups and compliance with treatments were complete. In the treatment group, a clinical response (≥55% reduction in the mean number of nocturnal voids from 5 to 2 times) was achieved in 12 (60%) patients compared to 2 (10%) in the placebo group with voids from 5 to 4 times (OR=13.5, 95% CI 2.4 - 74.8; P=0.0014) (Figure 2).
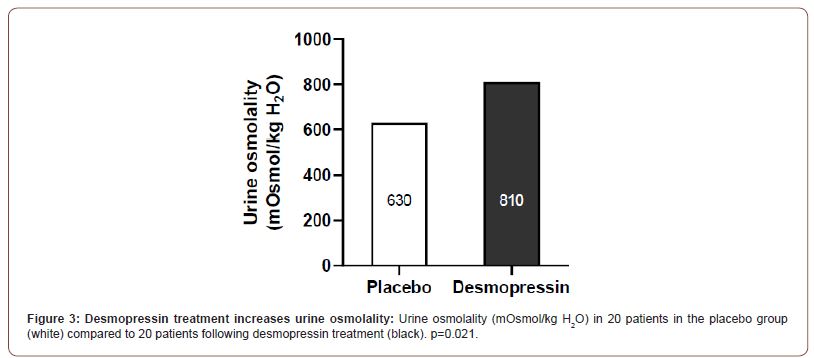
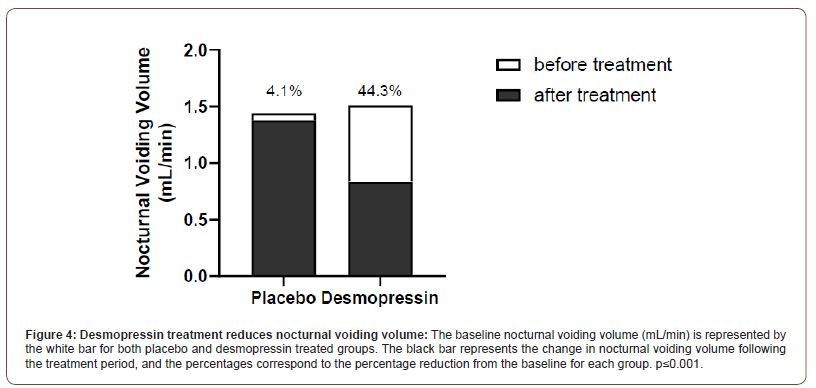
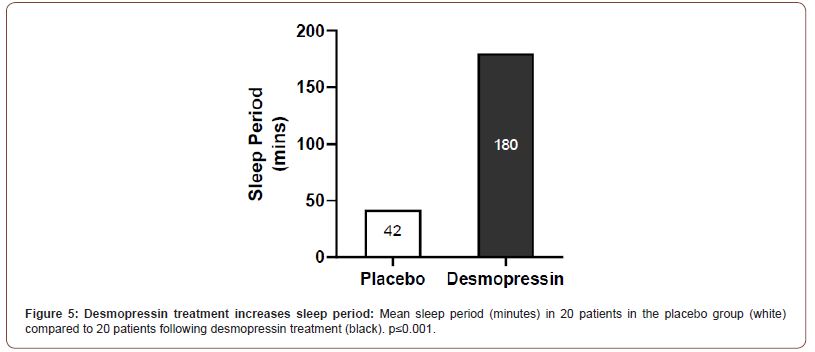
Patients in the desmopressin treatment group had a significantly higher urine osmolality (810 ± 210 mOsmol/kg H2O) compared to the placebo group (630± 61 mOsmol/kg H2O, P=0.021; Figure 3). Nocturnal voiding volume was reduced by 44.3% (mean: 1.51mL/min to 0.84mL/min) in the treatment group compared to 4.1% (1.44mL/min to 1.38mL/min) in the placebo group (Figure 4). Patients in the active treatment group had improved sleep parameters, with 60% of them experiencing more than 5 hours unbroken sleep compared to 6% in the placebo group. The overall sleep duration was also significantly more in the desmopressin group (180±46 min) compared to placebo (42±12 min; P<0.001; Figure 5). No adverse events were recorded in the treatment group during the study duration.
Discussion
Nocturia, if left untreated, can severely impact a patient’s quality of life by reducing the quality of sleep, and is associated with increased morbidity and mortality [8]. A clear understanding of the underlying etiology of nocturia is essential for choosing the correct treatment options and whilst lifestyle and behavioral changes may help some patients, pharmacotherapies remain the predominant option [15]. Nocturia is often attributed to nocturnal polyuria [13], which in turn is often a consequence of dysregulated ADH secretion through the night, especially in the elderly [24]. It is known that the pituitary gland decreases in size with age and corresponds to altered levels of ADH [25]. In elderly patients with nocturnal polyuria, ADH levels are suppressed to very low or undetectable levels at night resulting in 85% of 24hr-diuresis in extreme cases [16,26]. The majority of studies investigating nocturnal polyuria were designed in aging male subjects. In the present study we demonstrate that treatment of elderly patients of both genders with nocturnal polyuria using Desmopressin results in a reduction in nocturnal voids and voiding volume while increasing urine osmolality and improving sleep duration as a potential mechanism to improve quality of life and reduce associated comorbidities. The placebo and the treatment groups in this study suffered from nocturia and nocturnal polyuria (nocturia index score>1) and were well-matched for age, gender and other baseline characteristics including comorbidities diabetes insipidus and CVD. Desmopressin has shown to be effective in treating vasopressin-sensitive cranial diabetes insipidus and nocturnal enuresis, and since 2002, has been licensed for the treatment of nocturia in adults [15].
The observed effects of desmopressin in this study are comparable with previous reports. A similar two-phased study in men with a dose-titration and double-blind placebo-controlled period by [20], showed decreased nocturnal voids in 34% patients in the desmopressin group compared to 3% in the placebo [19]. performed a similar study in women and observed a 46% reduction in nocturnal voids compared to 7% in the placebo group [19]. A more recent, gender-independent study by [27], reported reduction of nocturnal voids in 33% following desmopressin administration compared to 11% in the placebo group [27]. All these studies were conducted in an older population akin to the patient cohort used in our study, and the results presented here extend the increasing evidence for the clinical use of desmopressin in improving the primary end points for nocturia in an elderly population. Supporting these findings, the present study observed that 60% patients in the desmopressin treatment group had a reduction in nocturnal voids in comparison to the placebo group where 10% patients experienced a reduction. Furthermore, we observed a reduction of 44.3% in nocturnal voiding volume from baseline following desmopressin administration, compared to 4.1% in the placebo group. This is comparable to previous reports of 44% reduction in a cohort of females [19], and 36% reduction observed in a male cohort [20]. To our knowledge, there has been no double-blind placebo-controlled study that reported a decrease in nocturnal voiding volume in a cohort consisting of both genders, where this pilot study pave the road toward a double-blind placebo-controlled study. Systematic review of studies in male patients with benign prostatic hyperplasia concluded that low-dose oral desmopressin therapy alone is an effective treatment for nocturia associated with lower urinary tract symptoms (LUTS) [28].
The increased reabsorption of water from the renal nephrons with desmopressin treatment, and the subsequent decrease in the nocturnal voiding volume results in a more concentrated urine. Indeed, we demonstrate an increase in urine osmolality in the desmopressin group compared to placebo. Although this has been demonstrated in a pediatric cohort for nocturnal enuresis [29], previous double-blind placebo-controlled studies in older adult populations have not reported this parameter [19, 20, 27].
Whilst reduction in nocturnal voids and nocturnal diuresis are essential for assessing the clinical efficacy of diuretic treatment, the changes in sleep parameters are vital to improving the QoL for the patients. In this study, treatment with desmopressin allowed 60% of patients more than 5 hours unbroken sleep, compared to 6% in the placebo group and significantly improved their sleep duration. This would undoubtedly benefit the patients QoL, but the use of established questionnaires to monitor work productivity and QoL would have been valuable for assessing the scale of the impact [30,31]. It has been demonstrated that 1 less nocturnal void and as little as 1 hour increased duration of first uninterrupted sleep period was associated with significant increases in QoL score [32]. Furthermore, an observational study assessing the relationship between nocturia and QoL revealed that an increasing number of nightly voids was associated with a deterioration of QoL scores, with significant differences particularly noted between 0-1 and ≥2 voids per night [33]. This suggests that to improve QoL, the treatment goal for patients should be to reduce nocturia to or below the threshold of 2 voids per night.
The present study has a few limitations. Following the initial open-labelled dose titration phase, the washout period could have been monitored empirically by measuring the return of nocturia parameters to baseline. This would have ensured no residual effect of desmopressin is carried over in the double-blind phase. Nonetheless, the one-week washout period is in keeping with other studies on desmopressin with an open-labelled dose titration phase [19, 20, 27]. Moreover, with a terminal half-life of 3.1hr and a clearance of 2.6mL/min/kg body weight [34,35], it is highly unlikely that desmopressin is retained past the wash-out period. The use of desmopressin has been associated with hyponatremia as serious potential adverse event, especially in the elderly [34]. Regular measurements of sodium levels during the open-labelled dose titration phase could have helped identify the patients that may have been at risk. Nevertheless, no adverse events were reported in this study, and close monitoring of symptoms such as headaches and weight increase would have helped identify any cases of hyponatremia. The efficacy of desmopressin in our patient cohort suggests that high proportion of them experienced nocturnal polyuria secondary to impaired ADH signaling. A baseline measurement of ADH concentrations in our patient cohort would have offered an insight into the etiology for nocturia in nonresponders from the treatment group.
In conclusion, in this two-phased randomized double-blind placebo-controlled study oral desmopressin treatment was welltolerated and effective in managing nocturia compared to placebo in an elderly population with nocturnal polyuria. The primary endpoint of nocturnal voiding was significantly improved along with voiding volume which resulted in increased duration of the first sleep period in the treatment group. These beneficial effects have the potential to improve quality of life, consistent with previous studies, and possibly reduce associated comorbidities in elderly patients with nocturnal polyuria.
Acknowledgement
Editorial support for this manuscript was provided by Astra- Health.
Conflict of Interest
Aksam Yassin has received partial compensation for data entry and occasional honoraria from Bayer Pharma, Ferring Pharmaceuticals and GSK. No other conflicts of interest to report.
References
- Kobelt G, F Borgstrom, A Mattiasson (2003) Productivity, vitality and utility in a group of healthy professionally active individuals with nocturia. BJU Int 91(3): 190-195.
- Everaert K, Peter Anderson, Robert Wood, Fredrik L Andersson, Tove Holm-Larsen (2018) Nocturia is more bothersome than daytime LUTS: Results from an Observational, Real-life Practice Database including 8659 European and American LUTS patients. Int J Clin Pract 72(6): e13091.
- Vaughan CP, R Eisenstein, D L Bliwise, Y K Endeshaw, Z J Nagamia, et al. (2012) Self-rated sleep characteristics and bother from nocturia. Int J Clin Pract 66(4): 369-373.
- Matias JR, N Orentreich (1988) The effect of testosterone, cyproterone acetate, and minoxidil on hair loss in the androchronogenetic alopecia mouse. Clin Dermatol 6(4): 169-176.
- Kupelian V, John T Wei, Michael P O'Leary, Jens Peter Norgaard, et al. (2012) Nocturia and quality of life: results from the Boston area community health survey. Eur Urol 61(1): 78-84.
- Stewart RB, M T Moore, F E May, R G Marks, W E Hale, et al. (1992) Nocturia: a risk factor for falls in the elderly. J Am Geriatr Soc 40(12): 1217-1220.
- Weiss JP, JG Blaivas (2000) Nocturia. J Urol 163(1): 5-12.
- Asplund R (1999) Mortality in the elderly in relation to nocturnal micturition. BJU Int 84(3): 297-301.
- Bosch JL, JPWeiss (2013) The prevalence and causes of nocturia. J Urol 189(1 Suppl): S86-92.
- Weiss JP (2012) Nocturia: focus on etiology and consequences. Rev Urol 14(3-4): 48-55.
- Weiss JP, AC Weinberg, JG Blaivas (2008) New aspects of the classification of nocturia. Curr Urol Rep 9(5): 362-367.
- Bing MH, L A Moller, P Jennum, S Mortensen, G Lose (2007) Pathophysiological aspects of nocturia in a Danish population of men and women age 60 to 80 years J Urol 178(2): 552-557.
- Weiss JP, Philip E V van Kerrebroeck, Bjarke M Klein, Jens Peter Norgaard (2011) Excessive nocturnal urine production is a major contributing factor to the etiology of nocturia. J Urol 186(4): 1358-1363.
- Asplund R (1995) The nocturnal polyuria syndrome (NPS). Gen Pharmacol 26(6): 1203-1209.
- Hashim H, P Abrams (2008) Desmopressin for the treatment of adult nocturia. Therapy 5(5): 667-683.
- Asplund R, H Aberg (1991) Diurnal variation in the levels of antidiuretic hormone in the elderly. J Intern Med 229(2): 131-134.
- Richardson DW, AG Robinson (1985) Desmopressin. Ann Intern Med 103(2): 228-239.
- Slusarz MJ, R Slusarz, J Ciarkowski (2006) Investigation of mechanism of desmopressin binding in vasopressin V2 receptor versus vasopressin V1a and oxytocin receptors: molecular dynamics simulation of the agonist-bound state in the membrane-aqueous system. Biopolymers 81(5): 321-328.
- Lose G, Othon Lalos, Robert M Freeman, Philip van Kerrebroeck (2003) Efficacy of desmopressin (Minirin) in the treatment of nocturia: a double-blind placebo-controlled study in women. Am J Obstet Gynecol 189(4): 1106-1113.
- Mattiasson A, P Abrams, P Van Kerrebroeck, S Walter, J Weiss (2002) Efficacy of desmopressin in the treatment of nocturia: a double-blind placebo-controlled study in men. BJU Int 89(9): 855-862.
- Weiss JP, Sender Herschorn, Cerasela D Albei, Egbert A van der Meulen (2013) Efficacy and safety of low dose desmopressin orally disintegrating tablet in men with nocturia: results of a multicenter, randomized, double-blind, placebo controlled, parallel group study. J Urol 190(3): 965-972.
- Hvistendahl GM, Anders Riis, Jens P Norgaard, Jens C Djurhuus (2005) The pharmacokinetics of 400 microg of oral desmopressin in elderly patients with nocturia, and the correlation between the absorption of desmopressin and clinical effect. BJU Int 95(6): 804-809.
- Juul KV, Bjarke Mirner Klein, Rikard Sandstrom, Lars Erichsen, Jens Peter Norgaard (2011) Gender difference in antidiuretic response to desmopressin. Am J Physiol Renal Physiol 300(5): F1116-1122.
- Norgaard JP, Hashim Hashim, Lars Malmberg, Dudley Robinson (2007) Antidiuresis therapy: mechanism of action and clinical implications. Neurourol Urodyn 26(7): 1008-1013.
- Terano T, A Seya, Y Tamura, S Yoshida, T Hirayama (1996) Characteristics of the pituitary gland in elderly subjects from magnetic resonance images: relationship to pituitary hormone secretion. Clin Endocrinol (Oxf) 45(3): 273-279.
- Asplund R (2004) Nocturia, nocturnal polyuria, and sleep quality in the elderly. J Psychosom Res 56(5): P 517-25.
- Van Kerrebroeck P, Masoumeh Rezapour, Ariane Cortesse, Joachim Thüroff, Anders Riis, et al. (2007) Desmopressin in the treatment of nocturia: a double-blind, placebo-controlled study. Eur Urol 52(1): 221-229.
- Taha DE, OM Aboumarzouk, AA Shokeir (2018) Oral desmopressin in nocturia with benign prostatic hyperplasia: A systematic review of the literature. Arab J Urol 16(4): 404-410.
- Akagawa S, Shoji Tsuji, Yuko Akagawa, Sohsaku Yamanouchi, Takahisa Kimata, et al. (2020) Desmopressin response in nocturnal enuresis showing concentrated urine. Pediatr Int 62(6): 701-704.
- Prasad M, Peter Wahlqvist, Rich Shikiar, Ya-Chen Tina Shih (200)4 A review of self-report instruments measuring health-related work productivity: a patient-reported outcomes perspective. Pharmacoeconomics 22(4): 225-244.
- Abraham L, Asha Hareendran, Ian W Mills, Mona L Martin, Paul Abrams, et al. (2004) Development and validation of a quality-of-life measure for men with nocturia. Urology 63(3): 481-486.
- Weiss JP, Norman R Zinner, Bjarke M Klein, Jens Peter Norgaard (2012) Desmopressin orally disintegrating tablet effectively reduces nocturia: results of a randomized, double-blind, placebo-controlled trial. Neurourol Urodyn 31(4): 441-447.
- Andersson F, Peter Anderson, Tove Holm-Larsen, James Piercy, Karel Everaert, et al. (2016) Assessing the impact of nocturia on health-related quality-of-life and utility: results of an observational survey in adults. J Med Econ 19(12): 1200-1206.
- Rembratt A, Charlotte Graugaard-Jensen, Thomas Senderovitz, Jens Peter Norgaard, Jens Christian Djurhuus (2004) Pharmacokinetics and pharmacodynamics of desmopressin administered orally versus intravenously at daytime versus night-time in healthy men aged 55-70 years. Eur J Clin Pharmacol 60(6): 397-402.
- Vilhardt H S, Lundin J (1986) Falch Plasma kinetics of DDAVP in man. Acta Pharmacol Toxicol (Copenh) 58(5): 379-381.
-
Mustafa Alwani, Aksam Yassin*, Bassam Albaba, Raidh Talib, Aftab Mohammad Azad and Mohamed M Arous. Oral Desmopressin in The Treatment of Nocturia in Aging Population: A Pilot Study. Annals of Urology & Nephrology. 3(1): 2022. AUN. MS.ID.000560.
-
Nocturia, Anti-diuretic hormone, Vasopressin, Desmopressin, Sleep, Aging.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






