 Research Article
Research Article
Inflammatory Myofibroblastic Bladder Tumor: A Case Report and Systematic Literature Review
Daniel Oberneck1, Charles Chatzopoulos2*
1MD, Resident at Chirec Delta Hospital, Université libre de Bruxelles, Brussels, Belgium
2MD, Head of Urology Department, CHIREC Delta Hospital, Université libre de Bruxelles, Brussels, Belgium
Charles Chatzopoulos, MD, Head of Urology Department, CHIREC Delta Hospital, Université libre de Bruxelles, Brussels, Belgium
Received Date: September 26, 2025; Published Date: October 06, 2025
Abstract
Introduction: Inflammatory myofibroblastic tumors (IMT) of the bladder are rare, challenging to diagnose, and can be mistaken for malignant tumors. This study presents an additional case and assesses the effectiveness of various treatment options by conducting a systematic review of the literature.
Methods: The patient was chosen due to their unique anatomopathological analysis and surgical considerations. A multidisciplinary oncology committee approved the appropriate treatment. A systematic literature review was conducted following INESSS standards to evaluate the effectiveness of various treatment options for patients with IMT of the bladder.
Results: The patient was successfully treated with robot-assisted partial cystectomy with ureterovesical reimplantation. Our review identified a total of 75 patients with a similar lesion. Men accounted for 45% of the patients, with an average age at presentation of 34.6 years and an average tumor size of 4.86 cm. The most common location was the anterior wall, observed in 24.3% of cases, followed by the dome, which accounted for 22.9% of cases. The majority of patients were treated with partial cystectomy (56.7%), and the average recurrence-free follow-up period was 17.11 months.
Conclusion: The patient experienced excellent post-treatment progress. Partial cystectomy is the most commonly described treatment for this lesion and offers low morbidity and reduced hospitalization time. The literature review revealed that complete excision with clear margins is recommended, with the caveat of the interpretational limitations of our results. Robot-assisted surgery may be considered an innovative approach, but further research is needed to establish standardized treatment protocols for this rare condition.
Keywords: Myofibroblastic bladder tumor; inflammatory pseudotumors; myofibroblastic cell; tumor delimitation
Abbreviations: CASP: Critical Appraisal Skills Programme; Cebam: Belgian Centre for Evidence-Based Medicine; Cismef: Catalogue et Index des Sites Médicaux Francophones; FISH: Hybridation in situ en fluorescence; KCE: Belgian Health Care Knowledge Centre; Lissa: La structure d’Indexation et de Recherche pour une Bibliographie Sélectionnée; HAS: Haute Autorité de Santé; IMT: Inflammatory myofibroblastic tumor; INESSS: Institut National d’Excellence en Santé et en Services Sociaux; MeSH: Medical Subject Headings; NICE: National Institute for Health and Care Excellence; PMP: Pseudosarcomatous myofibroblastic proliferation; TURBT: Trans urethral removal of bladder tumour; ALK: Anaplastic lymphoma kinase; HPW: High Power Field; MLH1: MutL Homolog 1; MSH2: MutS Homolog 2; MSH6: MutS Homolog 6; PMS2: Postmeiotic Segregation Increased 2; SMA: Smooth muscle actin
Introduction
Context
In this final study, we compare the management of a case of inflammatory myofibroblastic tumor (IMT) of the bladder with the existing literature. We report the case of a 49-year-old patient treated with robot-assisted partial cystectomy with ureterovesical reimplantation. IMT is a rare mesenchymal tumor of unknown etiology [1-3] with a potential for local recurrence and rare metastases [4]. The first case reported in the literature dates back to 1980 [5]. Since the diagnosis is not precise, several denominations have been used in the literature to describe this group of tumors. Names such as inflammatory pseudotumors, pseudosarcomatous myofibroblastic proliferations (PMP), and pseudosarcomatous fibromyxoid tumors refer to similar lesions [1,6]. The relationship between IMT and PMP can show overlaps, and their distinction is unclear [7]. At the time, it was important to distinguish between IMT, which mainly occurs in children and has malignant potential [6], and PMP. These distinctions and characterizations of the different similar tumors seem to have evolved over the past decade in the literature.
IMTs present a particular diagnostic challenge due to their shared characteristics with malignant neoplasms [1]. Despite often being described as benign, these lesions can easily be misdiagnosed (both clinically and histologically) as malignant tumors [8], especially due to their resemblance to spindle cells [9]. They can occur in multiple locations and are more common in the lungs. When present in the urinary tract, IMTs most frequently occur in the bladder [10], are indolent, and clinically manifest as hematuria, dysuria, or abdominal pain [1,710,11]. Their diagnosis is based on histology, immunohistochemistry, and molecular biology analysis. Partial cystectomy is the key treatment for this type of lesion [1], requiring careful patient selection and long-term follow-up. The use of robotic surgery improves clinical, anatomical, and pathological outcomes with low morbidity and reduced hospitalization time [12,13].
Objective
The primary objective is to contribute an additional case of IMT of the bladder to the literature. In this study, we aim to improve the diagnostic approach and treatment decision- making by conducting a systematic review of the literature. Assessing the effectiveness of different available treatment options for patients with bladder IMT in terms of recurrence rate, overall survival, and disease-free survival among patients with similar examination results. This review will facilitate the understanding of this rare tumor and the more effective management of affected patients.
Materials and Methods
Case selection
To include this case in our study, we respected certain inclusion criteria. First, the patient was chosen due to its uniqueness; the specific anatomopathological analysis, the absence of guidelines or recommendations. The case was discussed in a multidisciplinary oncology committee to ensure that the treatment was appropriate and that all options had been considered. Although we did not request ethics committee approval for this study, we respected ethical standards in terms of confidentiality and respect for patient rights. We also presented this case at a robotic surgery congress to share our results with other healthcare professionals.
Systematic literature review
We conducted a systematic literature review following the production standards of the National Institute for Excellence in Health and Social Services (INESSS) 2. To assess the need for such a study, we checked PROSPERO (international prospective register of systematic reviews) to verify if any similar study was already available using the term “myofibroblastic”.
Research Question
We aim to define the efficacy of different available treatment options for patients with IMT of the bladder
The question was formulated using the PICO (Population, Intervention, Comparator, Out- come) format:
a) P: patients with IMT, or PMP, or inflammatory pseudotumor, or myxofibrosarcomatous tumor of the bladder.
b) I: Robot-assisted partial cystectomy.
c) C: Robot-assisted total cystectomy, total cystectomy, partial cystectomy, transurethral removal of bladder tumor (TURBT), medical treatment.
d) O: recurrence rate, overall survival, and disease-free survival
Regarding the search on Pubmed (Table 1), we searched for relevant MeSH (Medical Subject Headings) terms using the Hetop (Health Terminology/Ontology Portal) platform.
The inclusion criteria for the studies were as follows: study conducted on humans, full-text accessible, pathology related to IMT, bladder as the primary site of the lesion, article in English or French, information on the treatment followed for case reports. The exclusion criteria were: primary site of the lesion outside the bladder, inflammatory lesion appearing within three months of a previous urological intervention (for case reports). We did not restrict the search to a specific period due to the low number of reported cases in the literature.
Databases
Special attention was given to the level of evidence of the identified studies to ensure a rigorous and reliable analysis of the collected data. For this purpose, we searched for guidelines or recommendations, followed by randomized clinical trials, metaanalyses, systematic reviews, and observational studies such as case series or case reports. For the search of guidelines, several databases and specialized websites, such as the Haute Autorité de Santé (HAS), Catalogue et Index des Sites Médicaux Francophones(Cismef), Belgian Centre for Evidence-Based Medicine (Cebam), Belgian Health Care Knowledge Centre (KCE), La structure d’Indexation et de Recherche pour une Bibliographie Sélectionnée (Lissa), the National Institute for Health and Care Excellence (NICE), and UpToDate, were examined to find relevant guidelines for our study. For the studies, the MEDLINE database was analyzed via PubMed. The Cochrane and Trip database were also searched (Table 1). Due to access constraints, we did not search CENTRAL and EMBASE.
Table 1: Table showing the search equations by database.
In addition to the systematic search, the PubMed, Cochrane, Scopus, and ScienceDirect databases were informally analyzed to find additional case reports using the following search equations:
a) Tumor AND myofibroblastoma OR myofibroblastic AND pseudosarcomatous AND bladder.
b) Tumor AND myofibroblastoma OR myofibroblastic AND bladder.
Results
Clinical Case
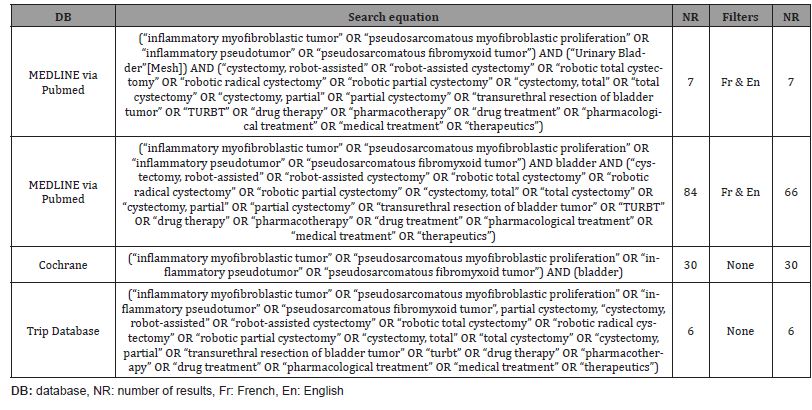
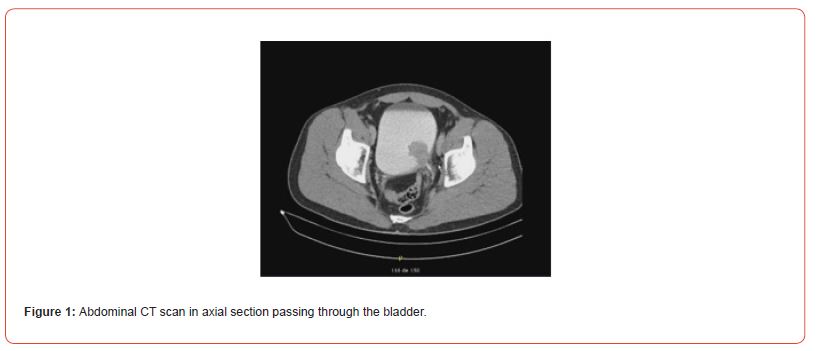
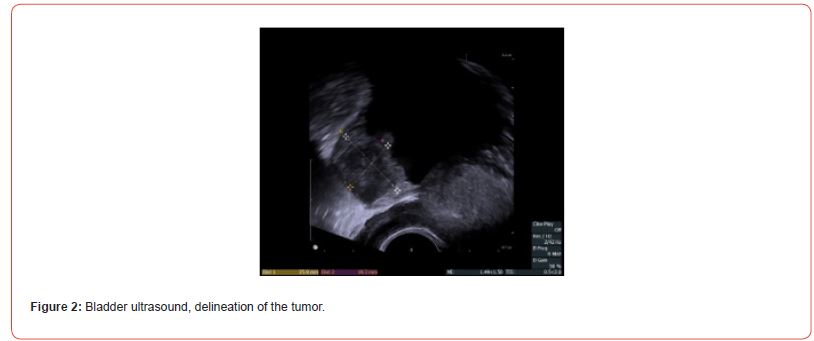
A 49-year-old patient presented with macroscopic hematuria. The CT scan revealed a protruding mass measuring 4 cm on the left postero-lateral wall of the bladder (Figures 1&2). Cystoscopy showed a fleshy polypoid mass near the left ureteral meatus. Bladder cancer was suspected, and an endoscopic resection was performed. Microscopic examination showed that the different samples involved the bladder wall, with a predominantly fusiform myofibroblastic cell proliferation arranged in a fascicular pattern and associated with inflammatory cells. At other locations, there were more star-shaped cells arranged in a myxoid stroma associated with inflammatory cells (mixed infiltrate characterized by neutrophils, eosinophils, plasma cells, lymphocytes). The myofibroblastic cells infiltrated the bladder wall diffusely. There was extensive invasion of the submucosa and invasion of the muscular layers. Some areas of tumor necrosis were present. The mitotic index ranged from 4 to 6 mitoses per 10 high-power fields. Many hemorrhagic changes were associated with this tumor proliferation. The tumor was invasive and poorly defined. Surface ulceration occurred.
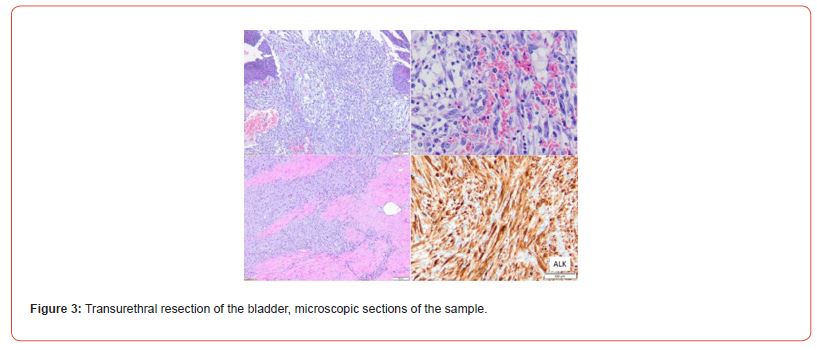
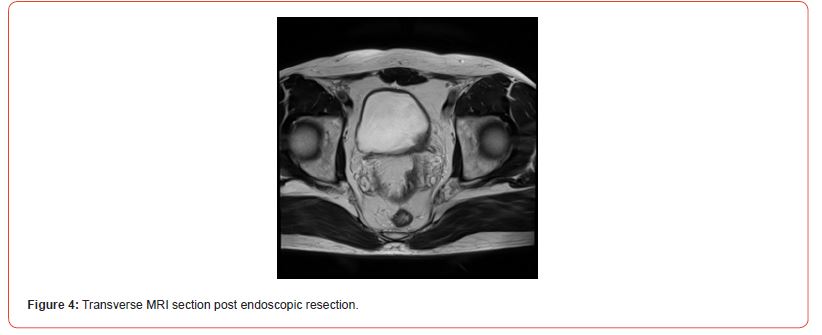
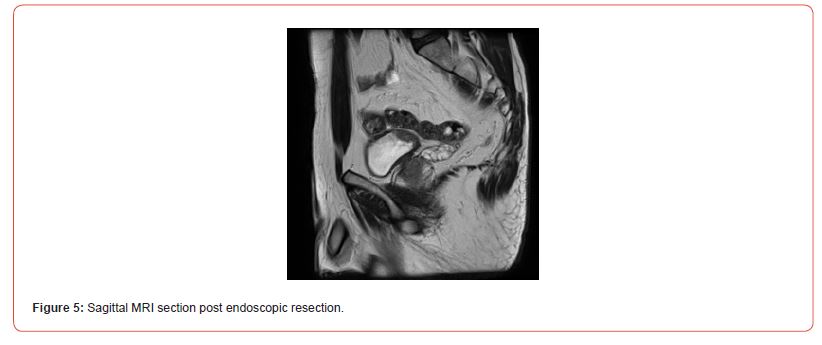
Immunostaining of the tumor cells showed heterogeneous positivity for keratin AE1E3. This positivity was lower in terms of intensity compared to the adjacent normal urothelial lining. There was no positivity for anti-p63 antibody. The tumor cells were focally positive for anti- Desmin and SMA antibodies. The proliferation index, evaluated using anti-Ki67 antibody, was estimated to be between 5 and 10% depending on the analyzed areas. There was strong and diffuse immunostaining for anti- ALK antibody. Fluorescence In Situ Hybridization (FISH) analysis showed rearrangement of the ALK gene in 84% of cells (Figure 3). The microscopic and immunohistochemical examinations are suggestive of IMT or pseudosarcomatous IMT. Microsatellite instability testing was performed. The MLH1, PMS2, MSH2, and MSH6 antibodies showed nuclear positivity, which is not suggestive of microsatellite instability for this tumor. A postoperative pelvic MRI (Figures 4&5) showed a heterogeneous enhancing lesion on the left postero-lateral wall of the bladder, measuring 26 x 22 x 14 mm, near the uretero-vesical junction, without extravesical extension.
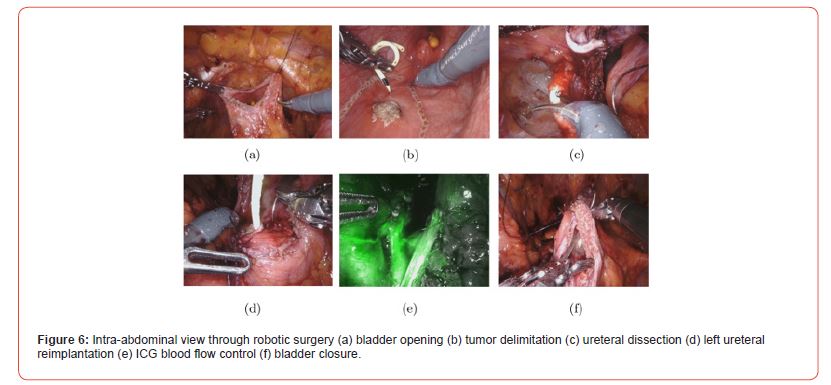
After discussion at the urology oncology clinique, complete resection of the lesion with clear surgical resection margins is recommended. Partial cystectomy with left segmental ureterectomy and ureteral reimplantation is proposed. The procedure was performed using robot-assisted laparoscopy (Figure 6). The patient was placed in the dorsal decubitus position with 30° Trendelenburg. Transperitoneal approach. Placement of 4 DaVinci Xi robot trocars in line at the level of the umbilicus and a 12 mm Air seal trocar in the right iliac fossa. Intra- abdominal pressure reduced to 8 mmHg. Incision of the peritoneum and dissection of the Retzius space. Opening of the anterior aspect of the bladder (Figure 6a) and identification of the tumor and ureteral meatus (Figure 6b). Placement of ureteral stents. Widen block resection of the tumor (Figure 6c) on the left postero-lateral aspect of the bladder and the lower left ureter, which is involved in the lesion. Closure of the posterior bladder wall using a V-lock 3/0 suture. Mobilization of the bladder dome, which is fixed to the left Psoas muscle using 2 Vicryl 2/0 sutures, and reimplantation of the left ureter (Figure 6d) with submucosal tunneling. Verification of ureteral vascularization using indocyanine green fluorescence angiography (Figure 6e). Placement of a JJ stent in the left ureter and closure of the anterior aspect of the bladder (Figure 6f) using a V-lock 3/0 suture. Placement of a bladder catheter and drain in the left iliac fossa.
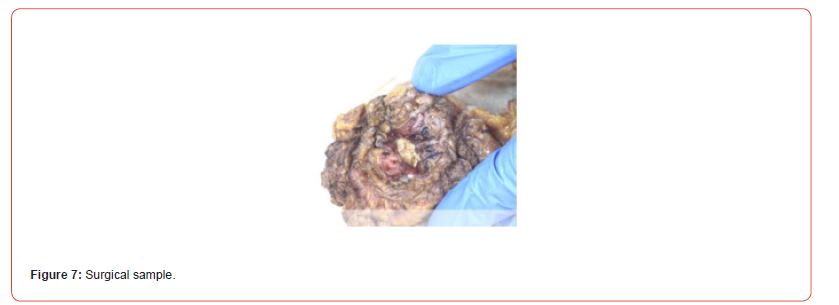
Postoperative recovery was uneventful. The patient was discharged from the hospital on the 4th postoperative day with the bladder catheter. The bladder catheter was removed on the 15th postoperative day. Microscopic examination of the surgical specimen (Figure 7) showed an inflammatory myofibroblastic proliferation involving the muscular layers and occasionally the peri-vesical adipose tissue. There were significant postoperative changes with calcifications and necrosis. No urothelial carcinoma in situ or invasive lesion was visualized. The surgical resection margins were clear. After a follow-up of 2 years, cystoscopy and PET-Scan showed no evidence of recurrence.
Systematic Review
During our search for guidelines, the French National Authority for Health (HAS) website did not yield conclusive results for search terms such as “bladder tumor,” “myofibroblastic,” “pseudotumor,” and “pseudosarcomatous.” Searches on Cismef, Cebam, and KCE also proved unsuccessful for these same search terms. However, searches on Lissa revealed 59 results for myofibroblastic tumors, none of which were selected. Searches on NICE were also unproductive, as there was only one result for myofibroblastic tumors, and the result is not yet available. The terms “pseudosarcomatous” and “pseudotumor” did not yield any relevant results. Likewise, no results were found on UpToDate.
A total of 232 studies were identified through the literature search method. After excluding duplicates (n=30), studies excluded using filters (n=18), and studies that did not meet the selection criteria (n=110), the remaining articles (n=74) were analyzed based on their full content (Figure 8). Case reports did not need to be analyzed using an evaluation grid, but they were selected based on criteria of relevance to the subject, content, and available information to understand the case, keeping in mind the analysis points of the CARE (CAse REport) checklist available on the Equator platform (Enhancing the QUAlity and Transparency Of health Research).
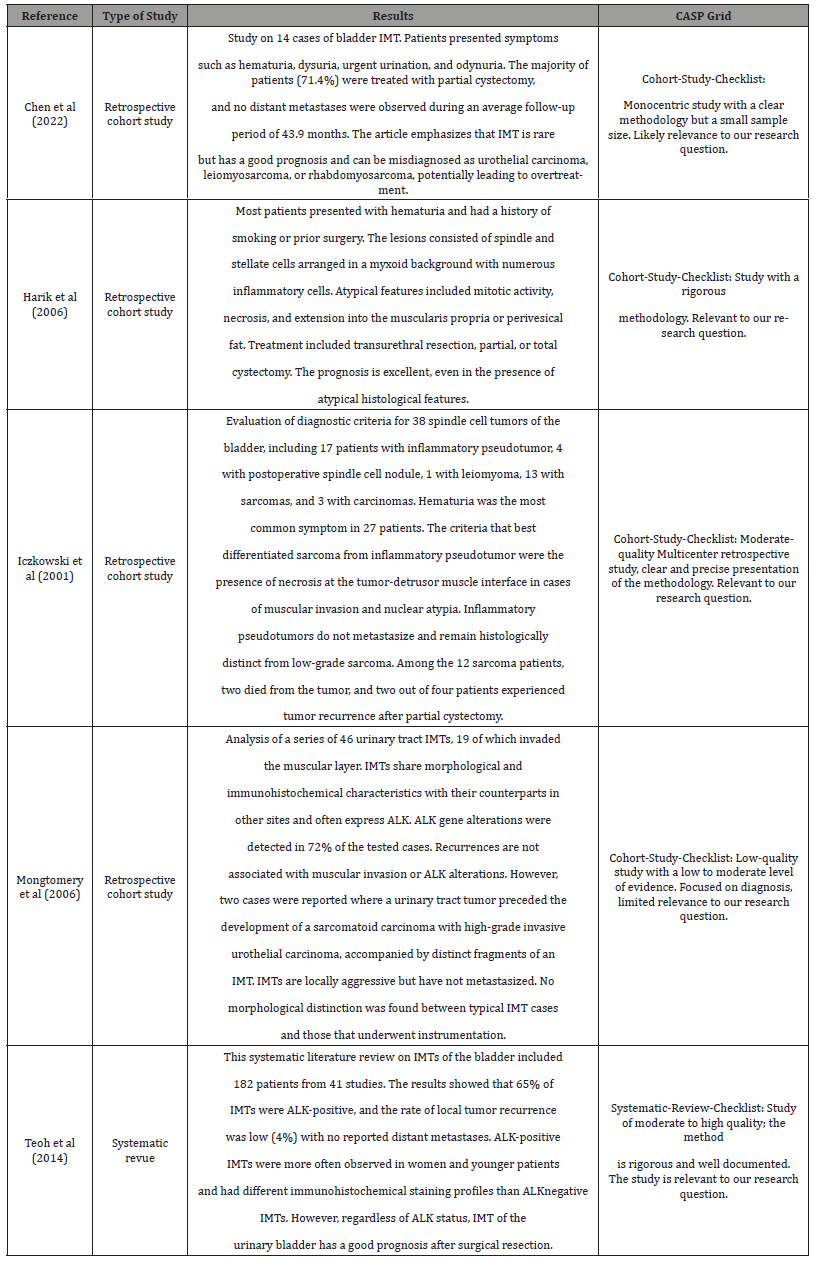
Five studies were included in the results and were evaluated using the Critical Appraisal Skills Programme (CASP) grids to ensure the quality of the presented data. Table 2 provides information on these studies. The first study, published in March 2022, was a retrospective cohort study conducted over an elevenyear period (Table 2) [1]. The second study, published in July 2006 (Table 2) [2], was a retrospective study. The third study, publishedin October 2001 (Table 2) [3], focused on differentiating between inflammatory pseudotumors and bladder sarcomas. The fourth study (Table 2) [4], published in December 2006, examined 46 cases of IMT, including an example of inflammatory fibrosarcoma and a subset associated with high-grade urothelial carcinoma. Finally, a systematic review (Table 2) [5] published in September 2014 was also evaluated.
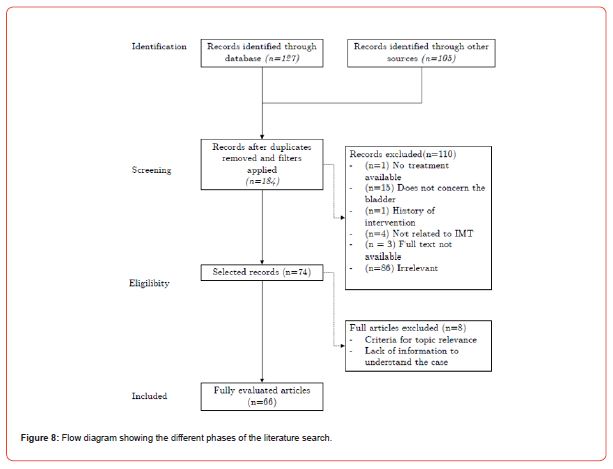
Table 2: Table of the studies.
Analysis of the Table
Table 3: Table of the studies.
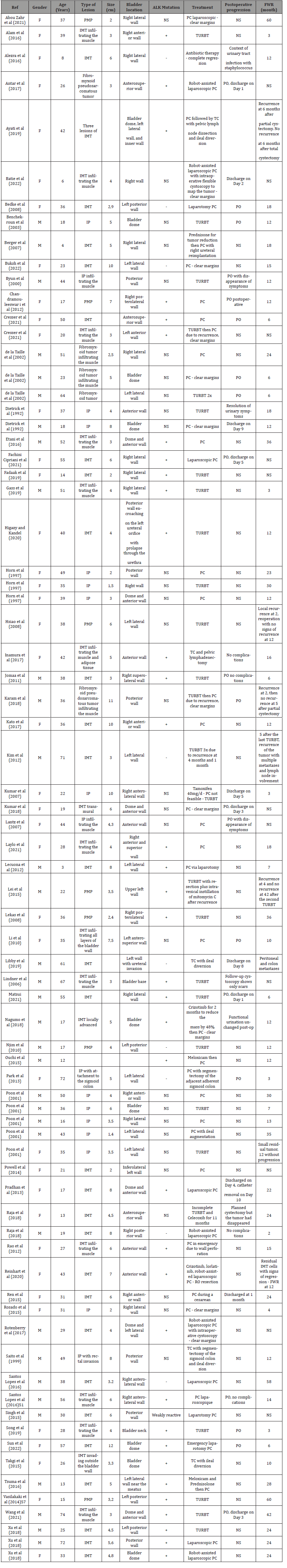
We compiled a table of 75 patients based on 61 case report articles (Table 3).
In total, 34 patients were male (45%) and 41 were female (55%). The average age of presentation for patients was 34.6 years. The average tumor size was 4.86 cm. The most common location was the anterior wall with 18 cases (24.3%), followed by the dome with 17 cases (22.9%), the posterior wall with 14 cases (18.9%), the right lateral wall with 13 cases (17.5%), and finally the left lateral wall with 12 cases (16.2%). Other bladder locations were not included in the calculation. In cases of multiple locations, the predominant component was considered. Regarding the ALK mutation, 37 cases (78.7%) were positive, 10 (21.3%) were negative, and 28 cases (37%) were not specified. The majority of patients underwent partial cystectomy (43, 57.3%). Transurethral removal of bladder tumor (TURBT) was performed for 27 cases (36.5%), and total cystectomy for 4 cases (5.4%). Medical treatment (1.3%) was recorded. Among those treated with partial cystectomy, 6 underwent robot-assisted surgery (14.2%). Among those treated with TURBT, 5 (18.5%) underwent additional treatment: 2 with partial cystectomy and 3 with a second TURBT. One patient treated with partial cystectomy received additional treatment with total cystectomy. The average duration of recurrence-free follow-up was found to be 17.11 months.
Discussion
Comparative Analysis of Our Clinical Case with The Literature Review
By comparing our case with the results of the literature review, we note that the average tumor size was 4.86 cm, similar to that of our patient. The most common locations were the anterior wall and dome of the bladder, while in our case, the location was the posterior wall with involvement of the left ureteral meatus, making conservative surgery more challenging from a technical point of view. The majority of patients (78.7%) in the literature review had a positive ALK mutation, similar to our case. The most commonly used treatment was partial cystectomy (56.7%), followed by TURBT (36.5%). In our case, a robot-assisted partial cystectomy was performed. Interestingly, only 14.2% of patients who underwent partial cystectomy benefited from robotic assistance. This difference is associated with technological advancements in robotic
surgery in recent years compared to what could be offered to patients from older cases. The literature review also showed that some patients who underwent TURBT required ad- ditional treatment with partial cystectomy or a second TURBT, while no additional treatment was necessary in our case.
Diagnosis
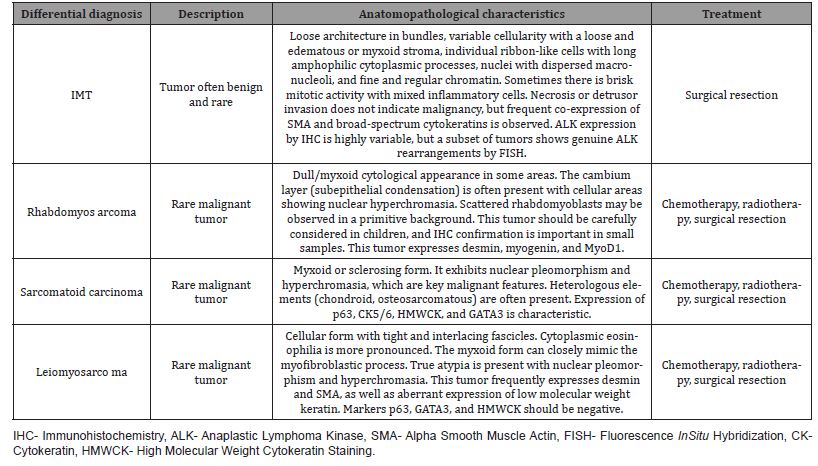
Biopsy is fundamental for establishing a definitive histological diagnosis considering the differ- ential diagnoses and significant confounding factors such as the aggressive and invasive nature that these tumors can have with other malignancies (Table 4). According to the reference book by Mahul B. Amin, PMP is the general term used and IMT is used in cases where an ALK gene rearrangement is present [14]. The initial diagnosis for our patient included the term “pseudosarcomatous”, which was important for determining the management. Although imaging can assist in the diagnosis, its nonspecific characteristics limit its utility [1].
The essential diagnostic criteria are a mass-like proliferation of spindle cells with myofibroblastic appearance, cytologically benign nuclei, scattered inflammatory cells, and diffuse immunohistochemical staining for SMA (Alpha Smooth Muscle Actin) [1,15]. To meet the diagnostic criteria for IMT, it is desirable for the tumor not to coexist with urothelial carcinoma and for an ALK rearrangement to be demonstrated [16]. The expression of ALK-1 protein and/or ALK gene rearrangements (detected by molecular analysis such as FISH) can help in the differential diagnosis between IMT and sarcomatoid carcinomas, as these genetic rearrangements are not found in the latter [16]. A recent study has shown that TERT promoter mutations are characteristicof sarcomatoid urothelial carcinomas but not PMPs [17]. This could help improve the means of diagnosing these tumors with close similarities. No staging system is currently available for IMT, as the tumors generally have a favorable behavior, regardless of their size or involvement of the muscular layer [3,11].
Table 4: Comparison table of differential diagnoses [14].
Treatments and Prognosis
The five studies found in our search, aimed at evaluating different treatment options, have limited usefulness. Given the significant advancements in surgical and diagnostic techniques over the past ten years, studies from 2006 and 2001 appear to be less relevant. The systematic review conducted by Teoh et al. [18] indicated a low recurrence rate (4%) without metastasis, information that has since been refuted. The most recent study, from 2022, has limitations due to its retrospective and singlecenter nature, as well as its small sample size (14 patients). All their patients underwent minimally invasive surgery, and none of the patients treated with partial cystectomy experienced recurrence [19] over a mean follow-up of 5.5 years. The main treatments for IMTs are TURBT, total or partial cystectomy, and medical treatment (Table 5). Incomplete resection of IMTs has even been proposed because the remaining cells remain stable or regress spontaneously [2], and rare recurrences after resection generally follow an indolent course [1]. A recent study of 35 patients with bladder IMT treated endoscopically showed no recurrence at 6 months [20].
Table 5: Comparison table of treatments.
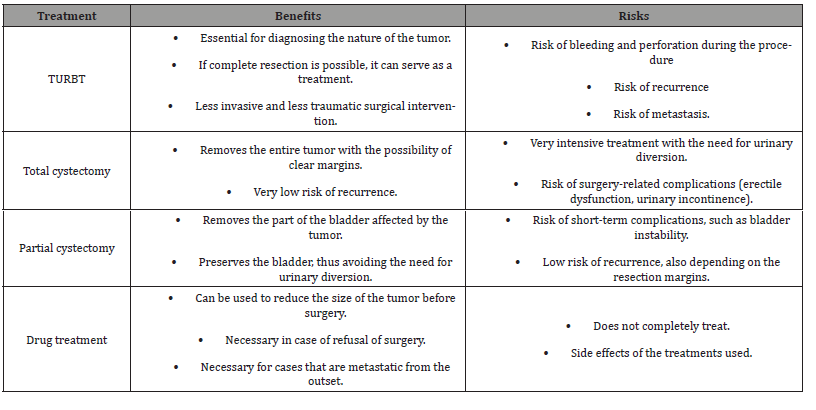
Despite the rarity of recurrences, IMTs deserve close follow-up due to their recently recognized malignant potential and the difficulty in excluding the diagnosis of sarcomatoid carcinoma. Therefore, complete surgical resection with clear margins and close followup remains the recommended treatment for urinary tract IMTs [1,4,21]. Partial cystectomy seems to be a better option for patients with tumor invasion of the muscular layer or ureter Robot-assisted surgery offers several advantages over pure laparoscopic approach for this type of treatment, including 3D visualization, greater and more stable freedom of movement, simpler intracorporeal suturing, instrument precision, surgeon ergonomics, and reduced morbidity [2,12, 22]. The da Vinci surgical robot system, described as technically feasible for this type of surgery, has been used [23]. Patient quality of life is improved without compromising survival [24]. Operating time is reduced [22,25], but this remains to be clarified as other studies report the opposite [12]. Personalized treatments are possible as several strategies are feasible [26]. Thanks to the progress of robotic surgery, the minimally invasive approach allows for the preservation of adequate bladder capacity while reducing long-term perioperative morbidity [2].
It also allows for shorter hospital stays [12]. For our patient, since the tumor had invaded the ureter, it was necessary to perform a Psoas Hitch. This procedure aimed to bridge the gap between the dissected bladder and ureter to allow for complete tumor resection with healthy margins. In addition, ureteral reimplantation was performed in a submucosal tunnel to prevent ureteral reflux. For cases that are not operable, neoadjuvant treatments such as crizotinib can be effective in reducing the size of large, locally advanced, and difficult-to-resect tumors [27,28]. For ALK- positive IMTs that do not respond to ALK inhibition with crizotinib, they can be treated with newer agents such as the next-generation ALK inhibitor, lorlatinib [28], facilitating subsequent partial cystectomy [27]. However, these treatments have not yet been reported regarding their use for initially resectable cases [27]. Treatment with anti-inflammatory agents or antibiotics (anecdotal) has also been used in certain circumstances [29,30].
Strengths and Limitations
The limitations of this systematic review include selection bias of retrospective data drawn from reported cases in the literature. Nomenclature is varied, and not all existing cases may have been described. Long-term outcomes of different available treatments and risk factors for recurrence were not examined. The strengths of this study include the systematic search method that allowed us to obtain a rigorous selection of publications, clear and structured presentation of results, and the integration of studies in addition to case reports. We present an innovative approach to treatment, namely the use of robot-assisted surgery. Recently, a research protocol has been registered on Prospero 3, which evaluates diagnostic and therapeutic modalities for IMTs of the genitourinary organs, confirming the timeliness and relevance of the topic of our study.
Conclusion
Inflammatory myofibroblastic tumors of the bladder are rare and difficult to diagnose. Partial cystectomy is the most commonly described treatment for this lesion and allows for low morbidity and reduced hospitalization time. Complete resection with clear margins is recommended. Robot-assisted surgery can be considered as an innovative approach, but further research is needed to establish standardized treatment protocols. These results appear to be of interest for clinical practice and future research. A multicenter, prospective study is needed to obtain evidence-based recommendations.
References
- Alam R, Johnson MH, Caldwell T, Pavlovich CP, Bivalacqua TJ, et al. (2016) Diagnosing and Treating Inflammatory Myofibroblastic Tumor of the Bladder. Gallucci M, é Case Rep Urol 2016: 5724020.
- Rotenberry C, Dowd K, Russell D, DeRiese W, Teeple S, et al. (2017) Robot-assisted Partial Cystectomy for Treatment of Inflammatory Myofibroblastic Tumor of the Bladder. Urol Case Rep 11: 25-27.
- Montgomery EA, Shuster DD, Burkart AL, Esteban JM, Sgrignoli A, et al. (2006) Inflammatory myofibroblastic tumors of the urinary tract: a clinicopathologic study of 46 cases, including a malignant example inflammatory fibrosarcoma and a subset associated with high-grade urothelial carcinoma. Am J Surg Pathol 30(12): 1502-1512.
- Libby EK, Ellis LT, Weinstein S, Hammer RD, Murray KS (2019) Metastatic inflammatory myofibroblastic tumor of the bladder. Urol Case Rep 23: 10-12.
- Song D, Jiao W, Gao Z, Liu N, Zhang S, et al. (2019) Inflammatory myofibroblastic tumor of urinary bladder with severe hematuria: A Case report and literature review. Medicine 98(1): e13987.
- Njim L, Dhouibi A, Binous Y, Touil N, Zakhama A, et al. (2010) Prolifération myofibrob- lastique pseudosarcomateuse de la vessie. Progrèsen Urologie (4): 307-310.
- Acosta AM, Demicco EG, Dal Cin P, Hirsch MS, Fletcher CDM, et al. (2021) Pseudosarcoma- tous myofibroblastic proliferations of the urinary bladder are neoplasms characterized by recurrent FN1-ALK fusions. Mod Pathol 34(2): 469-477.
- Vasilakaki T, Koulia K, Tsavari A, Arkoumani E, Liaropoulos D, et al. (2014) Pseudosarcomatous Myofibroblastic Proliferation of the Urinary Bladder: A Rare Entity. Urology 83(6): 1409-1411.
- Etani T, Naiki T, Nagai T, Iida K, Ando R, et al. (2016) Inflammatory Myofi- broblastic Tumor of the Urinary Bladder: A Case Report. Case Rep Oncol 9(2): 464-469.
- Teoh JYC, Chan NH, Mak SM, Lo AWI, Leung CY, et al. (2015) Inflammatory my-ofibroblastic tumours of the urinary bladder: multi-centre 18-year experience Urol Int 94(1): 31-36.
- Collin M, Charles A, Barker A, Khosa J, Samnakay N (2015) Inflammatory myofibroblastic tumour of the bladder in children: a review. J Pediatr Urol 11(5): 239-245.
- Bailey GC, Frank I, Tollefson MK, Gettman MT, Knoedler JJ (2018) Perioperative outcomes of robot-assisted laparoscopic partial cystectomy. J Robot Surg 12(2): 223-228.
- Golombos DM, O’Malley P, Lewicki P, Stone BV, Scherr DS (2017) Robot-assisted par-tial cystectomy: perioperative outcomes and early oncological efficacy. BJU Int 119(1): 128-134.
- Mahul B A, Satish K T (2022) Diagnostic Pathology Genitourinary. Elsevier 3rd Edition.
- (2022) WHO Classification of Tumours Editorial Board. Urinary and Male Genital Tumours. 5th Edition. Volume 8.
- Takagi K, Takai M, Kameyama K, Horie K, Kikuchi M, et al. (2015) ALK Gene Translocation in Inflammatory Myofibroblastic Tumor of the Urinary Bladder: A Case Report. Urology Case Reports 3(5): 138-140.
- Bertz S, Stöhr R, Gaisa NT, Wullich B, Hartmann A, et al. (2020) TERT promoter mutation analysis as a surrogate to morphology and immunohistochemistry in problematic spindle cell lesions of the urinary bladder. Histopathology 77(6): 949-962.
- Teoh JYC, Chan NH, Cheung HY, Hou SSM, Ng CF (2014) Inflammatory myofibroblastic tumors of the urinary bladder: a systematic review. Urology 84(3): 503-508.
- Chen C, Huang M, He H, Wu S, Liu M, et al. (2022) Inflammatory Myofibroblastic Tumor of the Urinary Bladder: An 11-Year Retrospective Study from a Single Center. Front Med 9: 831952.
- Hensley PJ, Bree KK, Guo CC, Lobo N, Campbell MT, et al. (2022) Clinicopathological analysis and outcomes of inflammatory myofibroblastic tumours of the urinary bladder. BJU Int 130(5): 604-610.
- Yagnik V, Chadha A, Chaudhari S, Patel K (2010) Inflammatory myofibroblastic tumor of the urinary bladder. Urol Ann 2(2): 78-79.
- Mikhail D, Sarcona J, Mekhail M, Richstone L (2020) Urologic Robotic Surgery. Surg Clin North Am 100(2): 361-378.
- Allaparthi S, Ramanathan R, Balaji KC (2010) Robotic partial cystectomy for bladder cancer: a single-institutional pilot study. J Endourol 24(2): 223-227.
- Knoedler J, Frank I (2015) Organ-sparing surgery in urology: partial cystectomy. Curr Opin Urol. mars 25(2): 111-115.
- Novara G, Catto JWF, Wilson T, Annerstedt M, Chan K, et al. (2015) Systematic review and cumulative analysis of perioperative outcomes and complications after robot- assisted radical cystectomy. Eur Urol 67(3): 376-401.
- Hamad J, McCloskey H, Milowsky MI, Royce T, Smith A (2020) Bladder preservation in muscle- invasive bladder cancer: a comprehensive review. Int Braz J Urol 46(2): 169-184.
- Nagumo Y, Maejima A, Toyoshima Y, Komiyama M, Yonemori K, et al. (2018) Neoadjuvant crizotinib in ALK-rearranged inflammatory myofibroblastic tumor of the urinary bladder: A case report. Int J Surg Case Rep 48: 1-4.
- Reinhart S, Trachsel Y, Fritz C, Wagner U, Bode-Lesniewska B, et al. (2020) Inflam-matory Myofibroblastic Tumor of the Bladder with FN1-ALK Gene Fusion: Different Response to ALK Inhibition. Urology 146: 32-35.
- Tsuma Y, Miyachi M, Ouchi K, Tsuchiya K, Iehara T, et al. (2016) Neoadjuvant Treat- ment With Cyclooxygenase-2 Inhibitor and Prednisolone Allows Conservative Surgery for Inflammatory Myofibroblastic Tumor of the Bladder. J Pediatr Hematol Oncol 38(8): e283-e285.
- Alezra E, Delforge X, Buisson P, Gourmel A, Cordonnier C, et al. (2016) [Complete resolution of inflammatory myofibroblastic tumor of the bladder after antibiotic therapy]. Arch Pediatr 23(6): 612-615.
-
Daniel Oberneck, Charles Chatzopoulos*. Inflammatory Myofibroblastic Bladder Tumor: A Case Report and Systematic Literature Review. Annals of Urology & Nephrology. 5(2): 2025. AUN.MS.ID.000612.
-
Myofibroblastic bladder tumor; inflammatory pseudotumors; myofibroblastic cell; tumor delimitation; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






