 Research Article
Research Article
In-Hospital and Postdischarge Outcomes of Patients with Cardiorenal Syndrome Type 1
Hamzic-Mehmedbasic A1*, Rebic D1, Durak Nalbantic A2, Dzubur A2, Ribic Mrkonja A1 and Hasanspahic S1
1Nephrology Clinic, Clinical Center University of Sarajevo, Sarajevo, Bosnia and Herzegovina
2Cardiology Clinic, Clinical Center University of Sarajevo, Sarajevo, Bosnia and Herzegovina
Hamzic-Mehmedbasic A, Nephrology Clinic, Clinical Center University of Sarajevo, Sarajevo, Bosnia and Herzegovina.
Received Date: December 01, 2022; Published Date: December 19, 2022
Abstract
Introduction: Cardiorenal syndrome type 1 (CRS1) is a syndrome characterized by a rapid worsening of cardiac function leading to acute kidney injury (AKI) and causing high mortality rates. We aimed to evaluate in-hospital and postdischarge outcomes of patients with CRS1 and to identify the risk factors for adverse outcomes of kidney function and mortality in CRS1 patients.
Methods: This prospective cohort study included a total of 56 acute heart failure (AHF) and acute coronary syndrome (ACS) patients diagnosed with CRS1. Patients were followed up during the hospital stay and six months after hospital discharge. In-hospital and six-month mortality as well as AKI severity, AKI progression, postdischarge development or progression of chronic kidney disease (CKD) were collected for analysis.
Results: The commonest stage of AKIN was stage 1 (46.4%), followed by stage 2 (28.6%) and stage 3 (25.0%). Progression of AKI occurred in 44.6% of patients, and 21.8% of patients underwent hemodialysis. Occurrence of CKD three months after discharge was observed in 55.2% of patients. The in-hospital and six-month mortality were 12.5% and 34.7%, respectively. Cox regression analysis revealed that the Charlson comorbidity index (CCI) score and AKIN stage 3 were independent prognostic factors of six-month mortality, while increased plasma B-type natriuretic peptide (BNP) levels, Acute Physiology and Chronic Health Evaluation (APACHE) II score and hemodialysis were independent predictors of overall mortality. Multivariate regression analysis identified plasma BNP as the independent prognostic factor of CKD development and serum cystatin C as the independent predictor of AKIN stage 3 and the need for hemodialysis.
Conclusion: Development of CRS1 in patients with AHF and ACS is associated with poor postdischarge survival and adverse outcomes of kidney function. Plasma BNP, serum cystatin C, and AKIN severity staging criteria may be a useful tool for predicting mortality and adverse kidney outcomes in CRS1.
Keywords: Cardiorenal syndrome type 1; Outcomes; Prognosis; Risk factors
Abbreviations: CRS: Cardiorenal syndrome; CRS1: Cardiorenal syndrome type 1; AKI: Acute kidney injury; AHF: Acute heart failure; ACS: Acute coronary syndrome; SNS: Sympathetic nervous system; RAAS: Renin-angiotensin-aldosterone system; BNP: B-type natriuretic peptide; sST2: Soluble suppression of tumorigenicity 2; NGAL: Neutrophil gelatinase-associated lipocalin; CICU: Coronary intensive care unit; CKD: Chronic kidney disease; AKIN: Acute Kidney Injury Network; CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; eGFR: Estimated glomerular filtration rate; COPD: Chronic obstructive pulmonary disease (COPD); CCI: Charlson comorbidity index; APACHE: Acute Physiology and Chronic Health Evaluation; KDIGO: Kidney Disease: Improving Global Outcomes; IL-18: Interleukin-18; EF: Ejection fraction; E/A: E-wave and A-wave ratio; MAP: Mean Arterial pressure; bpm: Beats per minute; BUN: Blood urea nitrogen; CRP: C-reactive protein; LVMI: left ventricular mass index; LAD: left atrial diameter; HD: Hemodialysis; ICU: Intensive Care units
Introduction
Heart-kidney interactions are being recognized as very important in the prognosis of each organ individually. Cardiovascular diseases are reported to be the main cause of mortality in over 50% of patients with renal failure, while impaired renal function significantly increases the mortality of patients with heart failure [1]. Syndromes that describe the interaction between the heart and kidneys are now defined and classified [2] but their prevention and treatment are fragmented and focused on one organ system. Cardiorenal syndromes (CRS) are defined as disorders of the heart and kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction of the other. Clinical CRS is classified into five distinct types. CRS type 1 (CRS1) is characterized by an acute worsening of cardiac function leading to acute kidney injury (AKI). The most common etiologies for acute cardiac events include acute heart failure (AHF), acute coronary syndrome (ACS), and cardiac surgeries. The presence of AKI in the setting of acute cardiac diseases is a very common finding. Cardiorenal syndrome type 1 has been described in 27-45% of hospitalized AHF patients, 9-54% of ACS patients [3], and 22.1% of patients that underwent cardiac surgery [4].
The mechanisms underlying the CRS1 are multifactorial, including hemodynamic alterations, neurohormonal effects, and inflammatory components. There are several major contributors, including the sympathetic nervous system (SNS), the reninangiotensin- aldosterone system (RAAS), the arginine vasopressin system, alterations in the immune response with apoptosis, and cytokine release. Reduced renal perfusion due to the presence of systolic dysfunction and decreased cardiac output is one of the most important etiological factors because compromised renal perfusion overactivates the RAAS. Moreover, elevated central venous pressure and renal venous congestion, which occurs as a response to hypotension and decreased cardiac output, may also be associated with the development of CRS1 [5].
AKI development may be the consequence of the basal clinical characteristics of patients, different primary kidney diseases, and hemodynamic status [6]. Patients who develop AKI after an acute cardiac event have a significantly high morbidity, an increased stroke risk, longer hospitalization, and a greater incidence of readmission. CRS1 is also correlated with increased risk for short-term and long-term mortality after an episode of AKI [4]. In the past decade, cardiac and renal biomarkers confirmed their predictive value in detecting AKI in the setting of acute cardiac disorders. High serum levels of B-type natriuretic peptide (BNP), soluble suppression of tumorigenicity 2 (sST2), neutrophil gelatinase-associated lipocalin (NGAL), and cystatin C were associated with an elevated risk of AKI in patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention [7]. In coronary intensive care unit (CICU) patients, serum cystatin, serum and urinary NGAL as well as serum IL-18 had good prognostic ability in detecting AKI [8]. Furthermore, renal and cardiac biomarkers were also evaluated in predicting mortality and severity of AKI in CRS1 patients in few studies. However, the only renal function outcome that was assessed was AKI progression [9-11]. Not many studies investigated a larger number of outcomes in CRS1 patients including not only in-hospital and postdischarge mortality but diverse renal function outcomes (staging of AKI, progression of AKI, and development of chronic kidney disease-CKD after AKI episode). Moreover, to our knowledge, the prognostic ability of renal and cardiac biomarkers to predict the development of de novo CKD and the progression of underlying CKD after AKI episode in CRS1 patients has not been the focus of interest yet. Identifying a useful and precise biomarker to predict in-hospital and postdischarge mortality, as well as diverse renal function outcomes after an episode of AKI in patients with acute cardiac illness, seems to be challenging.
The present study was designed to evaluate several in-hospital and postdischarge outcomes of patients with CRS1 and to identify the risk factors for adverse outcomes of kidney function and mortality in CRS1 patients.
Materials and Methods
Study cohort
This prospective cohort study was conducted at CICU in the Clinical Center University of Sarajevo. We included AHF and/or ACS patients hospitalized during the 18-month period but the study cohort consisted only of AHF and/or ACS patients diagnosed with CRS1 in the first 48 hours of hospitalization. CRS1 patients were followed up during the hospital stay and six months after hospital discharge. In-hospital and six-month mortality was collected for analysis. The outcome of kidney function was assessed three months after hospital discharge.
The inclusion criteria were adult patients diagnosed with AHF and/or ACS with a length of hospital stay longer than 24 hours. Exclusion criteria were as follows: history of end-stage renal disease requiring dialysis or with previous kidney transplantation and unable to provide informed consent. Written informed consent was obtained from all patients.
Definitions
Because the majority of patients in CICU received diuretics, AKI was defined and staged according to serum creatinine-based criteria per the Acute Kidney Injury Network (AKIN) criteria within the first 48 hours of hospitalization. We defined AKI in patients with an absolute increase in serum creatinine of 0.3 mg/dL (≥26.4 μmol/L) or more or a percentage increase in serum creatinine of 50% or more (1.5-fold from baseline). The diagnosis of CRS1 was confirmed in patients who developed AKI following acute cardiac illness (AHF, ACS, or both) [12]. The diagnosis of AHF was based on the European Society of Cardiology Criteria [13].
The estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI creatinine) equation [14]. Chronic kidney disease was recorded in patients with documented estimated glomerular filtration rate (eGFR) <60 mL/min and a history lasting >3 months. Patients who did not have medical records in Clinical Center University of Sarajevo were diagnosed with CKD using another institution`s data for the 3 months before admission. Antecedents of hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), cerebrovascular disease, and anemia were recorded for all patients. A diagnosis of hypertension was noted when the patients were taking antihypertensive medication or if their blood pressure in the hospital was ≥140/90 mmHg at least twice, and diabetes mellitus was recorded when the patients were receiving antidiabetic treatment or if their fasting serum glucose was > 6.9 mmol/L at least twice. Anemia was defined as a plasma hemoglobin concentration <135 g/L in men (<132 g/L in men over the age of 70) or <120 g/L in women. Comorbid conditions were calculated by the Charlson comorbidity index (CCI) score [15]. Illness severity was assessed and calculated using Acute Physiology and Chronic Health Evaluation (APACHE II) score [16].
In-hospital and postdischarge prognosis
The in-hospital outcomes of CRS1 patients were AKI progression and severity, need for acute hemodialysis, length of hospital stay, and in-hospital mortality. Postdischarge outcomes were the outcome of kidney function three months after hospital discharge and six-month mortality. AKI severity was defined by the AKIN classification which defines three stages of AKI [17]. Progression of AKI was defined by worsening AKIN stage in the first seven days of hospitalization. The outcome of renal function was evaluated three months after the hospital discharge of CRS1 patients. In CRS1 patients without pre-existing CKD, new onset CKD was recognized if eGFR sustained reduced <60 ml/min/1.73m2 three months after the AKI episode, according to Kidney Disease: Improving Global Outcomes (KDIGO) recommendations [18]. CKD progression was defined as incremental progression to a higher CKD stage (including the transition from stage 3A to stage 3B of CKD) in CRS1 patients with pre-existing CKD. CKD was classified into five stages according to KDIGO recommendations [19].
Measuring of acute kidney injury biomarkers
Serum cystatin C was measured by ELISA (R&D Systems). Serum and urine interleukin-18 (IL-18) were measured by ELISA (R&D Systems). Plasma BNP level was measured by immunodimetric assay method.
Statistical analysis
Categorical variables were expressed as numbers and percentages and compared by the Chi-square test or Fischer’s exact test. Continuous variables were presented as means ±SD, medians with interquartile ranges as appropriate, and compared with the Student t test and Man-Whitney U test. Multivariate logistic regression analysis was used to assess the determinants of renal function outcomes. Variables with p<0.05 in the univariate hazard analysis were further analyzed by multivariate hazard analysis to identificate the independent predictors of mortality. Data were presented as odds ratios (ORs) with 95% confidence intervals (CIs). A p-value less than 0.05 was considered to be statistically significant. Data were analyzed with the SPSS version 17.0 (SPSS, Chicago, Illinois).
Results
The patient cohort consisted of 56 CRS1 patients hospitalized at CICU of the Clinical Center University of Sarajevo. In the CRS1 patients, the average age was 74.4±10.0, male accounted for 39.5%. The mean eGFR was 64.9±20.7 mL/min/1.73 m2. A history of CKD was found in 48.2% of patients, and a history of diabetes and hypertension in 75.0% and 57.1% of patients, respectively. The mean ejection fraction (EF) was 37.9±11.2 with systolic dysfunction (EF <50%), and diastolic dysfunction (E-wave and A-wave ratio-E/ A<1) in 75% of patients. At the time of hospitalization, the mean arterial pressure (MAP) was 101.4±23.8 and heart rate 107.6±25.1 beats per minute (bpm), 48.2% of patients had atrial fibrillation and 23.2% of them had sinus tachycardia. The most common underlying factor for CRS1 development was AHF (89.3%) and the most common cause of AHF was coronary artery disease (53.8%), followed by cardiomyopathy (17%), valvular heart disease (16%) and hypertension (13.2%). One of the forms of the clinical presentation was pulmonary edema (25.5%) and pleural effusion (30.5%), and more than one-third of patients (37.5%) had low urine output <400 ml. The baseline characteristics (demographic data, laboratory values, clinical findings, and levels of AKI biomarkers) of CRS1 patients are presented in (Table 1).
Table 1:The baseline characteristics of patients with cardiorenal syndrome type 1.
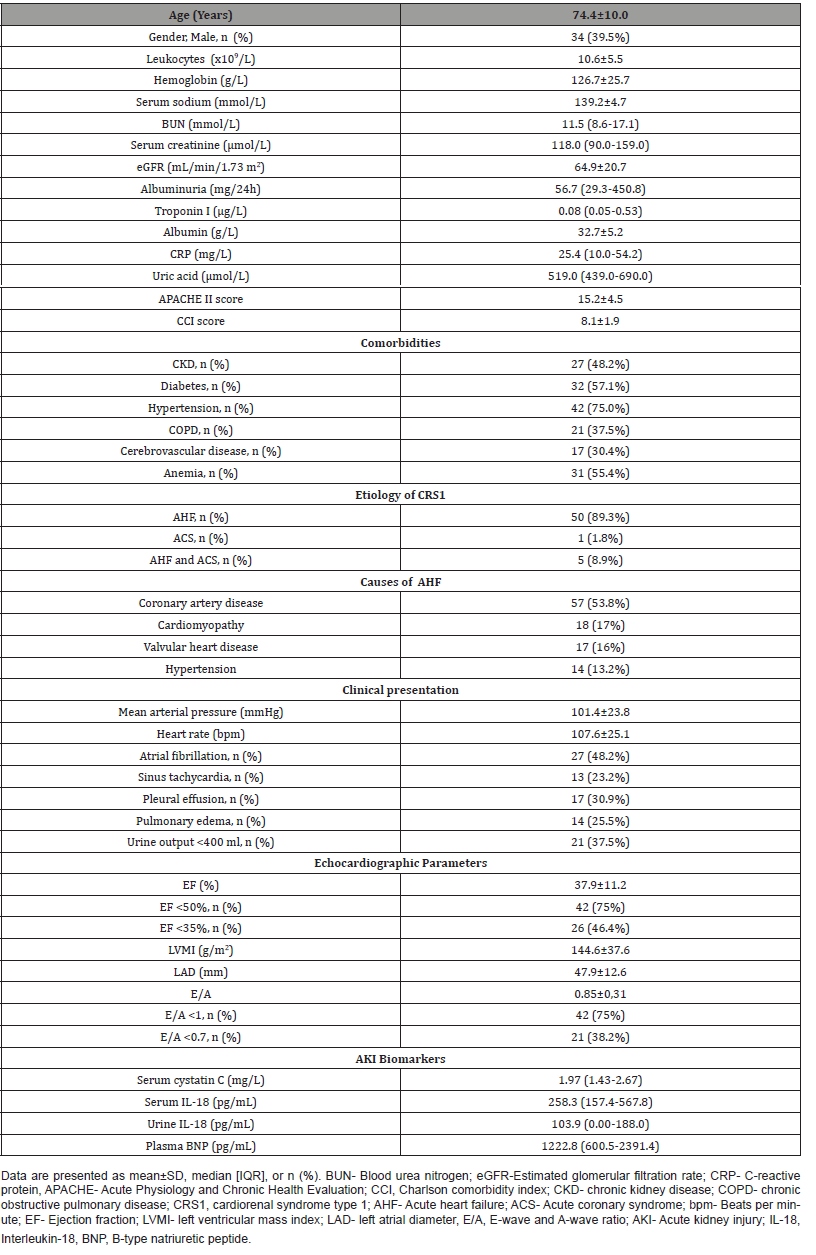
The outcomes of patients with CRS1 are presented in (Table 2). The commonest stage of AKIN was stage 1 (46.4%), followed by stage 2 (28.6%) and stage 3 (25.0%). Progression of AKI occurred in 44.6% of patients, and 21.8% of patients underwent hemodialysis during hospitalization. Assessment of kidney function three months after AKI episode revealed the occurrence of de novo CKD in 55.2% of CRS type 1 patients without underlying CKD, and progression of CKD in 60.0% of CRS1 patients with pre-existing CKD. The in-hospital and six-month mortality were 12.5% and 34.7%, respectively.
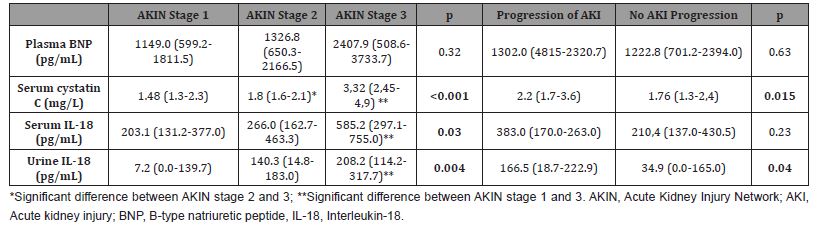
Levels of serum cystatin C, serum IL-18, and urine IL-18 were significantly increased in AKIN stage 3 in comparison to AKIN stage 1 (p<0.001, p=0.03, p=0.004, respectively) and serum cistatin C remained significantly elevated in AKIN stage 3 when compared to AKIN stage 2 (p<0.001). Serum cistatin C and urine IL-18 were significantly elevated in CRS1 patients with worsening of AKIN stage then in CRS1 patients without AKI progression (p=0.015, p=0.04, respectively) (Table 3).
Table 2:Outcomes of patients with cardiorenal syndrome type 1.
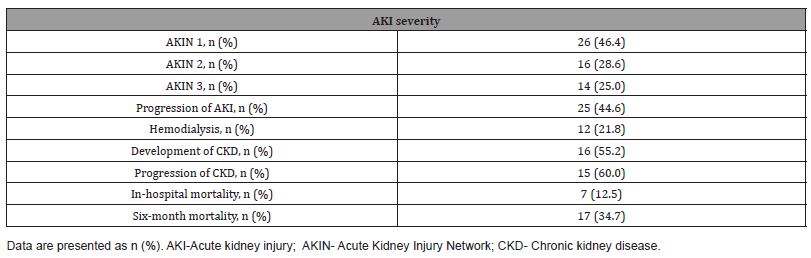
Table 3:Levels of acute kidney injury biomarkers according to the AKIN stages 1-3 and AKI progression.
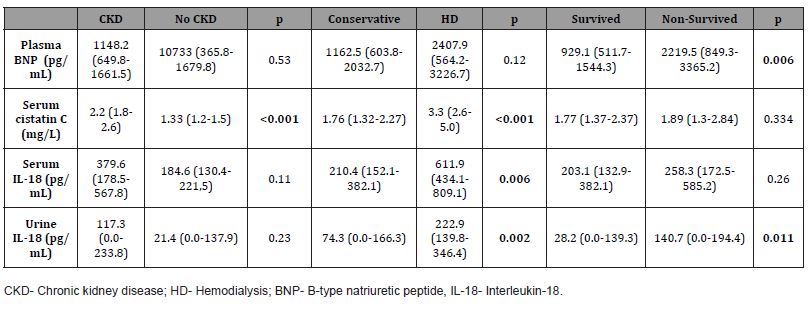
Serum cystatin C was significantly increased in patients who developed CKD three months after hospitalization in comparison to those without the occurrence of CKD (p<0.001). Levels of serum cystatin C, serum IL-18, and urine IL-18 were significantly more elevated in patients who underwent hemodialysis in comparison with patients treated with conservative treatment (p <0.001, p=0.006, p=0.002, respectively). Levels of plasma BNP and urine IL- 18 were significantly increased in patients who died in comparison to survived CRS1 patients (p=0.006, p=0.011, respectively) (Table 4).
Table 4:Levels of acute kidney injury biomarkers according to occurrence of CKD, type of in-hospital treatment and six-month survival.
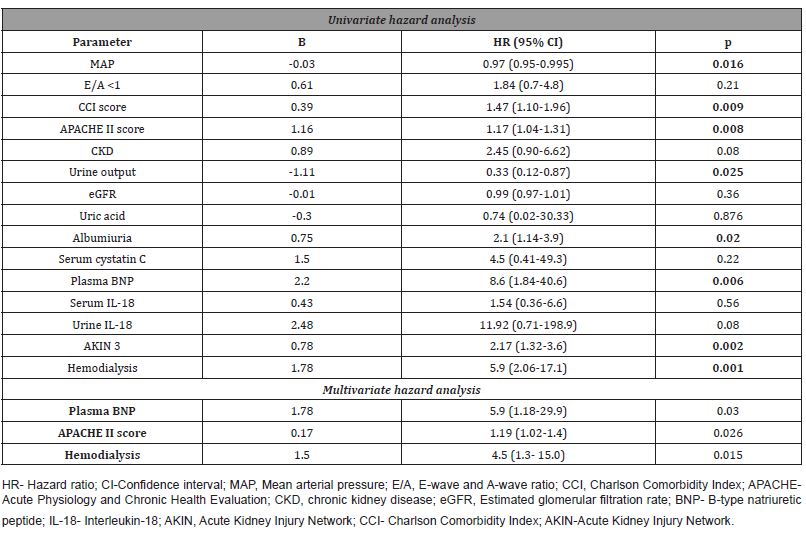
Multivariate logistic regression analysis identified BNP as the significant independent prognostic factor of the development of CKD (OR 1.001; 95% CI (1.000-1.001); p=0.037) and cystatin C as the significant independent predictor of AKIN stage 3 (OR 7.392; 95% CI (1.48-36.9); p=0.015) and need for hemodialysis (OR 5286.0; 95% CI (1.46-191100.0); p= 0.042) (Table 5).
Table 5:Predictors of adverse outcomes in cardiorenal syndrome type 1 patients.
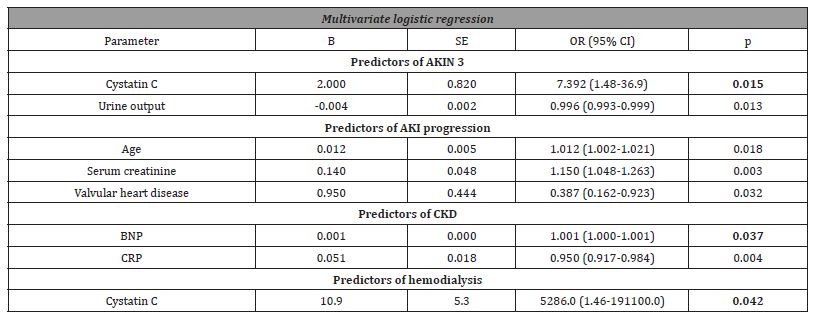
Univariate hazard analysis showed that increased CCI score and APACHE II score, elevated levels of BNP and albuminuria as well as AKIN stage 3, low urine output and low values of MAP were risk factors of six-month mortality. Multivariate hazard analysis showed that higher CCI score (HR 1.522; 95% CI (1.11-2.1); p=0.001), and AKIN stage 3 (HR=2.338; 95% CI (1.34-4.04); p=0.003) were independent predictors for six-month mortality (Table 6).
Table 6:Predictors of six-month mortality in patients with cardiorenal syndrome type 1.
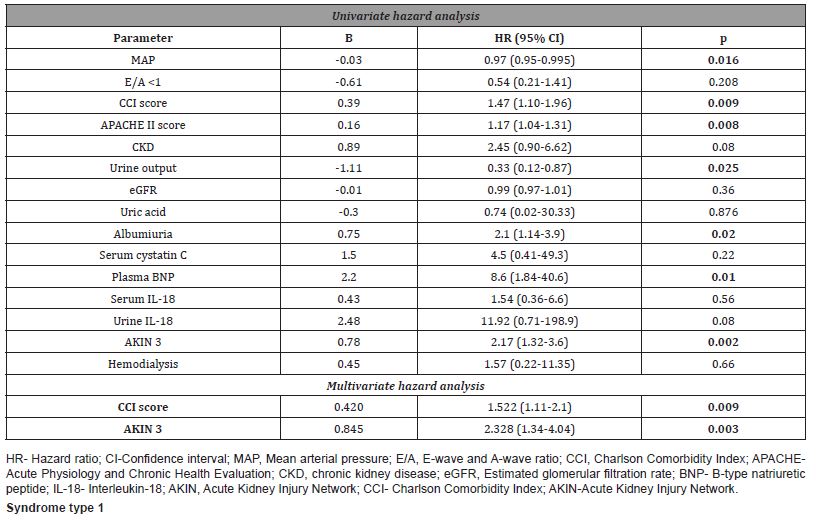
In univariate hazard analysis, increased CCI score and APACHE II score, elevated levels of BNP and albuminuria, AKIN stage 3, hemodialysis, low urine output and low values of MAP were risk factors for six-month mortality. Multiple Cox logistic regression hazard analysis revealed that increased plasma BNP levels (HR 5.9; 95% CI (1.18-29.9); p=0.03), APACHE II score (HR 1.19; 95% CI (1.02-1.4); p=0.026) and hemodialysis (HR 4.5; 95% CI (1.3-15.0); p=0.015) were independent predictors of overall (in-hospital and six-month) mortality (Table 7).
Table 7:Predictors of overall (intrahospital and six-month) mortality in patients with cardiorenal.
Discussion
In the present study, we analyzed the prognosis of CRS1 patients and identified the risk factors for adverse in-hospital and postdischarge outcomes of CRS1 patients. Consequences of CRS1 development showed a tendency to grow after hospitalization with greater six-month mortality (34.7%) than in-hospital mortality (12.5%) in patients diagnosed with CRS1. The occurrence of AKI after acute cardiac disorders was associated with adverse renal function outcome three months after discharge (development of CKD in 55.2% of patients without underlying CKD, and progression of CKD in 60.0% of patients with pre-existing CKD). AKIN severity classification, scoring systems for comorbidity and health evaluation, as well as emerging AKI biomarkers seem to be promising tools for predicting adverse outcomes of CRS1. In this study, CCI score and AKIN stage 3 proved to be independent prognostic factors of six-month mortality, while increased plasma BNP levels, APACHE II score and hemodialysis were independent predictors of overall (in-hospital and six-month) mortality. Plasma BNP was also identified as the significant independent prognostic factor of CKD development while serum cystatin C proved to be significantly associated with AKIN stage 3 and need for hemodialysis.
Occurrence of AKI in patients with acute cardiac events represents a risk factor for prolonged hospitalization and increased mortality [4]. In this research 12.5% of CRS1 patients died during hospitalization which was similar to percentage of in-hospital mortality in the study of Japanese AHF patients who developed AKI (13.8%) [20], but less than in elderly Chinese patients with CRS1 (23.2%) [21]. The mortality rate of CRS1 patients raised from 12.5% during hospitalization to 34.7% after discharge in this study which is in accordance with increased mortality rate after hospitalization (from 12% to 28%) in the study of Cowie at al, [22]. One metaanalysis showed that risk for mortality after initial hospital survival is two times higher one year after CRS1 [4].
The identification of risk factors for adverse outcomes in CRS1 is very important, but not many studies have been done to address this issue. In particular, prognostic significance of AKI biomarkers for the development of severe AKI stages and, especially, occurrence of CKD after hospitalization has not yet been widely discussed. In our study, elevated CCI score and AKIN stage 3 showed the independent predictive significance for six-month mortality of CRS1 patients, while the independent predictors of overall mortality were hemodialysis, elevated plasma BNP values and increased APACHE II score. One study indicated that, during admission for AHF, there was a stepwise increase in adverse outcome with increasing stages of AKI severity and more severe degrees of AKI were associated with increased short-term and longterm outcome of AHF patients [23]. In accordance with this finding, we have identified AKIN stage 3 as an independent predictor of six-month mortality in patients with CRS1. CRS1 patients with AKIN stage 3 had a 2.3 times higher risk of six-month mortality in comparison to the patients with AKIN stages 1 and/or 2. In addition to the AKIN classification, the APACHE II score showed an independent prognostic significance in predicting the overall mortality of CRS1 patients in our study. General illness severity scores are widely used in the intensive care units (ICU) to predict outcome. The APACHE II scoring system is important in treating disease and maintaining physiologic homeostasis in ICU patients. Mineral, metabolic and hemodynamic disturbances are common in patients with acute heart events, and the APACHE II score reflects the extent of these disorders in severe patients. Its ability to predict mortality has already been established in the AKI patients that were hospitalized in ICU [24], and in the population of acute cardiac patients in the CICU [8]. In accordance with these results, our study also confirmed that increased APACHE II score is an independent risk factor of overall mortality in patients hospitalized for the acute heart disorders with acute worsening of renal function. Murad et al. found that the increased risk of mortality in patients with acute heart disorders could partly be explained by the older age of these patients and presence of a large number of comorbidities [25]. Our CRS1 patients were also older (mean age of 74.4±10.0 years) with rather high mean CCI score (8.1±1.9). Furthermore, we proved that the CCI score, which is an important tool for evaluating the number and severity of comorbidities, have its usefulness in the prediction of six-month mortality in CRS1. Harel and al. also pointed out the importance of CCI score in predicting mortality of AKI patients [26]. Dialysis proved to be predictive for overall mortality of CRS1 patients in present study which is similar with results of the retrospective study of Hu et al. In this retrospective study multivariate analysis showed dialysis during hospitalization was risk factor for in-hospital all-cause mortality in CRS1 patients [21].
Natriuretic peptides have proven prognostic significance in patients with ACS [27]. In addition, BNP has proven to be a strong predictor of mortality in patients with congestive heart failure [28] and was also an important predictor of adverse outcomes in CKD patients [29]. However, to our knowledge, the prognostic significance of BNP as a predictor of mortality and/or renal function outcome in CRS1 patients has not been discussed earlier. In our study increased plasma BNP has shown an association with the sixmonth mortality of CRS1 patients, with significantly higher median plasma BNP concentrations in deceased in comparison to survived CRS1 patients. BNP was also confirmed to be an independent predictor of the overall (in-hospital and six-month) mortality in CRS1 patients. Its prognostic significance was so robust that with each increase in plasma BNP values of 1 pg/mL, the mortality risk in CRS1 patients increased 5.9 times. This negative effect of plasma BNP on the outcome of the CRS1 patient population could be explained, among others, by the presence of BNP in the coronary arteries and by its association with the extent and severity of coronary atherosclerotic plaques [30]. Increased plasma BNP in CRS1 patients could be a consequence of the increased activity of SNS in the left ventricle [31].
The occurrence of CRS1 is also associated with an increased risk of developing advanced stages of CKD [32]. In this research, evaluation of renal function outcome three months after an AKI episode revealed that de novo CKD occurred in 16/29 (55.2%) of CRS1 patients who had no pre-existing CKD. CKD progression was found in 15/25 (60.0%) of CRS1 patients with underlying CKD. The association of BNP and NT-proBNP with the progression of CKD has been demonstrated earlier in the population of pre-dialysis, nondiabetic patients [33]. However, in this study plasma BNP proved to be a significant prognostic marker of CKD development in the population of CRS1 patients.
Quantifying the AKI severity using the AKIN classification system may be helpful in risk stratification of an adverse outcome not only in AKI patients in ICU but in CRS1 patients too. In this research, we found that AKIN stage 1 was the most common in CRS1 patients (46.4%), while AKIN stage 2 (28.6%) and AKIN stage 3 (25.0%) were near equally represented. A greater incidence of AKIN stage 1 compared to other stages of AKI was one of the reasons for the significantly higher frequency of conservative treatment compared to dialysis (78.2% versus 21.8). Other authors have reported the possible role of AKI biomarkers in the prediction of severe AKI stages, and in guiding decisions on when to initiate renal replacement therapy in different heterogeneous populations of AKI patients [34]. Our study showed the association of serum cystatin C with more severe stages of AKI and hemodialysis treatment in patients with CRS1. Significantly higher serum cystatin C levels were recorded in CRS1 patients who underwent hemodialysis compared to patients treated conservatively. In the logistic regression analysis model, cystatin C was confirmed as an independent predictor of AKIN stage 3 and an independent predictor of hemodialysis. Similarly, in cardiac surgery patients, serum cystatin C levels were significantly increasing with an increasing class of AKI, and serum cystatin C also showed excellent ability in predicting composite outcomes (dialysis or death) [35, 36].
The prognostic ability of serum IL-18 and urine IL-18 to predict the adverse outcome of critically ill patients and patients with ACS has been established in the past decade [37,38]. However, to our knowledge significance of serum and urine IL-18 in predicting the outcome of CRS1 patients was not widely investigated. In present study, urine IL-18 was significantly associated with AKIN stage 3, the progression of AKI, the need for hemodialysis, and increased six-month mortality, while serum IL-18 was significantly associated with AKIN stage 3 and the need for dialysis. However, in multivariate analysis serum and urine IL-18 were not independent predictors of adverse outcomes in CRS type 1. The negative prognostic impact of urine IL-18 on renal function outcome of patients with acute heart disorders is not an unexpected finding, since urine IL-18 represents an inflammatory mediator of ischemic acute tubular necrosis, and its expression increases in response to ischemic kidney injury. The association of higher urine IL-18 concentrations with the progression of AKI has been confirmed in cardiac surgery patients and urine IL-18 proved to be a reliable biomarker of AKI progression [39]. Furthermore, elevated urine IL-18 levels were associated with a higher risk for dialysis in the early postoperative period after cardiac surgery [40] and urine IL- 18 remained independently predictive of the composite outcome of death or acute dialysis in ICU patients [37]. The association of higher urine IL-18 levels with a higher risk of postdischarge death in CRS1 patients has been demonstrated in our study. The negative prognostic influence of IL-18 in CRS1 could be explained by proven adverse IL-18 activity on the myocardium. Animal studies have shown that daily administration of IL-18 appears to induce interstitial fibrosis and myocyte hypertrophy, resulting in increased ventricular stiffness. The negative impact of IL-18 on the contractile function of the human myocardium was confirmed earlier [41]. In addition to urinary IL-18, our study showed an association of serum IL-18 with adverse outcomes of CRS1 patients (AKIN stage 3 and need for hemodialysis). In the research of Harford et al., serum IL-18 was also of particular interest as a predictor of future events (cardiovascular events and mortality) in patients with ACS [38]. Furthermore, Chen and al. confirmed that serum IL-18 is an independent prognostic factor of six-month mortality in patients hospitalized in CICU [8]. The association of serum IL-18 with these adverse outcomes maybe can be explained by the potential role of IL-18 in atherosclerotic plaque progression and destabilization that was confirmed by earlier investigation [42].
This study has several limitations. First, it is a single-center study with a rather small sample size. Second, besides plasma BNP, serum cystatin C, serum IL-18, and urine IL-18, there are more renal and cardiac biomarkers that could be evaluated in this study. Third, the postdischarge outcome that was evaluated might target a wider time period than six months in order to evaluate the long-term outcome of CRS1 patients. Despite these limitations, we believe that our study provides more evidence about a broad number of adverse outcomes related to CRS1 and the prognostic significance of cardiorenal biomarkers in predicting those outcomes.
Conclusion
Consequences of CRS1 development show a tendency to grow after hospitalization with higher six-month mortality (34.7%) than in-hospital mortality (12.5%) as well as the development of CKD in 55.2% of patients, and progression of CKD in 60.0% of CRS1 patients. AKIN classification, APACHE II and CCI scoring systems, as well as emerging AKI biomarkers seemed to be promising tools for predicting adverse outcomes in CRS1. AKIN stage 3, increased CCI score and APACHE II score are associated with higher mortality in CRS1 patients. Higher plasma BNP concentrations are associated with mortality and CKD development while higher serum cystatin C levels are linked to more advanced stages of AKI and the need for hemodialysis in CRS1.
Acknowledgement
None.
Conflict of interest
The authors report no conflicts of interest in this work.
References
- Hawkins R (2011) New biomarkers of acute kidney injury and the cardiorenal syndrome. Korean J Lab Med 31(2): 72-80.
- Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, et al. (2010) Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J 31(6): 703-711.
- Cruz DN (2013) Cardiorenal syndrome in critical care: the acute cardiorenal and renocardiac syndromes. Adv Chronic Kidney Dis 20(1): 56-66.
- Vandenberghe W, Gevaert S, Kellum JA, Bagshaw SM, Peperstraete H, et al. (2016) Acute kidney injury in cardiorenal syndrome type 1 Patients: A systematic review and meta-analysis. Cardiorenal Med 6(2): 116-128.
- Gembillo G, Visconti L, Giusti MA, Siligato R, Gallo A, et al. (2021) Cardiorenal syndrome: new pathways and novel biomarkers. Biomolecules 11(11): 1581.
- Palazzuoli A, McCullough PA, Ronco C, Nuti R (2015) Kidney disease in heart failure: the importance of novel biomarkers for type 1 cardio-renal syndrome detection. Intern Emerg Med 10(5): 543-554.
- Tung YC, Chang CH, Chen YC, Chu PH (2015) Combined biomarker analysis for risk of acute kidney injury in patients with ST-segment elevation myocardial infarction. PLoS One 10(4): e0125282.
- Chen TH, Chang CH, Lin CY, Jenq CC, Chang MY, et al. (2012) Acute kidney injury biomarkers for patients in a coronary care unit: a prospective cohort study. PLoS One 7(2): e32328.
- Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, et al. (2012) TRIBE-AKI Consortium Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 23(5): 905-914.
- Arthur JM, Hill EG, Alge JL, Lewis EC, Neely BA, et al. (2014) SAKInet Investigators Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int 85(2): 431-438.
- Chen C, Yang X, Lei Y, Zha Y, Liu H, et al. (2016) Urinary biomarkers at the time of AKI diagnosis as predictors of progression of AKI among patients with acute cardiorenal syndrome. Clin J Am Soc Nephrol 11(9): 1536-1544.
- Ronco C, McCullough P, Anker SD, Anand I, Aspromonte N, et al. Acute Dialysis Quality Initiative (ADQI) consensus group (2010) Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J 31(6):703-711.
- McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, et al. (2021) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 21 42(36): 3599-3726.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, et al. (2009) CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9): 604-612.
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, et al. (2005) Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 43(11):1130-1139.
- Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13(10): 818-829. PMID: 3928249.
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, et al. (2007) Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 11(2): R31.
- Fliser D, Laville M, Covic A, Fouque D, Vanholder R, et al. (2012) A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant 27(12): 4263-4272.
- Stevens PE, Levin A (2013) Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158(11): 825-830.
- Shirakabe A, Hata N, Kobayashi N, Shinada T, Tomita K, et al. (2013) Prognostic impact of acute kidney injury in patients with acute decompensated heart failure. Circ J 77(3): 687-696.
- Hu W, He W, Liu W, Fang X, Wu Y, et al. (2016) Risk factors and prognosis of cardiorenal syndrome type 1 in elderly Chinese patients: A retrospective observational cohort study. Kidney Blood Press Res 41(5): 672-679.
- Cowie MR, Komajda M, Murray-Thomas T, Underwood J, Ticho B, et al. (2006) POSH Investigators Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: results of the prospective outcomes study in heart failure (POSH). Eur Heart J) 27(10): 1216-1222.
- Roy AK, Mc Gorrian C, Treacy C, Kavanaugh E, Brennan A, et al. (2013) A Comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med 3(1): 26-37.
- Cruz MG, Dantas JG, Levi TM, Rocha Mde S, de Souza SP, et al. (2014) Septic versus non-septic acute kidney injury in critically ill patients: characteristics and clinical outcomes. Rev Bras Ter Intensiva 26(4): 384-391.
- Murad K, Goff DC Jr, Morgan TM, Burke GL, Bartz TM, et al. (2015) Burden of comorbidities and functional and cognitive impairments in elderly patients at the initial diagnosis of heart failure and their impact on total mortality: The Cardiovascular Health Study. JACC Heart Fail 3(7): 542-550.
- Harel Z, Bell CM, Dixon SN, McArthur E, James MT, et al. (2014) Predictors of progression to chronic dialysis in survivors of severe acute kidney injury: a competing risk study. BMC Nephrol 15: 114.
- Bassan F, Bassan R, Esporcatte R, Santos B, Tura B (2016) Very Long-term prognostic role of admission BNP in non-ST segment elevation acute coronary syndrome. Arq Bras Cardiol 106(3): 218-225.
- Choudhary R, Gopal D, Kipper BA, De La Parra Landa A, Aramin H, et al. (2012) Cardiorenal biomarkers in acute heart failure. J Geriatr Cardiol 9(3): 292-304.
- Abdel-Qadir HM, Chugh S, Lee DS (2011) Improving prognosis estimation in patients with heart failure and the cardiorenal syndrome. Int J Nephrol 2011: 351672.
- Gong H, Wang X, Ling Y, Shi Y, Shi H, et al. (2014) Prognostic value of brain natriuretic peptide in patients with heart failure and reserved left ventricular systolic function. Exp Ther Med 7(6): 1506-1512.
- Sakata K, Iida K, Mochiduki N, Nakaya Y (2009) Brain natriuretic peptide (BNP) level is closely related to the extent of left ventricular sympathetic overactivity in chronic ischemic heart failure. Intern Med 48(6): 393-400.
- Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, et al. (2008) Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med 168(6): 609-616.
- Spanaus KS, Kronenberg F, Ritz E, Schlapbach R, Fliser D, et al. Mild-to-Moderate Kidney Disease Study Group (2007) B-type natriuretic peptide concentrations predict the progression of nondiabetic chronic kidney disease: the Mild-to-Moderate Kidney Disease Study. Clin Chem 53(7): 1264-1272.
- Cruz DN, de Geus HR, Bagshaw SM (2011) Biomarker strategies to predict need for renal replacement therapy in acute kidney injury. Semin Dial 24(2): 124-131.
- Haase M, Bellomo R, Devarajan P, Ma Q, Bennett MR, et al. (2009) Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg 88(1): 124-130.
- Haase-Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, et al. (2009) Novel and conventional serum biomarkers predicting acute kidney injury in adult cardiac surgery-a prospective cohort study. Crit Care Med 37(2): 553-560.
- Siew ED, Ikizler TA, Gebretsadik T, Shintani A, Wickersham N, et al. (2010) Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol 5(8): 1497-1505.
- Hartford M, Wiklund O, Hultén LM, Persson A, Karlsson T, et al. (2010) Interleukin-18 as a predictor of future events in patients with acute coronary syndromes. Arterioscler Thromb Vasc Biol 30(10): 2039-2046.
- Koyner JL, Garg AX, Coca SG, Sint K, Thiessen-Philbrook H, et al. TRIBE-AKI Consortium (2012) Biomarkers predict progression of acute kidney injury after cardiac surgery. J Am Soc Nephrol 23(5): 905-914.
- Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, et al. TRIBE-AKI Consortium (2011) Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol 22(9): 1748-1757.
- Platis A, Yu Q, Moore D, Khojeini E, Tsau P, et al. (2008) The effect of daily administration of IL-18 on cardiac structure and function. Perfusion 23(4): 237-242.
- Hulthe J, McPheat W, Samnegård A, Tornvall P, Hamsten A, et al. (2006) Plasma interleukin (IL)-18 concentrations is elevated in patients with previous myocardial infarction and related to severity of coronary atherosclerosis independently of C-reactive protein and IL-6. Atherosclerosis 188(2): 450-454.
-
Hamzic-Mehmedbasic A*, Rebic D, Durak Nalbantic A, Dzubur A, Ribic Mrkonja A and Hasanspahic S. In-Hospital and Postdischarge Outcomes of Patients with Cardiorenal Syndrome Type 1. Annals of Urology & Nephrology. 3(3): 2022. AUN.MS.ID.000561.
-
Cardiorenal syndrome type 1, Outcomes, Prognosis, Risk factors, C-Reactive protein, serum cystatin C, apoptosis
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






