 Review Article
Review Article
Exploring Novel Therapeutic Strategies for Calciphylaxis: A Focus on Vitamin K2 Supplementation
Iyad Abuward Abusharkh1*, Chadia Beaini2, Reema Saffarini3, Saja Al-Abbasi1, Hala Yamout4, Nisrine Arhda5, Imane Guermah2, Ahmed Murad1
1AMSA Medical Center and Kidney Care, UAE
2Mediclinic Welcare Hospital, UAE
3Tomoh Al Amal Home Healthcare, UAE
4Clemenceau Medical Center Hospital, UAE
5Hospital Clinico Universitario de Santiago de Compostela, Spain
Iyad Abuward Abusharkh, AMSA Medical Center and Kidney Care, UAE
Received Date:April 13, 2024; Published Date:April 23, 2024
Abstract
Calciphylaxis, a rare and potentially life-threatening condition characterized by ischemic skin lesions and subcutaneous tissue necrosis, presents significant challenges in management due to its complex pathophysiology and limited treatment options. This article explores innovative therapeutic strategies for calciphylaxis, focusing on a novel approach that targets key molecular pathways implicated in its pathogenesis. Additionally, a case report is presented to illustrate the clinical application and efficacy of this new approach in a real-world setting. The case highlights the successful management of calciphylaxis with vitamin K2 (Menaquinone-7) in a treatment-naïve patient with severe affectation, underscoring the potential of this emerging therapeutic approach. Through this discussion and case presentation, we aim to contribute to the evolving understanding and improved management of calciphylaxis, offering insights into promising avenues for future research and clinical practice.
Keywords: Chronic kidney disease (CKD); hemodialysis; calciphylaxis; vascular calcification; vitamin k2; menaquinone-7
Introduction
Calciphylaxis, also referred to as calcific uremic arteriolopathy, is a rare life-threatening vasculopathy, characterized by deposition of calcium in the arteriolar microvasculature of the deep dermis and subcutaneous adipose tissue, resulting in intensely painful, ischemic skin lesions [1]. Although uremic calciphylaxis typically affects patients with end-stage chronic kidney disease, it can also occur in patients with earlier stages of chronic kidney disease (CKD) [2], acute kidney injury [3-5], prior receipt of a kidney transplant [6] and even with normal kidney function. The largest nationwide study to date estimates an incidence rate of 3.49 per 1,000 patientyears among patients receiving maintenance hemodialysis [7]. The incidence of calciphylaxis in this population has increased in the past decade, however, whether this is truly an increase in incidence or enhanced awareness remains unclear [8].
Painful evolving skin lesions (livedo reticularis, livedo racemosa, and retiform purpura) typically involving the areas of cutaneous and subcutaneous adiposity and rapidly progressing to ulcers with black eschar are the hallmarks of calciphylaxis presentation [9]. Although reported in relatively uncommon sites, including the penis, the digits, and the breasts [10-13], the lesions are most commonly seen on the abdomen and proximal lower extremities. Visceral calciphylaxis without primary cutaneous manifestations has also been reported, such as in mesenteric and colonic arteries [14,15]. The one-year mortality in calciphylaxis patients is reported at 45-80% with ulcerated lesions associated with higher mortality compared to non-ulcerated lesions. Sepsis is the leading cause of death [16].
Risk Factors
The major risk factor of calciphylaxis is impaired kidney function. Patients with calciphylaxis have high parathyroid hormone (PTH) levels at the start of treatment with dialysis. Compromised calcium reabsorption and phosphate excretion in patients with impaired kidney function results in secondary hyperparathyroidism, promoting bone remodeling, thereby increasing serum calcium levels and facilitating arteriolar microcalcification. Calciphylaxis may develop in patients with chronic kidney disease (CKD) despite normal serum calcium and phosphate parameters [17]. Risk factors are classified into modifiable and non-modifiable factors. Non-modifiable factors include longer dialysis vintage, caucasian race, and female sex. Postmenopausal women, probably due to genetic and hormonal reasons like estrogen deficiency, have a predisposition to a calcification pattern involving small vessels. Longer dialysis vintage of over 6-7 years has been reported as a risk factor for calciphylaxis [18]. This relationship has been inconsistent across studies. In a large cohort of 172 patients with uremic calciphylaxis patients undergoing maintenance hemodialysis, the median dialysis vintage was 3.1 years [19].
Modifiable factors include obesity, diabetes mellitus, hypoalbuminemia, hyperparathyroidism, vitamin K deficiency and vitamin K antagonist (VKA), corticosteroids, vitamin D deficiency, protein C and S deficiencies, Crohn’s disease, autoimmune disorders, substantial weight loss, recurrent hypotension, and malignant neoplasms (cholangiocarcinoma, hematologic malignancies, and melanoma). Although obesity was found to be associated with calciphylaxis, a definitive correlation has yet to be established. It can be categorized as one of many risk factors but not causation for the development of calciphylaxis. It is mainly associated with proximal calciphylaxis (involving trunk, thighs, breasts, etc) [20]. Obesity, due to the expansion of the subcutaneous compartment by adipose tissue, subjects the fibroelastic septa that anchor the skin to the body and arterioles to increased tensile stress, further reducing the blood flow in already calcified arterioles in dialysis patients [21].
Obese patients with calciphylaxis, have not only a high mortality rate, but also an increased morbidity with the sequelae. Diabetes mellitus is reported as a comorbidity in patients with calciphylaxis. However, no data are available on whether diabetes control or duration influences the development of calciphylaxis [22]. Malnutrition affects one-third of hemodialysis patients and is associated with a higher risk of morbidity and mortality. Serum albumin is a marker of nutrition and inflammation; and predicts mortality particularly when the level is below 3.8 g/dl [23]. Casecontrol studies report lower albumin levels in calciphylaxis patients when compared to dialysis patients without calciphylaxis [24,25]. Whether hypoalbuminemia is pathogenic, whether it is merely a marker of malnutrition or chronic inflammation, or whether it is a result of calciphylaxis itself, is not clear in the literature. However, there is no doubt that malnutrition is a determining factor in the recovery of wounds and the effectiveness of the immune response. Chronic kidney disease-mineral bone disorder axis abnormalities including hyperphosphatemia, hypocalcemia, hyperparathyroidism, elevated calcium-phosphorus product, and vitamin D deficiency are prevalent in advanced CKD patients [26].
Hyperparathyroidism has been considered an important risk factor for calciphylaxis. However, despite the high prevalence of mineral bone abnormalities in dialysis patients, calciphylaxis is relatively a rare disease and a number of reports in the literature describe calciphylaxis in dialysis patients despite the absence of significant mineral bone laboratory abnormalities and relatively normal or even low serum calcium, normal serum phosphorous levels, and PTH level at the time of calciphylaxis [27]. According to the German calciphylaxis registry, 47% of the reported cases of calciphylaxis had serum PTH values within the recommended target range [28]. On the other hand, cases of calciphylaxis secondary to hypoparathyroidism have been published in the literature [29,30]. The use of warfarin, a VKA, increases the risk of calciphylaxis by a factor of 3 to 13 [31,32]. About 40 to 50% of patients with end stage renal disease and calciphylaxis have been treated with warfarin and warfarin use is also linked with increased mortality among patients with this disease [33].
Even in the absence of warfarin use, vitamin K deficiency may play a role in the pathogenesis of calciphylaxis. Matrix Gla protein (MGP) is a potent inhibitor of vascular calcification. The ability of MGP to inhibit calcification requires the activity of a vitamin K–dependent enzyme, which mediates MGP carboxylation [34]. Fetuin-A and pyrophosphate are additional inhibitors of calcification whose expression is decreased in CKD and calciphylaxis. Ultimately, low levels of MGP, fetuin-A, and pyrophosphate result in a favorable environment for calcification to occur. Hypercoagulable conditions are frequently observed in patients with chronic kidney disease (CKD). In a matched case-control analysis of 152 individuals with chronic kidney disease (CKD), lupus anticoagulant, protein C deficiency, and combined thrombophilia were all found to be significantly associated with calciphylaxis development [35]. In another case-control study of 49 uremic calciphylaxis patients and 98 control patients on dialysis, no significant difference in protein C activity, protein S antigen, or antithrombin III activity was noted [36].
In all cases, evaluation for thrombophilic conditions in calciphylaxis patients should be considered since it has important treatment implications. Calcium supplements, calcium-based phosphate binders, and active vitamin D have been associated with increased calciphylaxis risk [37].
Diagnosis
The diagnosis is based mainly on clinical criteria, such as patients with severe CKD or who are on dialysis with painful purpura or ulcers, especially in the lower extremities or trunk. A skin biopsy can help confirm the diagnosis, especially in cases where it is not clear, and must be able to assess the arterioles of the subcutaneous tissue. Performing a skin biopsy should be approached with caution due to the risk of promoting new ulcers or infections [38]. Histopathological findings that can be seen include calcification of small vessels in the dermis and subcutaneous fat and, in some cases, ischemic necrosis of the dermis and epidermis as well as thrombosis of the vascular lumen [39]. Other diagnostic methods can be used to aid in diagnosis. Skin ultrasounds can confirm the presence of vascular calcifications of the subcutaneous tissue through the visualization of hyper-echogenic deposits with acoustic shadows. Simple X-rays can also visualize vascular calcifications in the affected areas, while mammography technique has greater definition and can show greater evidence of calcifications of small vessels with high sensitivity [40].
The differential diagnosis should be made by considering the patient’s clinical history, comorbidities, and clinical presentation. Martorell hypertensive ulcers are hypertensive ulcers caused by atherosclerosis of the peripheral arteries. They usually have rapid growth and with disproportionate amount of pain with involvement of the lateral-dorsal part of the calf and Achilles tendon. They are usually seen in those with normal renal function and have a palpable pulse with normal arterial studies, unless there is peripheral arterial disease. The presence of intermittent claudication and known arterial vascular disease, in the presence or absence of CKD, indicates that the origin of the ulcer could be vascular. In warfarin-induced necrosis, there is development of pain, erythema, petechiae, and ecchymosis, with subsequent gangrene. It usually appears early after starting warfarin. Cholesterol embolism usually occurs suddenly, after an intravascular procedure, and is accompanied by cyanosis. Ulcers due to vasculitis appear in the context of systemic involvement, accompanied by extracutaneous involvement such as fever, hemoptysis, abdominal pain, etc. [41].
Treatment
Due to its rapid progression and high mortality, early diagnosis and initiation of treatment are of great importance. Immediate medical-surgical management is needed. Debridement of necrotic tissue has shown improvement in 1-year survival in a retrospective analysis of 63 calciphylaxis cases from Mayo Clinic compared to patients who did not undergo surgical debridement. The use of appropriate antibiotic therapy is important since wound infections are one of the major factors that increase mortality in patients with calciphylaxis. The use of hyperbaric oxygen therapy can be useful in cases of calciphylaxis since it can promote the recovery of necrotic lesions. However, the high cost and difficult access in some countries may limit its use [42]. Calciphylaxis lesions are very painful and can become disabling for patients. Adequate pain relief is important to improve the patient’s quality of life. Options for treatments include opioids, ketamine, or other pain relievers including some antidepressants, which can be of great help in pain control. The choice of treatment should depend on the toxicity of the medications and the renal metabolism. Because of this, fentanyl, buprenorphine, and methadone could be preferred to opioids, as they are associated with less toxicity in CKD [43]. An important part of calciphylaxis treatment is the elimination of its precipitating factors [44]:
a) Control of secondary hyperparathyroidism.
b) Avoiding hypercalcemia and hyperphosphatemia.
c) Avoid the use of active metabolites of vitamin D.
d) Avoid calcium-based phosphorus binders.
e) Discontinue treatment with VKA.
f) Improve the patient’s nutritional status.
g) Intensify dialysis and increase the clearance of uremic toxins and phosphorus by increasing duration or frequency to achieve the recommended guidelines goals.
The use of calcification inhibitors is essential given the pathophysiology of this disease. The best-known agents to inhibit ectopic calcifications are sodium thiosulfate, bisphosphonates, and a new agent called SNF472. Sodium Thiosulfate is the most used measure to treat calciphylaxis although its use is still considered off-label. In addition to its calcification-inhibiting and vasodilating effect, it can also form water-soluble complexes with various metals and minerals. In one study of 172 hemodialysis patients with calciphylaxis and treated with sodium thiosulfate, 74% had total or partial improvement while 26% did not improve or had an unclear response. The treatment dose was 25 g in 100 mL of saline solution, administered during the last half hour of each hemodialysis. Bisphosphonates as treatment have been described in several retrospective studies. Although they can induce adynamic bone disease, the benefits that can provide are greater than the resulting risk [45]. SNF472 is a new formulation of myo-inositol hexaphosphate, which is still in the clinical development phase. It is administered intravenously and acts selectively and directly inhibiting the formation and growth of hydroxyapatite crystals [46,47].
Prognosis
We present a case of a 38-year-old woman known to have a history of hypertension, end-stage kidney disease due to hypertensive nephropathy, and secondary hyperparathyroidism, who developed bilateral ischemic lesions on the anterior aspect of the leg, which are painful and evolved into necrotic lesions. The lesions extended to the lethal aspects of the leg and foot within a month. The patient received multiple sessions of antibiotic therapy without improvement. The lesions eventually evolved from skin necrosis to necrosis of the subcutaneous tissue (Figures 1-3). The patient was only receiving hemodialysis twice a week for 18 months, then once weekly for 4 months due to financial reasons. She was on amlodipine 10mg qday, hydralazine 50mg, doxazosin, carvedilol 25mg, and paracetamol at home.
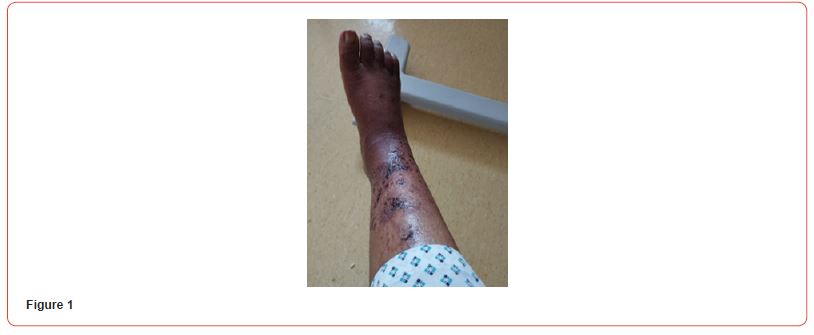
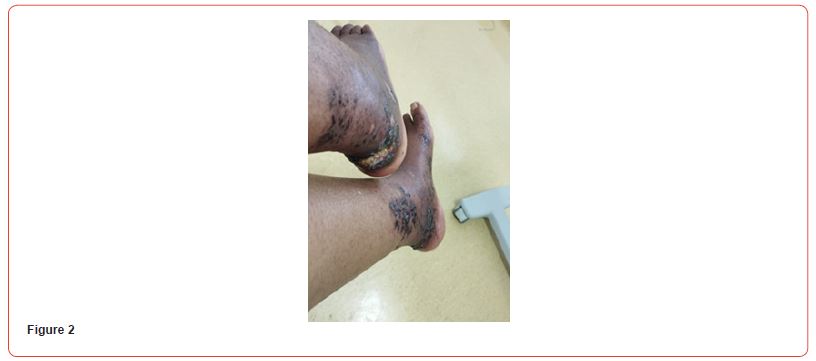
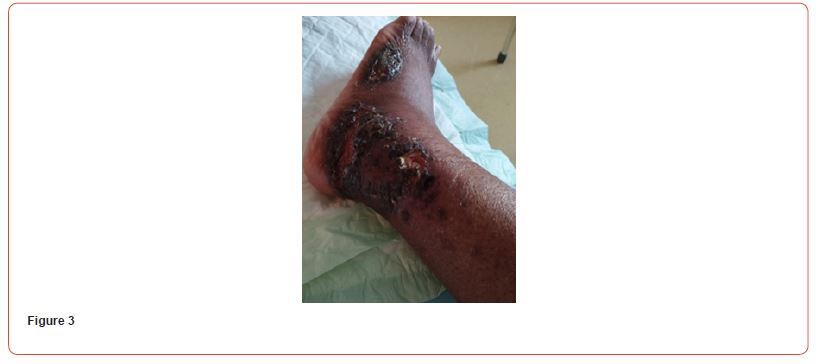
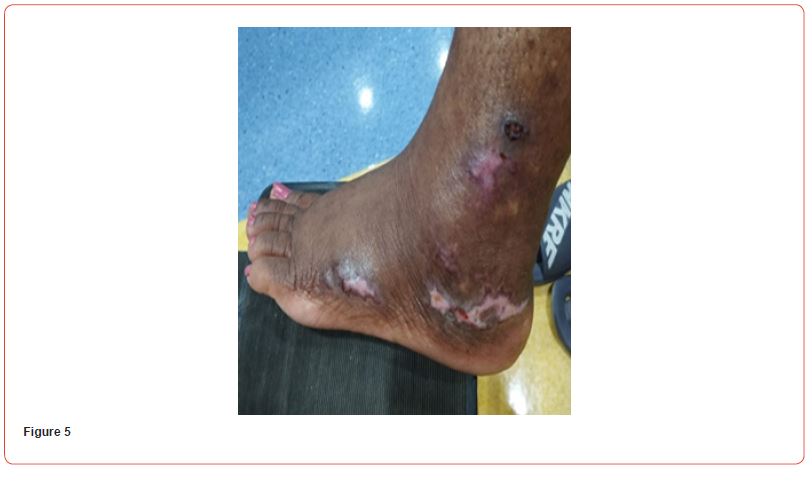
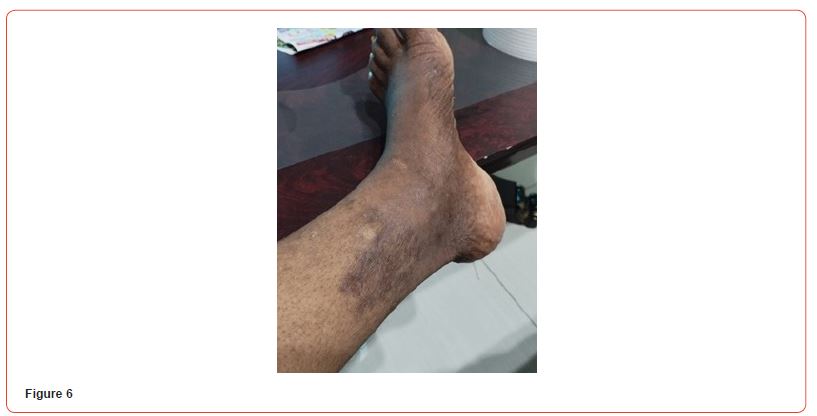
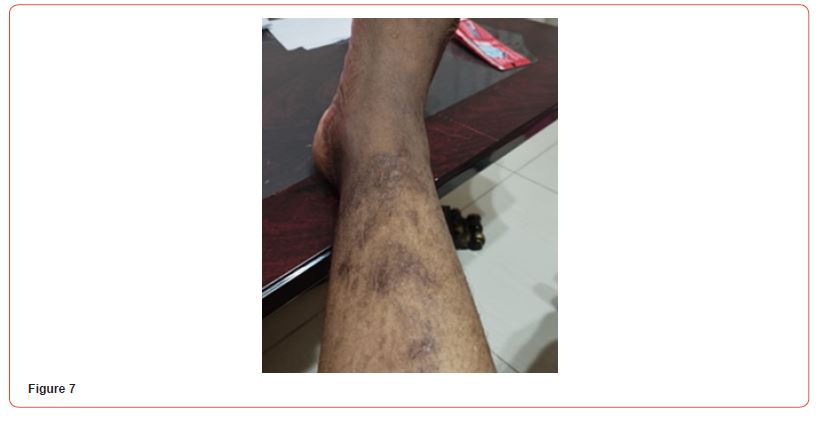
Blood tests showed: Creatinine 11,8 mg/dl, Urea 141 mg/dl, Hb 8,6 g/dL, Na 140, K 6,3 mEq/L, Calcium 7,9 mg/dl, Phosphorus 3,88 mg/dl. Albumin 3,6 g/L. PTH 2204 pg/ml. She initially refused to increase the frequency of her hemodialysis sessions or perform additional testing for her skin lesions. It was decided to start treatment with vitamin k2 (menaquinone-7) supplements at a high dose of 360 mcg/day. The patient eventually agreed to receive two sessions of hemodialysis per week. After a month of starting the treatment, the patient reported resolution of pain with clear improvement in the necrotic lesions. Two months after treatment, the ulcers have clinically improved and clear healing is evident (Figures 4&5). Three months after initiating treatment, there were no remaining ulcers and a complete resolution of the calciphylaxis lesions. Only pigmentary lesions are seen, probably due to postinflammatory hyperpigmentation (Figures 6&7).
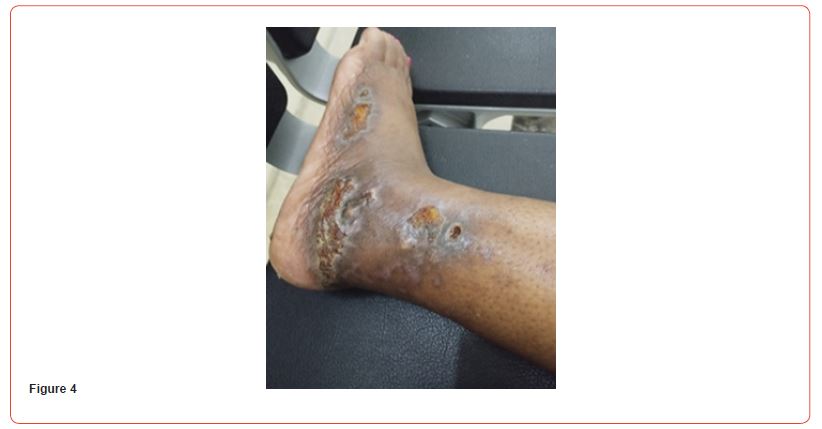
Discussion
Calciphylaxis is a rare and poorly understood disease with a high mortality rate and minimal treatment options. It is important to focus on inhibiting arterial calcification for the prevention and treatment of calciphylaxis. Matrix Gla protein (MGP) is a vitamin K-dependent protein that is synthesized in the bone and other mesenchymal cells and is also highly expressed in vascular smooth muscle cells (VSMCs) and chondrocytes. Studies have shown that MGP, in its active form, acts as a potent inhibitor of calcification. The regulation of calcification by MGP is potentially controlled in several ways, including direct inhibition of the calcium phosphate receptor, formation of calcium phosphate vesicles matrix, the formation of apoptotic bodies, and VSMCs transdifferentiation [48]. There is increasing evidence demonstrating that circulating dp-ucMGP (inactive form of MGP) increases in CKD and is further aggravated in patients on dialysis. Levels of dephosphorylated, non-carboxylated (dp-ucMGP) are used as a marker of vitamin K deficiency in the vasculature. Vitamin K2 supplementation can effectively activate MGP through carboxylation, thereby decreasing the inactive MGP levels in a dose-dependent manner [49-51]. Furthermore, its use has not been associated with adverse effects or toxicity [52].
Conclusion
Calciphylaxis is a highly morbid disease with high mortality for which treatment options are few. This case shows the potential use of vitamin K2 as a potential new treatment. Future studies should be conducted to evaluate the use of Vitamin K2 as treatment and prevention of calciphylaxis in patients receiving renal replacement therapy or patients at high risk for the disease.
Acknowledgment
We would like to acknowledge that this article did not receive any specific grant from funding agencies in the public, commercial, or nonprofit sectors.
References
- Nigwekar SU, Thadhani R, Brandenburg VM (2018) Calciphylaxis. N Engl J Med 378(18): 1704-1714.
- McCarthy JT, El-Azhary RA, Patzelt MT, Weaver AL, Albright RC, et al. (2016) Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc 91(10): 1384-1394.
- Nseir V, Bradauskaite G, Pedroza M, Minimo C, Zaki R, et al. (2021) A rare case of calciphylaxis in an orthotopic liver transplant recipient with acute kidney injury. Exp Clin Transplant 19(4): 382-385.
- Honda Y, Endo Y, Tanizaki H, Fujisawa A, Kitoh A, et al. (2015) Calciphylaxis associated with acute renal failure in multicentric Castleman’s disease. Eur J Dermatol 25(5): 497-499.
- Russell R, Omer W, Mudabal N, Wanat KA (2021) Calciphylaxis in a young adult with acute kidney injury and recently diagnosed end-stage liver disease. Am J Med Case Rep 9(11): 544-547.
- Vanparys J, Sprangers B, Sagaert X, Kuypers DR (2013) Chronic wounds in a kidney transplant recipient with moderate renal impairment. Acta Clin Belg 68(2): 128-131.
- Nigwekar SU, Zhao S, Wenger J, Hymes JL, Maddux FW, et al. (2016) A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol 27(11): 3421-3429.
- Nigwekar SU, Solid CA, Ankers E, Malhotra R, Eggert W, et al. (2014) Quantifying a rare disease in administrative data: the example of calciphylaxis. J Gen Intern Med 29: S724-S731.
- Nigwekar SU (2017) Calciphylaxis. Curr Opin Nephrol Hypertens 26(4): 276-281.
- Smilnak G, Jiang M, Jain B (2022) Calciphylaxis of the penis and distal digits: a case report. J Med Case Rep 16(1): 18.
- Nisar US, Cheville JC, Sturgis CD (2022) Images - penile pain in the setting of end-stage renal disease: an unusual anatomic location for calciphylaxis. Can Urol Assoc J 16(5): E304-E305.
- Harada K, Araki J, Tokumasu K, Hagiya H, Otsuka F (2020) Calciphylaxis of the fingers. J Gen Fam Med 21(2): 25-26.
- Elghobashy M, Vaquas S, Elshafie M, Kaneri S, Shaaban AM (2020) Unusual presentation of mammary calciphylaxis in a patient on long-standing renal dialysis. Pathobiology 87(5): 317-321.
- Tan RYP, Juneja R, Gunawardane DN, Milton CA (2020) Isolated Mesenteric Calciphylaxis with Ischemic Colitis in a Hemodialysis Patient Without Active Cutaneous Calciphylaxis: A Case Report of Calcific Uremic Arteriolopathy. Kidney Med 2(2): 209-212.
- Mitchell TA, Hoffer ZS, Cancio LC (2020) Extracutaneous Manifestations of Calciphylaxis: Ogilvie's Syndrome with Perforation. Am Surg 86(5): 546-548.
- Nigwekar SU, Kroshinsky D, Nazarian RM, Goverman J, Malhotra R, et al. (2015) Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 66(1): 133-146.
- Gallimore GG, Curtis B, Smith A, Benca M (2014) Curious case of calciphylaxis leading to acute mitral regurgitation. BMJ Case Rep 2014: bcr2013201803.
- Angelis M, Wong LL, Myers SA, Wong LM (1997) Calciphylaxis in patients on hemodialysis: a prevalence study. Surgery 122(6): 1083-1089.
- Nigwekar SU, Brunelli SM, Meade D, Wang W, Hymes J, et al. (2013) Sodium thiosulfate therapy for calcific uremic arteriolopathy. Clin J Am Soc Nephrol 8(7): 1162-1170.
- Davis JM (2016) The Relationship Between Obesity and Calciphylaxis: A Review of the Literature. Ostomy Wound Manage 62(1): 12-18.
- Janigan DT, Hirsch DJ, Klassen GA, MacDonald AS (2000) Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J kidney Dis 35(4): 588-597.
- Ruderman I, Toussaint ND, Hawley CM, Krishnasamy R, Pedagogos E, et al. (2021) The Australian Calciphylaxis Registry: reporting clinical features and outcomes of patients with calciphylaxis. Nephrol Dial Transplant 36(4): 649-656.
- Sridhar NR, Josyula S (2013) Hypoalbuminemia in hemodialyzed end stage renal disease patients: risk factors and relationships--a 2-year single center study. BMC Nephrol 14: 242.
- Mazhar AR, Johnson RJ, Gillen D, Stivelman JC, Ryan MJ, et al. (2001) Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int 60(1): 324-332.
- Nigwekar SU, Bhan I, Turchin A, Skentzos SC, Hajhosseiny R, et al. (2013) Statin use and calcific uremic arteriolopathy: a matched case-control study. Am J Nephrol 37(4): 325-332.
- Magagnoli L, Cozzolino M, Caskey FJ, Evans M, Torino C, et al. (2023) Association between CKD-MBD and mortality in older patients with advanced CKD-results from the EQUAL study. Nephrol Dial Transplant 38(11): 2562-2575.
- Bleyer AJ, Choi M, Igwemezie B, Torre E, White WL (1998) A case control study of proximal calciphylaxis. Am J Kidney Dis 32(3): 376-383.
- Brandenburg VM, Kramann R, Rothe H, Kaesler N, Korbiel J, et al. (2017) Calcific uraemic arteriolopathy (calciphylaxis): data from a large nationwide registry. Nephrol Dial Transplant 32(1): 126-132.
- Heeswijk I, Ugur A, Havill L, Kinton R, Hughes D (2023) Calciphylaxis in a patient with hypoparathyroidism and MEN-1 syndrome. Endocrinol Diabetes Metab Case Rep 2023(4): 23-230009.
- Erdel BL, Juneja R, Evans-Molina C (2014) A case of calciphylaxis in a patient with hypoparathyroidism and normal renal function. Endocr Pract 20(6): e102-e105.
- Hayashi M, Takamatsu I, Kanno Y, Yoshida T, Abe T, et al. (2012) A case-control study of calciphylaxis in Japanese endstage renal disease patients. Nephrol Dial Transplant 27(4): 1580-1584.
- Zhang Y, Corapi KM, Luongo M, Thadhani R, Nigwekar SU (2016) Calciphylaxis in peritoneal dialysis patients: a single center cohort study. Int J Nephrol Renovasc Dis 9: 235-241.
- Santos PW, He J, Tuffaha A, Wetmore JB (2017) Clinical characteristics and risk factors associated with mortality in calcific uremic arteriolopathy. Int Urol Nephrol 49(12): 2247-2256.
- Nigwekar SU, Bloch DB, Nazarian RM, Vermeer C, Booth SL, et al. (2017) Vitamin K-dependent carboxylation of matrix Gla protein influences the risk of calciphylaxis. J Am Soc Nephrol 28(6): 1717-1722.
- Dobry AS, Ko LN, St John J, Sloan JM, Nigwekar S, et al. (2018) Association Between Hypercoagulable Conditions and Calciphylaxis in Patients with Renal Disease: A Case-Control Study. JAMA Dermatol 154(2): 182-187.
- Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR (2007) Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol 56(4): 569-579.
- Hayashi M, Takamatsu I, Kanno Y, Yoshida T, Abe T, et al. (2012) Japanese Calciphylaxis Study Group. A case-control study of calciphylaxis in Japanese end-stage renal disease patients. Nephrol Dial Transplant 27(4): 1580-1584.
- Chang JJ (2019) Calciphylaxis: Diagnosis, Pathogenesis, and Treatment. Adv Skin Wound Care 32(5): 205-215.
- Gallo Marin B, Aghagoli G, Hu SL, Massoud CM, Robinson-Bostom L (2023) Calciphylaxis and Kidney Disease: A Review. Am J Kidney Dis 81(2): 232-239.
- Bonchak JG, Park KK, Vethanayagamony T, Sheikh MM, Winterfield LS (2016) Calciphylaxis: a case series and the role of radiology in diagnosis. Int J Dermatol 55(5): e275-e279.
- Cucchiari D, Torregrosa JV (2018) Calciphylaxis in patients with chronic kidney disease: A disease which is still bewildering and potentially fatal. Calcifilaxis en pacientes con enfermedad renal crónica: una enfermedad todavía desconcertante y potencialmente mortal. Nefrologia 38(6): 579-586.
- Basile C, Montanaro A, Masi M, Pati G, De Maio P, et al. (2002) Hyperbaric oxygen therapy for calcific uremic arteriolopathy: a case series. J Nephrol 15(6): 676-680.
- Nayak-Rao S (2011) Achieving effective pain relief in patients with chronic kidney disease: a review of analgesics in renal failure. J Nephrol 24(1): 35-40.
- Fine A, Zacharias J (2002) Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int 61(6): 2210-2217.
- Torregrosa JV, Sánchez-Escuredo A, Barros X, Blasco M, Campistol JM (2015) Clinical management of calcific uremic arteriolopathy before and after therapeutic inclusion of bisphosphonates. Clin Nephrol 83(4): 231-234.
- Perelló J, Ferrer MD, Mar Pérez M, Kaesler N, Brandenburg VM, et al. (2020) Mechanism of action of SNF472, a novel calcification inhibitor to treat vascular calcification and calciphylaxis. Br J Pharmacol 177(19): 4400-4415.
- Brandenburg VM, Sinha S, Torregrosa JV, Garg R, Miller S, et al. (2019) Improvement in wound healing, pain, and quality of life after 12 weeks of SNF472 treatment: a phase 2 open-label study of patients with calciphylaxis. J Nephrol 32(5): 811-821.
- Bjørklund G, Svanberg E, Dadar M, Card DJ, Chirumbolo S, et al. (2020) The Role of Matrix Gla Protein (MGP) in Vascular Calcification. Curr Med Chem 27(10): 1647-1660.
- Westenfeld R, Krueger T, Schlieper G, Cranenburg EC, Magdeleyns EJ, et al. (2012) Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: a randomized trial. Am J Kidney Dis 59(2): 186-195.
- Shanahan CM (2005) Mechanisms of vascular calcification in renal disease. Clin Nephrol 63(2): 146-157.
- Dalmeijer GW, Schouw YT, Magdeleyns E, Ahmed N, Vermeer C, et al. (2012) The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis 225(2): 397-402.
- Pucaj K, Rasmussen H, Møller M, Preston T (2011) Safety and toxicological evaluation of a synthetic vitamin K2, menaquinone-7. Toxicol Mech Methods 21(7): 520-532.
-
Iyad Abuward Abusharkh*, Chadia Beaini, Reema Saffarini, Saja Al-Abbasi, Hala Yamout, Nisrine Arhda, Imane Guermah, Ahmed Murad. Exploring Novel Therapeutic Strategies for Calciphylaxis: A Focus on Vitamin K2 Supplementation. Annals of Urology & Nephrology. 4(4): 2024. AUN.MS.ID.000593.
-
Chronic kidney disease; hemodialysis; calciphylaxis; vascular calcification; vitamin k2; menaquinone-7; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






