 Case report
Case report
Dilemma and its Silver Liner between HCV and ANCA-associated Vasculitis: A Case Report and Literature Review
Wen Wen, Yuehong Li*, Zhen Zhuang
Department of Nephrology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China
Yuehong Li, Department of Nephrology, Beijing Tsinghua Changgung Hospital, School of Clinical Medicine, Tsinghua University, Beijing, China
Received Date: July 24, 2024; Published Date: August 02, 2024
Abstract
The occurrence of ANCA-associated vasculitis (AAV) in the setting of hepatitis C virus (HCV) remains uncommon, especially without cryoglobulinemias. Here, we report a case of a 58-year-old woman diagnosed with both HCV infection and AAV concurrently. Clinical presentations included proteinuria, microscopic hematuria, elevated creatinine, and positive MPO-ANCA, with the absence of cryoglobulin. Renal biopsy revealed pauci-immune crescentic glomerulonephritis. Treatment involved anti-viral medications, followed by prednisone and cyclophosphamide. The renal function showed improvement during the follow-up.
Keywords: Renal dysfunction; lumbar puncture; cranial magnetic resonance imaging; electroencephalogram
Introduction
Antineutrophil cytoplasmic antibodies (ANCA) associated vasculitis (AAV) is a systemic inflammatory disease that typically affects the kidneys, manifesting as rapidly progressive glomerulonephritis with pauci-immune crescents formation in histology. Treatment with immunosuppressive medications (e.g. corticosteroids, cyclophosphamide, rituximab, etc.) is always necessary, and decisions can be challenging when dealing with complicating infections since severe infections may be triggered by immunosuppressants and indicate increased mortality [1]. Here, we present a case of a patient who was diagnosed with hepatitis C virus (HCV) infection and AAV simultaneously, and we discuss the relationship between HCV and ANCA and how to manage such a situation.
Case Presentation
This is a 58-year-old Chinese woman who noticed persistent foaming in her urine and high blood pressure a month before admission, but she did not seek medical attention. Twenty days ago, she was admitted to the neurology department due to delirium after taking levofloxacin for 3 days to treat her urinary tract infection symptoms. Lumbar puncture, cranial magnetic resonance imaging, and electroencephalogram were conducted, ruling out any organic lesions. However, renal dysfunction was observed, with a serum creatinine level of 171umol/L. Urine analysis revealed proteinuria and microscopic hematuria. Further investigation of autoimmune antibodies revealed a positive MPO-ANCA (187AU/ml) with a pANCA of 1:40. Screening for infections showed a positive result for anti-HCV. Subsequently, she was transferred to the nephrology department for the treatment of her kidney disease. She had a history of cesarean section and appendectomy 30 years ago, with a blood transfusion during surgery. Physical examinations indicated that she was conscious, with a blood pressure of 153/65mmHg. The skin and mucosa appeared normal throughout her body. Bilateral lung breath sounds were clear, and heart examination was unremarkable. There was no swelling in the extremities.
The Results of Laboratory Examinations are as follows:
a) Hemoglobulin 94 g/L, alanine transaminase 41.6 U/L, glutamic oxalacetic transaminase 47.8 U/L, phosphorus 1.59 / L, iPTH 34.5ng/L.
b) Routine urinalysis showed SG 1.004 left, the WBC 25 cells/uL, PRO 0.3 g/L (1 +), BLD 250 (3 +) cells/uL, RBC 492.1/ uL, WBC 26.7/uL. 24-hour urine protein 1192.8mg.
c) HCV-RNA 7.08×10^4 IU/ml. Cryoglobulin: Negative. ANA and ENA spectrum were negative. Lupus anticoagulant 1.35↑.β2-GPI was reactive (69) AU/ml. ACL-IgM was suspicious (8.27) MPLU/ml. Anti GBM antibodies were negative. Direct Coombs’ test was 1+ while the indirect Coombs’ test was negative. Tumor markers including CA19-9, SCCAg, CA15-3, CEA, AFP, CYFRA21-1, and CA-125 were negative. Blood and urine immunofixation electrophoresis was negative.
d) Chest CT revealed chronic inflammation in both lungs and multiple solid nodules. Mild emphysema was observed in both lungs, along with partial pleural thickening on both sides, and enlarged lymph nodes in the mediastinum.
Kidney biopsy was conducted, revealing the following findings:
a) Light Microscopy: 3 fibrotic crescents with sclerosis in 21 glomeruli, mild segmental proliferation of mesangial cells and stroma in the remaining glomeruli, along with cellular, cellular fibrous, and fibrous crescent formation. Additionally, observations included vacuolar and granular degeneration of renal tubular epithelial cells, focal exfoliation of the brush border, cell disintegration, bare basement membrane formation, focal atrophy, cystic dilatation of the renal tubular lumen, a few red blood cells, and PAS-positive protein cast in the lumen. Focal renal interstitial lymphocytes, monocytes, and a few eosinophils infiltration with fibrosis were noted. Thickening of small artery walls was observed. Congo red stain yielded negative results. Immunohistochemistry revealed granular deposition in the capillary wall, mesangial area, and arteriolar wall of C4d glomerulus (Figures 1A-1D).
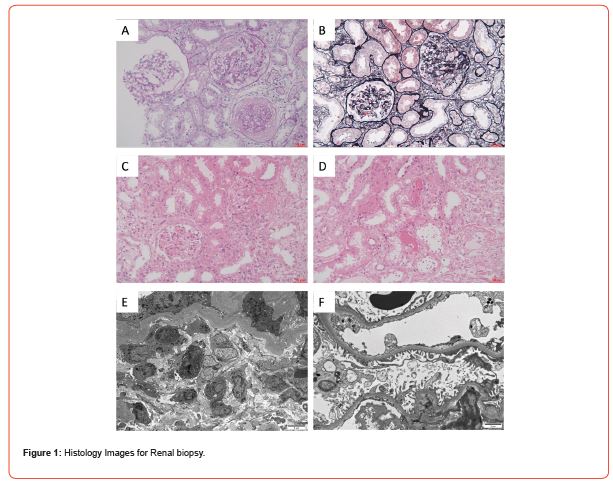
b) Immunofluorescence: IgA(-), IgG(-), IgM(±), C1q(-), C3(-), FRA(-), Alb(±), IgG1(-), IgG2(-), IgG3(-), IgG4(-).
c) Electron microscopy: slight segmental proliferation of mesangial cells and stroma, lose or widened basement membrane stage, segmental fusion of epithelial foot processes, and no electron density deposition. In renal tubular epithelial cells, increased lysosomes, shedding of some microvilli, and atrophy were observed. Renal interstitial edema, lymphocytes, monocytes, a small number of neutrophils, and plasma cell infiltrates with collagen fiber hyperplasia were present (Figures 1E&1F).
d) Histological Diagnosis: Focal proliferative glomerulonephritis with multiple crescents formation, consistent with ANCAassociated polyangiitis renal damage.
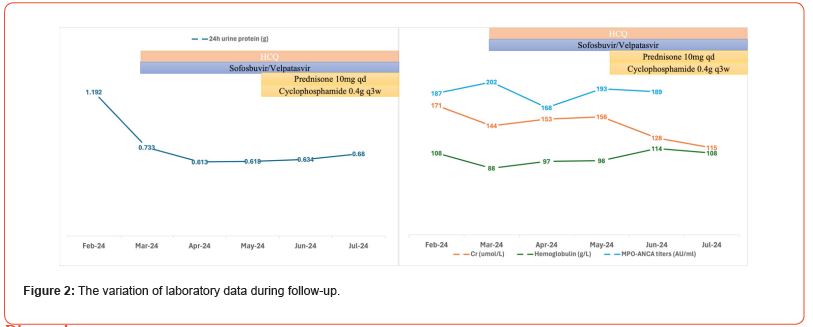
Antiviral therapy with Sofosbuvir/Velpatasvir 1# qd was initiated. Hydroxychloroquine (HCQ) was given at 0.2g bid. Blood pressure remained well controlled with felodipine 5 mg qd. After one month, HCV-RNA tested negative. Low dose prednisone (10mg qd) and cyclophosphamide were introduced. The changes in related parameters during follow-up are detailed in Figure 2.
Discussion
HCV infection is a unique condition that easily triggers an uncontrolled immune response and leads to systemic vasculitis. Various autoantibodies (e.g. ANA, AMA, SMA, RF, ANCA, anti-ds-DNA, anti-LKM-1, etc) have been detected in patients with HCV infection [2]. ANA, RF, and SMA are more common than ANCA, with ANCA present in 42.3% of HCV patients who have had a stroke [3]. c-ANCA is more prevalent compared to p-ANCA. Despite the presence of autoantibodies, the diagnosis of an autoimmune disease may not be confirmed. Cryoglobulinemia (CG) is the primary manifestation of HCV-related vasculitis [4]. The specific mechanisms by which HCV induces vasculitis may involve mimicking human immunoglobulins, enhancing T and B cells, activating innate immunity, causing mutations in host genes, and impacting epigenetic changes. HCVrelated vasculitis without cryoglobulinemias is uncommon. There have been only a few reported cases, with the most common being HCV-related polyarteritis nodosa (PAN). The development of AAV in the context of HCV is still rare, particularly in the absence of cryoglobulinemias. In Table 1, we have summarized the characteristics of case reports on HCV complicating AAV [5-10].
Table 1: Summary of Case Reports on HCV infection with AAV.
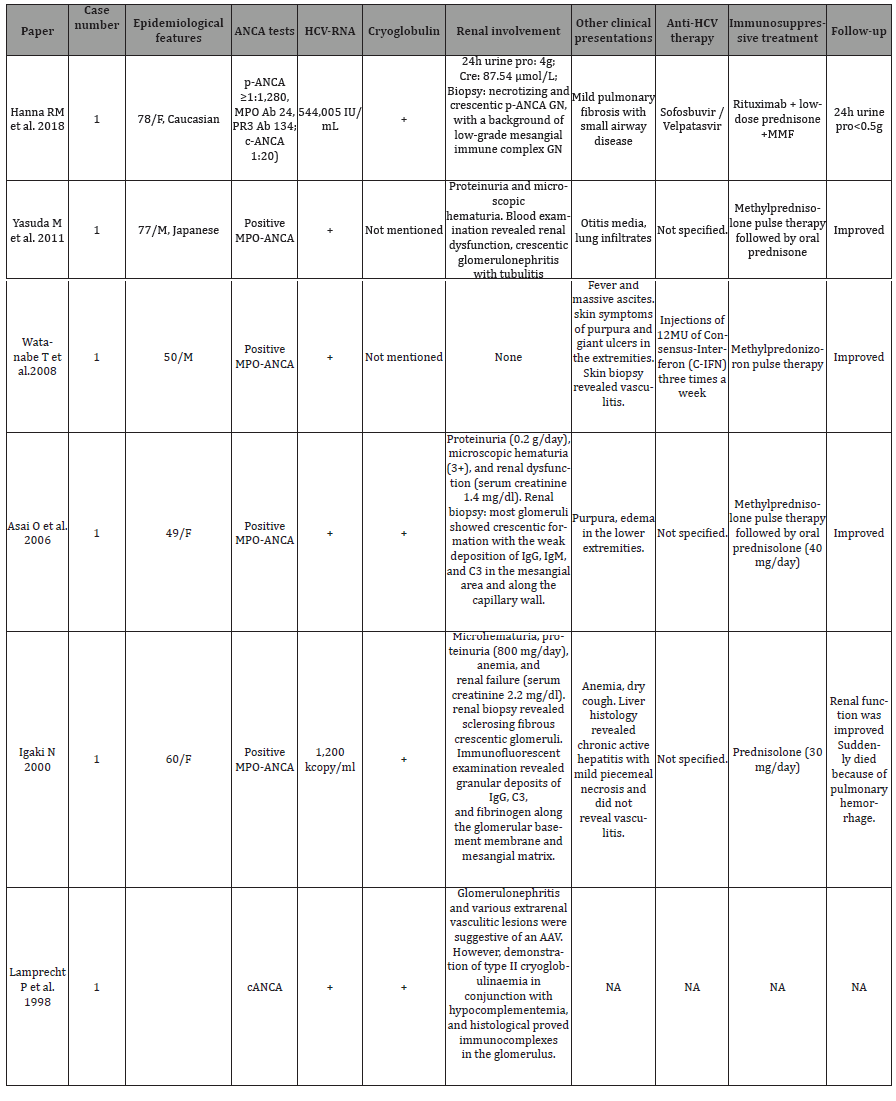
Out of the 7 cases reviewed, only 2 did not have cryoglobulinemias, and most of them tested positive for MPO-ANCA or p-ANCA. Some instances of vasculitis in HCV patients have been attributed to anti-HCV treatment [11], although this contradicts the accumulating evidence of HCV’s role in triggering immune responses itself.
There is no consensus on the treatment for AAV in HCV patients. According to the guideline for HCV-related cryoglobulinemic vasculitis, direct-acting antiviral agents (DAAs) may play a crucial role in treating mild or moderate disease with stable renal function and proteinuria. DAAs have been shown to achieve rapid and sustained virological response, and some studies have observed clearance of autoantibodies [12,13]. DAAs have been proven effective in treating cryoglobulinemic vasculitis, potentially eliminating the need for renal biopsy [14]. For severe disease indicated by clinical or histological findings, immunosuppressive therapies and plasma exchange are recommended. HCQ has immunoregulatory effects on various autoimmune diseases without exacerbating infections. A clinical trial (HAVEN study, NCT04316494) is currently underway to investigate HCQ’s role in AAV.
Our patient maintained stable renal function and urine protein levels while being treated without the use of immunosuppressive agents. However, upon the addition of corticosteroids and cyclophosphamide, there was an improvement in renal function, although the ANCA titers did not decrease. The prognosis of lung fibrosis in this case still requires further observation. The optimal timing for aggressive immunosuppressive therapy remains unknown. In this instance, we waited until one month after HCV treatment initiation, but the virus tested negative after just one week of treatment. Whether initiating immunosuppressive treatment earlier would lead to better outcomes for AAV and not worsen HCV infection requires more evidence. A more thorough explanation of this issue will ultimately reveal the connection between HCV and AAV in the real world.
Authors’ Contributions
Wen Wen, Yuehong Li, and Zhen Zhuang were involved in the patient case, collected the necessary data, and drafted and finalized the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgements
The authors appreciate the patient for her cooperation in the diagnostic procedure and follow-up.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Pena C, Costi AC, Garcia L, Garcia M (2024) Severe infections in systemic necrotizing vasculitis. Reumatol Clin (Engl Ed) 20(5): 237-242.
- Duran A, Cetin Duran A, Taskiran I (2022) Investigation of the Contribution of Autoantibodies to Clinical Diagnosis in Liver Pathologies and the Identification of Accompanying Autoimmune Diseases. Mikrobiyol Bul 56(1): 81-94.
- Mohamed AB, Hefny HM, Saif-Al-Islam M, Zaghloul AM, Khalaf S, et al. (2021) Association of anti-neutrophil cytoplasmic antibody in ischemic stroke Egyptian patients with hepatitis C virus. Egypt J Immunol 28(1): 33-45.
- Ragab G, Hussein MA (2017) Vasculitic syndromes in hepatitis C virus: A review. J Adv Res 8(2): 99-111.
- Hanna RM, So N, Kaldas M, Hou J, Arman F, et al. (2018) Case Report: Patient with Hepatitis C, p-ANCA, and Cryoglobulin Antibodies Presenting with Necrotizing Crescentic p-ANCA Glomerulonephritis. Case Rep Nephrol Dial 8(2): 161-170.
- Yasuda M, Araki H, Fujitomo Y, Morita Y, Urasaki K, et al. (2011) Case of MPO-ANCA-positive Wegener's granulomatosis with hepatitis C virus infection. Nihon Jinzo Gakkai Shi 53(7): 1053-1058.
- Watanabe T, Oono Y, Takeshita E, Kobayashi Y, Tanaka Y, et al. (2008) Case of ANCA associated vasculitis induced by interferon therapy for HCV infection. Nihon Shokakibyo Gakkai Zasshi 105(12): 1787-1793.
- Asai O, Nakatani K, Yoshimoto S, Akai Y, Nishino T, Iwano M, et al. (2006) A case of MPO-ANCA-related microscopic polyangiitis with mixed cryoglobulinemia. Nihon Jinzo Gakkai Shi 48(4): 377-384.
- Igaki N, Nakaji M, Moriguchi R, Akiyama H, Tamada F, et al. (2000) A case of hepatitis C virus-associated glomerulonephropathy presenting with MPO-ANCA-positive rapidly progressive glomerulonephritis. Nihon Jinzo Gakkai Shi 42(4): 353-358.
- Lamprecht P, Schmitt WH, Gross WL (1998) Mixed cryoglobulinaemia, glomerulonephritis, and ANCA: essential cryoglobulinaemic vasculitis or ANCA-associated vasculitis? Nephrol Dial Transplant 13(1): 213-221.
- Ahmad YK, Tawfeek S, Sharaf-Eldin M, Elbatea HE, Kobtan A, et al. (2017) Anti-Nuclear Cytoplasmic Antibody-Associated Vasculitis: A Probable Adverse Effect of Sofosbuvir Treatment in Chronic Hepatitis C Patients. Hosp Pharm 52(4): 294-301.
- Lauletta G, Cicco S, Dammacco F (2024) Hepatitis C virus-related autoimmunity before and after viral clearance: a single center, prospective, observational study. Minerva Med 115(3): 284-292.
- Rossotti R, Merli M, Baiguera C, Bana NB, Rezzonico LF, et al. (2023) Impact of treatment with direct-acting antivirals on inflammatory markers and autoantibodies in HIV/HCV co-infected individuals. J Viral Hepat 30(6): 530-539.
- Kidney Disease: Improving Global Outcomes Hepatitis CWG (2022) KDIGO 2022 Clinical Practice Guideline FOR the Prevention, Diagnosis, Evaluation, and Treatment of Hepatitis C in Chronic Kidney Disease. Kidney Int 102(6S): S129-S205.
-
Wen Wen, Yuehong Li*, Zhen Zhuang. Dilemma and its Silver Liner between HCV and ANCA-associated Vasculitis: A Case Report and Literature Review. Annals of Urology & Nephrology. 4(5): 2024. AUN.MS.ID.000596.
-
Renal dysfunction; lumbar puncture; cranial magnetic resonance imaging; electroencephalogram; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






