 Case Report
Case Report
Congenital Pelvi-ureteric Junction Obstruction Associated with Crossing Lower Pole Vessels
Abubakar Sadiq Muhammad1,2*
1Department of Surgery, IMAN Hospital Runjin Sambo, Sokoto
2Usmanu Danfodiyo University Teaching Hospital Sokoto, Nigeria
Abubakar Sadiq Muhammad, Usmanu Danfodiyo University Teaching Hospital Sokoto, Nigeria.
Received Date: January 29, 2021; Published Date: February 23, 2021
Abstract
Background: Congenital pelvi-ureteric junction obstruction [PUJO] occurs when there is impairment of flow of urine from the renal pelvis to the proximal ureter due to inherited abnormalities of pelvi-ureteric junction [PUJ]. The presentation is usually at the 4th or 5th decade of life. It may rarely be associated with crossing lower pole vessel [CLPV]. Treatment is by Anderson- Hynes [dismembered] pyeloplasty which remove the abnormal PUJ and transposes the PUJ anterior to the offending vessels. I report a case of 45-year-old man with congenital PUJO associated with crossing lower pole vessel vessels who had dismembered Anderson -Hynes pyeloplasty.
Case report: This is a 45-year-old man who presented with 4 years history of recurrent colicky left flank pain which was relieved by passing large volume of urine. The general examination was non remarkable. The left kidney was ballotable. The abdominopelvic computerized urogram revealed severe left hydronephrosis and hyperdense structure in the region of the left kidney. He had exploration via left Gibson incision with finding of multiple crossing lower pole vessels and aperistaltic segment at PUJ. He had excision of the abnormal PUJ, transposition of the crossing vessels posteriorly and dismembered Anderson-Hynes pyeloplasty. He had uneventful recovery and was discharged home 5 days postoperatively.
Conclusion: Congenital PUJO associated with lower pole vessel is rare and may present in the 5th decade of life. Anderson- Hynes dismembered pyeloplasty is the procedure of choice and associated with good outcome.
Keywords: Congenital; Pelvi-ureteric junction obstruction; Crossing vessels; Dismembered pyeloplasty; Anderson hynes; Hydronephrosis
Abbreviations: PUJO: Pelvi-ureteric junction obstruction; PUJ: Pelvi-ureteric junction; CLPV: Crossing lower pole vessel
Introduction
Congenital pelvi-ureteric junction obstruction [PUJO] occurs when flow of urine is impeded from renal pelvis to the proximal ureter due to inherited abnormalities at pelvi-ureteric junction [PUJ] [1]. It may also be acquired from stone disease, inflammatory/ infective conditions and urothelial carcinoma of the renal pelvis [2]. The inherited causes may be intrinsic or extrinsic [2]. The intrinsic causes include aperistaltic segment due abnormal musculature, valves and folds. The extrinsic causes include crossing lower pole vessel, adhesions and bands [2]. The crossing lower pole vessel is the cause of obstruction in 11-20% of cases [3]. This is referred to currently to as vascular bar [4]. It may also co-exist with other causes. Even though a congenital problem, the presentation may occur at the 4th or 5th decade of life. The routine diagnostic investigations of intravenous or computerized tomographic scan urogram can only diagnose the PUJO with accuracy. The lucency of the crossing vessels or short segment sign [5] may suggest the presence of a crossing vessels in 20-60% of the cases [5]. The crossing vessel can be accurately detected on Helical CT angiogram or Magnetic Resonance Angiogram [MRA] [6,7]. Doppler ultrasound can also assist in making the diagnosis when angiography is not feasible [8]. The gold standard treatment is by Anderson- Hynes dismembered pyeloplasty which remove the abnormal PUJ and transposes the PUJ anterior to the offending vessels. This can be done laparoscopically, robotically or by open method [1, 9-11]. Alternatively, cranial relocation of the crossing polar vessel can be done by open, laparoscopic or robotic technique. This was first described by Hellstrom [12] and later modified by Chapman [13,14]. In the original procedure the polar vessels were anchored to the anterior pelvic wall using vascular adventitial sutures while in the modified technique the vessels were wrapped in a more superior position in the anterior pelvic wall without need of vascular adventitial sutures. No case of PUJO due to crossing lower pole vessel reported in our environment. I report a case of 45-yearold man with congenital PUJO associated with crossing lower pole vessels who had dismembered pyeloplasty.
Case Report
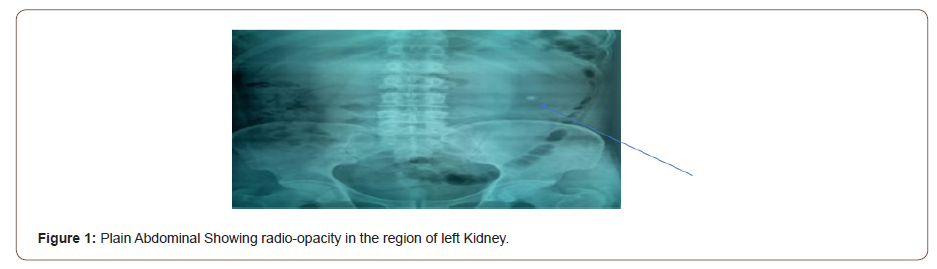
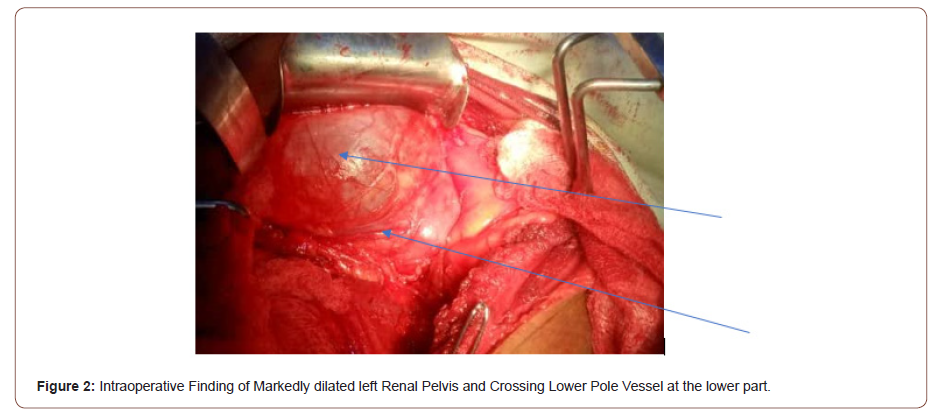
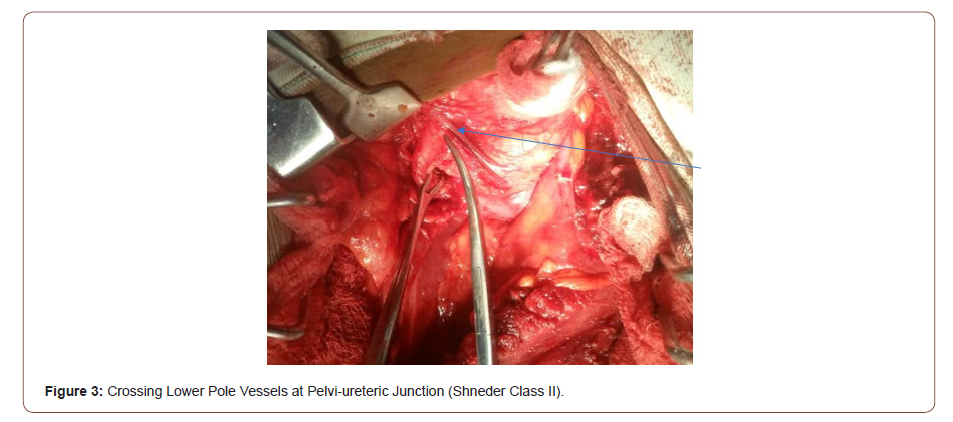
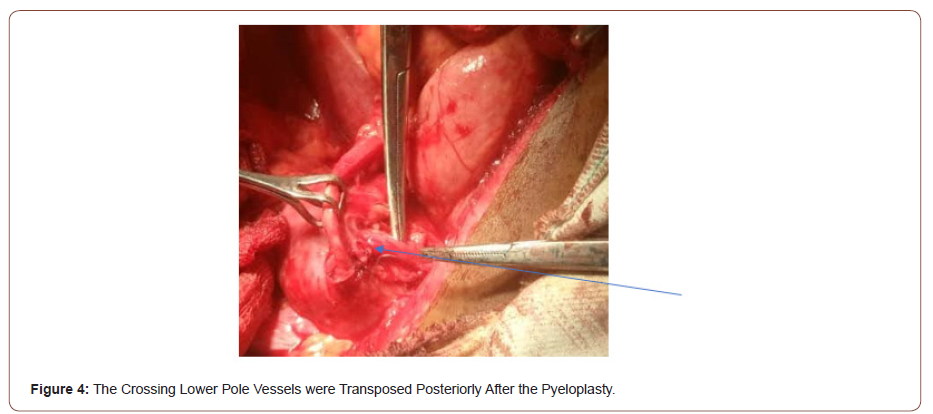
This is a 45-year-old man who presented with 4 years recurrent colicky left flank pain which is relieved by passing large volume of urine. The general examination was not remarkable. The left kidney was ballotable. The abdominopelvic ultrasound and computerized urogram revealed severe left hydronephrosis and a renal stone. Full blood count, urine microscopy, urea and creatinine were normal. Clotting profile, chest x ray and electrocardiogram were normal. Plain abdominal x ray revealed radiopacity in the in the region of the right kidney (Figure 1). The diagnosis of left hydronephrosis left renal pelvic stone done. He had exploration via left Gibson incision with findings of Moderate Hydronephrosis (Figure 2), Grade II multiple crossing lower pole vessels (Figure 3) at the level of an aperistaltic PUJ which did not drain the kidney even after the transposition of the vessels posteriorly. There was no stone found. He had excision of the aperistaltic PUJ with adjoining proximal ureter and redundant pelvis. Anderson-Hynes dismembered pyeloplasty was done with the neo PUJ anterior (Figure 4) to the crossing lower pole vessels. Double J stent was inserted and a retroperitoneal drain. He was placed on the intravenous Ceftriaxone 1 g daily for 48hours, pentazocine 60 mg for 24 hours and intravenous fluid Normal saline 1L hourly for 48 hours. He was started on oral feed 2 days post operatively and the retroperitoneal drain was removed 3 days post operatively. He had uneventful recovery and was discharged home 5 days postoperatively. Stitches were removed 10 days post operatively. He was asked to come for double J stent removal 2 weeks post operatively, but he absconded with the stent in situ (Figures 1-4).
Discussion
Pelvi-ureteric junction obstruction occur due to impairment of flow of urine from the renal pelvis to the proximal ureter [2]. The obstruction can be physiologic or anatomic [2], congenital or acquired. Acquired causes are due to stones, inflammation or tumors [2]. The congenital causes can be intrinsic or extrinsic. The intrinsic causes include aperistaltic segment due to abnormal musculature and valves while the extrinsic causes include adhesions, bands and crossing lower pole vessel which recently was referred to as vascular bar [2,4]. This accounted for the causes of PUJO in 11-20% [5] and 25-50% [15] in various studies in children and adult respectively. The obstruction can cause symptomatic progressive hydronephrosis, loss of cortical mantle, recurrent urinary tract infection, renal impairment, stone formation and hypertension warranting need for surgical intervention [2]. When there is CLPV Anderson-Hynes is indicated which will tackle all the abnormalities [2] unlike endopyelotomy which is not going to address the CLPV [2]. The diagnosis is usually incidental as doppler ultrasound of the renal vessels, computerized tomogram angiogram [CTA] or magnetic resonance angiogram [MRA] did not form routine part of PUJO investigations [5]. A low incidence of 5.6% was reported by a previous study and the authors did not recommend routine doppler or MRA to detect crossing lower pole vessel [6]. The predominant complaint of adult patients is colicky flank pain [5] which is in agreement with the present study. There was also Dietel’s crisis as reported in the literature. The finding of crossing lower pole vessels is usually incidental as observed in the present study. The working diagnosis was hydronephrosis due to renal pelvic stone. The stone was not found, the possibility is the presence of dystrophic calcification in a peristaltic segment. Co-existing renal pelvic abnormality was found in 33% of the cases [16]. This implies that the crossing lower pole vessels was the primary cause of the PUJO in 67% in the previous study [16]. In the present case there was co-existing PUJ abnormality which was supported by the absence of proximal ureteral dilatation and failure of urine flow after the transection of the ureter at the level of proximal ureter [5]. Anderson-Hynes dismembered pyeloplasty was done for the index as reported in the literature [2]. This allowed the transposition of the crossing vessels posteriorly and excision of the redundant renal pelvis. This can be laparoscopic, robotic or open [9-11]. Open pyeloplasty was done as we have no experience and expertise of laparoscopic pyeloplasty and no facility for robotic pyeloplasty in the area of our practice. Open pyeloplasty is still the standard of care as laparoscopic and robotic techniques though have comparable outcomes to the open technique, they are technically challenging and demanding [15]. Schneider et al [17] classified CLPV in to 3 based on intraoperative location, pelvis, PUJ and proximal ureter into class 1, 2 and 3 respectively. They advocated pyeloplasty for 1 and 2 and Hellstrom procedure for class 3 CLPV assuming the obstruction is primarily extrinsic. The index patient had class 2 CLPV which is appropriate for pyelopasty which he had. Presence of instrinsic abnormality made the index case unsuitable for Hellstrom procedure even if there is class III CLPV. The patients were mobilized early after 48 hours, drain removed in 7 hours and discharged 5 days post operatively which is comparable to the mini-invasive surgeries in terms of duration of admission and removal of drain [18].
Conclusion
Congenital Pelvi-ureteric junction obstruction associated with lower pole vessel is rare and may present in the 5th decade of life. The main presentation is colicky pain and Dietl’s crisis. The diagnosis may be incidental as doppler ultrasound and Magnetic Resonance Angiogram are not part of routine investigation for Pelvi-ureteric obstruction. Meticulous examination of vascular radiolucency and short segment sign may be done. Pelvi-uretic junction abnormality and crossing lower pole vessels may co-exist in some patients. Anderson- Hynes dismembered pyeloplasty is the procedure of choice and associated with good outcome.
Acknowledgement
I acknowledge the support of IMAN Hospital Runjin Sambo staff during management of the patients and preparation of the manuscript.
Conflict of Interest
No conflict of interest.
References
- Kaur G, Adhikari R, Cass P, Bown M, Gunatillake P (2015) Electrically conductive polymers and composites for biomedical applications. Rsc Advances 5(47): 37553-37567.
- Maity S, Chatterjee A (2015) Textile/polypyrrole composites for sensory applications. Journal of Composites.
- Ouyang J (2018) Recent advances of intrinsically conductive polymers. Acta Physicochimica Sinica 34(11): 1211-1220.
- Joshi M, Adak B (2019) Advances in nanotechnology based functional, smart and intelligent textiles: a review. In: Andrews DL, Lipson RH, Nann T (edts), Comprehensive Nanoscience and Nanotechnology, (2nd edn), Elsevier, pp. 253-290.
- Coyle S, Wu Y, Lau KT, De Rossi D, Wallace G, et al. (2007) Smart nanotextiles: a review of materials and applications. MRS bulletin 32(5): 434-442.
- Naghdi S, Rhee KY, Hui D, Park SJ (2018) A review of conductive metal nanomaterials as conductive, transparent, and flexible coatings, thin films, and conductive fillers: Different deposition methods and applications. Coatings 8(8): 278.
- Xue CH, Chen J, Yin W, Jia ST, Ma JZ (2012) Superhydrophobic conductive textiles with antibacterial property by coating fibers with silver nanoparticles. Applied Surface Science 258(7): 2468-2472.
- Karimi L, Yazdanshenas ME, Khajavi R, Rashidi A, Mirjalili M (2014) Using graphene/TiO2 nanocomposite as a new route for preparation of electroconductive, self-cleaning, antibacterial and antifungal cotton fabric without toxicity. Cellulose 21(5): 3813-3827.
- Atwa Y, Maheshwari N, Goldthorpe IA (2015) Silver nanowire coated threads for electrically conductive textiles. Journal of Materials Chemistry C 3(16): 3908-3912.
- Zhang F, Yang J (2009) Preparation of nano-ZnO and its application to the textile on antistatic finishing. International Journal of Chemistry 1(1): 18.
- Joshi M, Adak B (2018) Nanotechnology-based Textiles: A Solution for Emerging Automotive Sector. Rubber Nanocomposites and Nanotextiles; De Gruyter: Berlin, Germany: 207-266.
- Karim N, Afroj S, Malandraki A, Butterworth S, Beach C, et al. (2017) All inkjet-printed graphene-based conductive patterns for wearable e-textile applications. Journal of materials chemistry C 5(44): 11640-11648.
- Trovato V, Teblum E, Kostikov Y, Pedrana A, Re V, et al. (2020) Sol-gel approach to incorporate millimeter-long carbon nanotubes into fabrics for the development of electrical-conductive textiles. Materials Chemistry and Physics 240: 122218.
- Martin-Gallego M, Verdejo R, Khayet M, de Zarate JMO, Essalhi M, et al. (2011) Thermal conductivity of carbon nanotubes and graphene in epoxy nanofluids and nanocomposites. Nanoscale research letters 6(1): 1-7.
- Chen J, Gao X, Song W (2019) Effect of various carbon nanofillers and different filler aspect ratios on the thermal conductivity of epoxy matrix nanocomposites. Results in Physics 15: 102771.
- Yan J, Ma Y, Zhang C, Li X, Liu W, et al. (2018) Polypyrrole–MXene coated textile-based flexible energy storage device. RSC advances 8(69): 39742-39748.
- Ahmed A, Hossain MM, Adak B, Mukhopadhyay S (2020) Recent Advances in 2D MXene Integrated Smart-Textile Interfaces for Multifunctional Applications. Chemistry of Materials.
- Seyedin S, Uzun S, Levitt A, Anasori B, Dion G, et al. (2020) MXene composite and coaxial fibers with high stretchability and conductivity for wearable strain sensing textiles. Advanced Functional Materials 30(12): 1910504.
- Ahmed A, Jalil MA, Hossain MM, Moniruzzaman M, Adak B, Islam, et al. (2020) A PEDOT: PSS and graphene-clad smart textile-based wearable electronic Joule heater with high thermal stability. Journal of Materials Chemistry C 8(45): 16204-16215.
- Chatterjee K, Tabor J, Ghosh TK (2019) Electrically conductive coatings for fiber-based e-textiles. Fibers 7(6): 51.
- Abdali H, Ajji A (2017) Preparation of electrospun nanocomposite nanofibers of polyaniline/poly (methyl methacrylate) with amino-functionalized graphene. Polymers 9(9): 453.
- Fu KK, Padbury R, Toprakci O, Dirican M, Zhang X (2018) Conductive textiles. In: Miao, M., Xin, J. H. (edts), Engineering of High-Performance Textiles, Woodhead Publishing, UK, pp. 305-334.
-
Abubakar Sadiq Muhammad. Congenital Pelvi-ureteric Junction Obstruction Associated with Crossing Lower Pole Vessels. Annal Urol & Nephrol. 2(4): 2021. AUN.MS.ID.000543.
-
Congenital, Pelvi-ureteric junction obstruction, Crossing vessels, Dismembered pyeloplasty, Anderson-hynes, Hydronephrosis
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






