 Case Report
Case Report
Calciphylaxis in a Peritoneal Dialysis Patient
Sandeep Anand Padala1*, Veronica Moino2, Vidya Medepalli3, Azeem Mohammed1 and Stanley Jr Nahman1
1Department of Nephrology, Augusta University, USA
2Department of Medicine, The Edward Via College of Osteopathic Medicine, USA
3Department of Medicine, Medical College of Georgia, USA
Sandeep Anand Padala, Department of Nephrology, Augusta University, USA.
Received Date: November 14, 2019; Published Date: November 27, 2019/p>
Abstract
Calciphylaxis, also referred to as Calcific Uremic Arteriolopathy, is a rare and life-threatening disorder that should be considered as a differential diagnosis in end-stage renal disease (ESRD) patients presenting with painful skin lesions. It presents with livedo reticularis and painful subcutaneous nodules which ultimately progress to skin ischemia and necrosis. Although skin lesions tend to dominate the clinical presentation, this disorder is likely a systemic disorder with widespread calcifications involving the vasculature of the skeletal muscles, intestines, mesentery, optic nerves and lungs. Although it is largely described in patients with ESRD, it may also be seen with less advanced chronic kidney disease, acute kidney injury, and even those with normal kidney function.
In addition to CKD, other risk factors include female sex, white race, obesity, diabetes mellitus, hypercalcemia, hyperphosphatemia, metastatic cancers, recurrent hypotension, and warfarin therapy. We present the case of a 47-year-old white male on peritoneal dialysis who presented to the hospital with dusky discoloration of his skin with multiple well-demarcated black, necrotic painful skin lesions on his fingers and toes for three weeks. Given the laboratory and imaging findings there was a high clinical suspicion for calciphylaxis.
Keywords: Calciphylaxis; Calcific uremic arteriolopathy; End-stage renal disease (ESRD); Peritoneal Dialysis; Sodium thiosulfate; Vascular calcifications; Livedo reticularis; Matrix gla protein (MGP)
Case Report
A 47-year-old Caucasian male with a past medical history of ESRD secondary to diabetic nephropathy on peritoneal dialysis for eight years, Diabetes Type I, untreated hepatitis C, hypertension, and melanoma presented to the hospital with a chief complaint of skin discoloration and painful ulceration of his upper and lower extremities. On admission, his blood pressure was 117/50, heart rate 97, temperature 36.9. On physical examination well demarcated black eschar and necrosis was seen on all fingertips in addition to open lesions on toes (Figure 1,2) with palmar pallor and reticular erythematous patches. Additionally, there was one welldemarcated, clean ulceration on the right lower leg. He reported decreased sensation at the site of lesions. His upper and lower extremities were cool to palpation. His left dorsalis pedis pulse was diminished and non-palpable while the right dorsalis pedis pulse was faintly palpable and intact. Cardiac exam revealed regular rhythm and no murmurs. Pulmonary exam revealed breaths sounds that were clear to auscultation bilaterally. Laboratory studies at admission are demonstrated in (Figure 1,2) (Table 1).
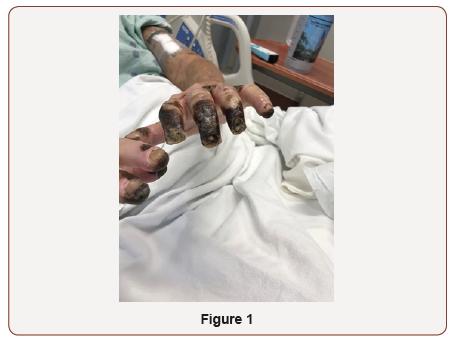
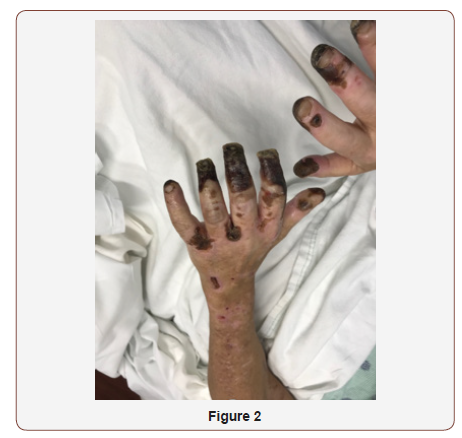
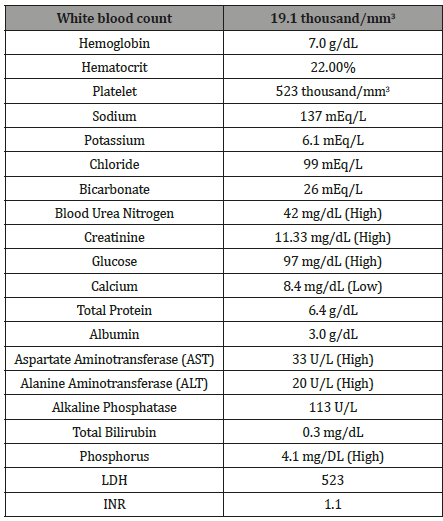
Table 1:Selected studies reported on the outcomes of SMA injuries.
The patient had a failed kidney and pancreas transplant allograft secondary to noncompliance with his immunosuppressive medications. He continued to be noncompliant with his peritoneal dialysis. Given the clinical presentation, our working diagnosis was atheroembolic disease, vasculitis, calciphylaxis, or Raynaud’s phenomenon. A transthoracic echo was performed and ruled out any evidence of thrombus. Computed tomography angiography (CT) of the chest with and without IV contrast demonstrated diffuse severe atherosclerotic calcification of the aorta and its major branches without evidence of occlusive disease. The workup for secondary causes was essentially unremarkable and included complements (C3 and C4), rheumatoid factor, antineutrophil cytoplasmic antibodies, and antinuclear antibodies.
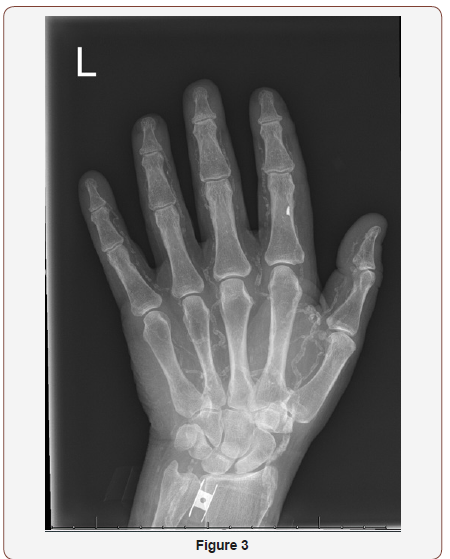
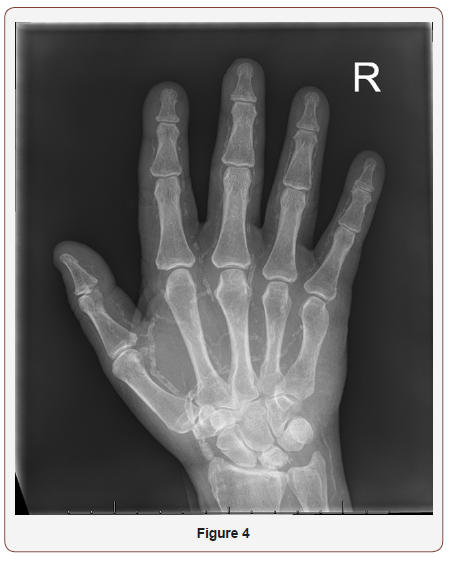
Given his significant vascular disease and extensive skin lesions, dermatology recommended holding off on a skin biopsy. During his hospital stay, the necrotic lesions continued to worsen. With all of the patient’s risk factors and clinical findings there was a high clinical suspicion for calciphylaxis. Right- and left-hand x-rays demonstrated diffuse and severe small vessel calcification throughout his hands (Figure 3,4). A metabolic bone scan further supported the clinical picture of calciphylaxis. Given the concern for calciphylaxis, we recommended hemodialysis for better clearance, with careful attention to prevent any hypotensive episodes (Figure 3,4).
Treatment was multidisciplinary. The patient was treated with opioids for pain control. General surgery was consulted and did not recommend any surgical intervention. Sodium thiosulfate was not administered in the hospital due to the regulatory restrictions in the state of Georgia. Ultimately, the patient was started on hemodialysis via a left upper extremity arteriovenous fistula and discharged in stable condition with his pain under control.
Discussion
Calciphylaxis is a fatal disease that begins with skin lesions and progresses rapidly to be a systemic illness. Early stages of this disease can include subcutaneous nodules without skin manifestations. These nodules typically progress to subcutaneous plaques in the absence of erythema and livedo reticularis. As calciphylaxis progresses, the plaques enlarge, livedo racemosa develops, and deep ulcerations with adjacent black necrotic eschars form [1]. The disease can be categorized into uremic (in patients with ESRD) and non-uremic (in patients with normal renal function or chronic kidney disease) and further subdivided into central or peripheral based on the location of the skin lesions. Central lesions involve adipose tissues of the abdomen or thighs, in contrast to peripheral lesions which mainly involve the digits [2]. Patients with ESRD and calciphylaxis have a higher 1-year mortality rate of 45% to 85%, compared to patients without ESRD [3]. 70% to 80% of the skin manifestations in ESRD patients are located centrally compared to those seen in patients without end-stage renal disease [2].
While the pathophysiology for this fatal disease remains unclear, it is thought that the calcifications develop due to vitamin K mediated deficiency resulting in decreased Matrix Gla Protein carboxylation. Matrix Gla proteins (MGP) are extracellular matrix proteins synthesized by vascular smooth muscles and endothelial cells and are potent inhibitors of vascular calcification. Carboxylated MGP is thought to inhibit the procalcifying factors bone morphogenetic protein 2 (BMP-2) and bone morphogenetic protein 4 (BMP- 4). Therefore, deficiency of MGP promotes increased cutaneous expression of BMP 2 and 4, leading to increased calcification of the vessels [2].
Reductions of MGP in the cutaneous tissue and circulation may be associated with a more than two times increase in calciphylaxis risk and may help explain why warfarin, a vitamin K antagonist, is a predisposing factor for the disease. Warfarin increases the risk of calciphylaxis by a factor of 3 to 13 and results in increased mortality for patients with this disease [2]. Additionally, vitamin K deficiency can also result from malabsorption, which develops in 80% of patients with ESRD and calciphylaxis [2]. Histological analysis suggests that calcified, narrowed microvessels lead to chronic low-grade ischemia. Most patients with one, or even multiple, risk factors do not have calciphylaxis. It is thought that there is a triggering event- such as repetitive trauma from subcutaneous injections- that induces the calciphylaxis.
Patients with calciphylaxis often have a high parathyroid level at the start of dialysis. At clinical presentation, however, 45% of these patients are found to have a parathyroid hormone level below the recommended target range, which suggests overuse of calcium and vitamin D supplements [2]. PTH suppression then results in low bone turnover which is thought to exacerbate the extra skeletal calcium deposits.
Although the pathogenesis of calciphylaxis continues to be poorly understood, microvascular calcification and thrombosis likely play pivotal roles. It is unclear whether mechanisms for calciphylaxis differ based on lesion characteristics and further studies are needed to determine that. Histological features of this disease include calcification, thrombosis, and fibro intimal hyperplasia of the arteries in the dermis and adipose tissue which leads to necrosis, ischemia, and panniculitis [3].
Calciphylaxis is associated with multiple risk factors. Dialysis patients are more affected because they are more likely to have bone mineral disease abnormalities such as hyperphosphatemia, elevated calcium phosphorus product, hypocalcemia, hyperparathyroidism and vitamin D deficiency. It is more commonly reported in patients in the 5th decade of life along with a 2:1 female predominance [3]. Furthermore, comorbid conditions including diabetes mellitus, obesity, autoimmune diseases, hypercoagulable conditions, hypoalbuminemia and longer dialysis treatments can also play a role. Medications such as warfarin, a Vitamin K antagonist, can increase the risk of calciphylaxis in patients with and without ESRD. Risk factors that could have contributed to the development of calciphylaxis in our patient included dependence on dialysis-specifically peritoneal dialysis for eight years, failed renal transplant, hyperphosphatemia, obesity, and Type I Diabetes Mellitus.
The gold standard for diagnosis of calciphylaxis is a skin biopsy, but there can be a risk of non-healing lesion following biopsy. Further investigation is needed in regards to the use of noninvasive diagnostics testing includes X-ray, computed tomography, mammography, nuclear bone scan and Raman spectroscopy [5-8]. Plain radiography typically portrays a net-like, superficial vascular calcification pattern. Careful attention should be paid to rule out other causes such as warfarin-induced skin necrosis, atherosclerotic vascular disease, cellulitis, and cholesterol embolization. Definitive diagnosis by biopsy will show basophilic calcium deposits in dermal and subcutaneous vessels with intimal fibroplasia and thrombosis [1]. Specialized stains such as Von Kossa and alizarin red stains can be utilized to focus on calcification.
Treatment and management of calciphylaxis requires the input from several specialties such as pain management, wound care, nephrology and dermatology. Pain is one of the main symptoms of this disorder and can be treated with opioids. However, morphine can cause toxic byproducts which may decrease tissue perfusion further [4]. Fentanyl patches can be used in addition to hydromorphone for breakthrough pain [4]. Wound care includes gentle debridement of necrotic tissue to promote successful wound healing and prevent infection. Deciding if and when to perform surgical debridement and skin grafting is controversial but it may be considered in certain patients [4].
Elimination of risk factors is an important component to the management of patients with calciphylaxis. The ideal parathyroid hormone level has not been determined, but extreme low or high levels should be avoided [2]. Correcting hypercalcemia and hyperphosphatemia are recommended by discontinuing Vitamin D and calcium- based phosphate binders. Warfarin should be discontinued, with the addition of a different anticoagulant based on each individual patient. In ESRD patients, it is recommended to transition from peritoneal dialysis to hemodialysis. Hemodialysis may advance wound healing when compared to peritoneal dialysis through better control of mineral metabolism [2].
Sodium thiosulfate is a pharmacotherapeutic agent that has become a component of the primary treatment plan. It is prescribed intravenously at 25-gram dose three times a week after hemodialysis. It works by inhibiting the ability of adipocytes to activate calcification of vascular smooth-muscle cells [2]. Adverse effects include nausea, vomiting, hypotension, headache and severe metabolic acidosis [4]. In a study of 53 patients with calciphylaxis on hemodialysis, 26% of patients had resolution of skin lesions after three weeks of treatment with sodium thiosulfate [2]. A systematic review and meta-analysis done by Udomkarnjananun et al. has shown that treatment with sodium thiosulfate, parathyroidectomy, hyperbaric oxygen, calcimimetics and bisphosphonate therapies failed to show mortality benefit in the pooled cohort studies [9]. More randomized controlled trails are needed to assess the benefits of this therapy and treatment of this fatal disease.
Conclusion
Calciphylaxis, also referred to as Calcific Uremic Arteriolopathy, is a rare and life-threatening disorder that should be considered as a differential diagnosis in end-stage renal disease (ESRD) patients presenting with painful skin lesions. Although skin lesions tend to dominate the clinical presentation, this disorder is likely a systemic disorder with widespread. Further studies are needed to identify the most appropriate treatment options for this fatal disease.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Ghosh T, Winchester DS, Davis MDP, El Azhary R, Comfere NI (2017) Early clinical presentations and progression of calciphylaxis. Int J Dermatol 56(8): 856-861.
- Nigwekar SU, Thadhani R, Brandenburg VM (2018) Calciphylaxis. N Engl J Med 378(18): 1704-1714.
- Nigwekar SU, Kroshinsky D, Nazarian RM, Goverman J, Malhotra R, et al. (2015) Calciphylaxis: risk factors, diagnosis, and treatment. Am J Kidney Dis 66(1): 133-146.
- Jeong HS, Dominguez AR (2016) Calciphylaxis: Controversies in Pathogenesis, Diagnosis and Treatment. Am J Med Sci 351(2): 217-227.
- Lloyd WR, Agarwal S, Nigwekar SU, Esmonde White K, Loder S, et al. (2015) Raman spectroscopy for label-free identification of calciphylaxis. J Biomed Opt 20(8): 80501.
- Paul WB, Ramya I, George R, Viggeswarpu S (2015) Diagnosis of calciphylaxis by imaging with low-energy-X-rays (mammographic technique). J Assoc Physicians India 63(12): 69.
- Bonchak JG, Park KK, Vethanayagamony T, Sheikh MM, Winterfield LS (2016) Calciphylaxis: a case series and the role of radiology in diagnosis. Int J Dermatol 55(5): e275-e279.
- Paul S, Rabito CA, Vedak P, Nigwekar SU, Kroshinsky D (2017) The role of bone scintigraphy in the diagnosis of calciphylaxis. JAMA Dermatol 153(1): 101-103.
- Suwasin Udomkarnjananun, Kitravee Kongnatthasate, Kearkiat Praditpornsilpa, Somchai Eiam Ong, Bertrand L Jaber, et al. (2018) Treatment of Calciphylaxis in CKD: A Systematic Review and Meta-analysis. Kidney Int Rep 4(2): 231-244.
-
Sandeep Anand Padala, Veronica Moino, Vidya Medepalli, Azeem Mohammed, Stanley Jr Nahman. Calciphylaxis in a Peritoneal Dialysis Patient. Annal Urol & Nephrol. 1(5): 2019. AUN.MS.ID.000521.
-
Calciphylaxis, Calcific Uremic Arteriolopathy, Chronic kidney disease, kidney Injury, Hepatitis C, Hypertension, Melanoma, Atheroembolic Disease, Tomography, Angiography, A Left Upper Extremity Arteriovenous Fistula, Bone Morphogenetic Protein 4, Warfarin, Vitamin K Antagonist, Calcium, Vitamin D Supplements
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






