 Research Article
Research Article
Acute and Chronic Kidney Disease and Their in- Hospital Outcomes During COVID-19 Pandemic Waves in the Pre-Vaccination Period
Rebic D1*, Hamzic-Mehmedbasic A1, Granov N2, Dzubur A2, Odobasic M1 and Hasanspahic S1
1Nephrology Clinic, Clinical Center University of Sarajevo, Bosnia and Herzegovina
2Cardiology Clinic, Clinical Center University of Sarajevo, Bosnia and Herzegovina
Damir Rebic, Nephrology Clinic, Clinical Center University of Sarajevo, Bosnia and Herzegovina.
Received Date: December 16, 2022; Published Date: January 09, 2023
Abstract
Introduction: Although diffuse alveolar damage and respiratory failure are the key features of COVID-19, the involvement of other organs such as the kidney has also been reported. The reports of the incidence of acute kidney injury (AKI) in COVID-19 patients vary widely. In this study, we report our unique experience with AKI in COVID-19 patients and provide its incidence, risk factors, and prognosis to expand the current understanding of this complication. Also, progressive loss of kidney function in chronic kidney disease (CKD) is a very common finding in patients with COVID-19. The study also aims to assess the impact of CKD on the severity of COVID-19 presentation and to identify possible risk factors for in-hospital mortality.
Methods: This retrospective observational study was performed at the Clinic of nephrology from March 2020 to April 2021 and approved by the local Ethics Committee. The earliest day of the serum creatinine change that met the KDIGO criteria for AKI was selected as day 1 of AKI. In the CKD group, according to the baseline kidney function, patients were divided into three groups: The estimated glomerular filtration rate (eGFR) <30mL/ min/1.73m2, 30-60mL/min/1.73m2, and >60mL/min/1.73m2. Statistical analyzes were performed using SPSS 21 Windows.
Results: The incidence of AKI during hospitalization was 28.99% and was significantly higher in patients who were presented with eGFR <60 mL/min/1.73 m2. In the multivariable model, AKI (stage 3) is associated with a higher risk of hospital mortality during the follow-up period. CKD was diagnosed in 27.2% patients of whom 64 presented severe stage, and 214 patients presented moderate CKD. The probability that the patient will experience in-hospital mortality was significantly increasing with deterioration of kidney function.
Conclusions: AKI and deterioration CKD in our hospitalized COVID-19 patients was common and carried high mortality, especially in patients with AKI stage 3.
Keywords: Coronavirus disease 19; Acute kidney injury, Chronic kidney disease; Hospital mortality
Abbreviations: COVID-19: Coronavirus disease 2019; Mers: Middle East Respiratory Syndrome; AKI: Acute kidney injuri; CKD: Chronic kidney disease; ESKD: End-stage kidney disease; KDIGO: The Kidney Disease: Improving Global Outcome; eGFR: The estimated glomerular filtration rate; MDRD: Modification of Diet in Renal Disease Study; IL-6: Interleukin-6; PCT: Procalcitonin; ARDS: Acute respiratory distress syndrome; TCZ: Tocilizumab; RDV: remdesivir; COPD: Chronic obstructive pulmonary disease; ACEI: Angiotensin-converting enzyme inhibitors; ARB: Angiotensin II receptor blocker; NSAID: Non-steroidal anti-inflammatory drugs; WBC: white blood cells; PLT: Platelet count; CRP: C-reactive protein; IL-6: interleukin-6; LDH: Lactate dehydrogenase; CK: Creatine kinase; RRT: Renal replacement therapy; LMWH: Low molecular weight heparin; ALT: Alanine aminotransferase; WHO: World Health Organization; CI -Confidence interval; HR -Hazard ration
Introduction
Coronavirus disease 2019 (COVID-19) is associated with a high morbidity and mortality [1]. Two other coronavirus infections, SARS 2002-03 and Middle East Respiratory Syndrome (MERS) in 2012 caused severe respiratory syndrome in humans. All three of these emerging infectious diseases are caused by coronaviruses. While COVID-19 is primarily an infection that can cause pneumonia and hypoxemia, other organs are involved, including the kidney, gastrointestinal tract, and heart. Reports are numerous, but the incidence of acute renal injury (AKI) secondary to COVID-19 (COVAKI) is high, with a prevalence level of as much as 68% in critically ill patients in New York City -USA [2]. Most AKI cases are mild to moderate. However, dialysis rates may be as high as 30% and survival may be reduced when AKI occurs. Kidney failure appears to occur late in the course of the disease, so there may be a window for treatment. Treatment currently consists mostly of preventive measures as no directed treatment for AKI is available. This makes AKI in general, and in the current COVID-19 pandemic in particular, an important condition to be addressed [3].
An enlarged risk of serious clinical presentation of coronavirus disease of 2019 (COVID-19) is associated with old age, immunosuppressive therapy, and comorbidities, including cardiovascular and lung diseases, diabetes, cancer, and CKD (chronic kidney disease)[4]. Progressive loss of kidney function in CKD results in altered adaptive and innate immune systems, including depletion of B lymphocytes and damaged cell-mediated immunity. The damaged immune response is associated with a higher prevalence and serious trend of infections that might be responsible for a high mortality rate, specifically in patients with end-stage kidney disease (ESKD). In addition to secondary immunodeficiency, activation of the immune system has also been observed in patients with CKD [2]. Enlarged production and decreased clearance of pro-inflammatory cytokines in patients with CKD and SARS-CoV-2 infection may lead to more serious systemic inflammation and oxidative stress [5].
The primary objective of the study was to determine the incidence of in-hospital AKI in COVID-19 patients and to study baseline characteristics and laboratory data associated with its development. The secondary objective of the study was to identify risk factors, and prognosis of AKI and thus provide a broader understanding of these complications in a hospitalized group of COVID patients at the Nephrology Clinic a year ago. Also, the present study aims to evaluate the impact of CKD on the severity of COVID-19 presentation and to identify possible risk factors for in-hospital mortality.
Material and Methods
AKI
All adult patients >18 years of age admitted to the Clinic for nephrology with COVID-19 infection from March 2020 through April 2021, were studied in this retrospective analysis. COVID-19 infection was diagnosed based on clinical presentation, radiographic lung abnormalities, and a positive result SARS CoV-2 of real-time PCR. We excluded COVID-19 patients from our study if they were on maintenance dialysis or were kidney transplant recipients. We collected the demographics, prior medical history including outpatient medications and prior level of kidney function, comorbidities, the presenting clinical symptoms when available, and in-hospital laboratory data from the electronic medical record. Laboratory data consisted of complete blood count, kidney and liver function, pulse oximetry, hemostasis parameters, creatinine kinase, lactate dehydrogenase, procalcitonin, and inflammatory markers including C-reactive protein (CRP), ferritin, interleukine-6, and D-dimer. The normal range of these measures was provided by our laboratory. The Kidney Disease: Improving Global Outcome (KDIGO) [6] the definition was used to identify AKI. The estimated glomerular filtration rate (eGFR) on admission was taken as baseline eGFR and was divided into <60 and ≥60 mL/min/1.73m2. eGFR was estimated using the Modification of Diet in Renal Disease Study (MDRD) equation. The earliest day of the serum creatinine change that met the KDIGO criteria for AKI was selected as day 1 of AKI. The peak serum creatinine value was used to determine the stage of AKI, with an increase in the serum creatinine of 1.5-1.9, 2.0-2.9, and >3 times the baseline serum creatinine defined as AKI stages 1, 2, and 3 respectively.
CKD
This retrospective observational study was performed at the Clinic of nephrology from March 2020 to April 2021 and approved by the Clinical center Ethics Committee (No 04-229-10-2021). We included all adult patients diagnosed with COVID-19. Patients with COVID-19 were divided into three groups according to the estimated glomerular filtration rate (eGFR) ( >60 ml/min/1.73 m2 ; 30-60 ml/ min/1.73 m2 ; <30 ml/min/1.73 m2 ). All included patients gave their informed consent. Exclusion criteria were diagnosis of acute kidney injury and lack of previous medical history or laboratory findings that could confirm the diagnosis of CKD.
COVID-19 was diagnosed by detection of SARS-CoV-2 RNA in nasopharyngeal swab sample using real-time reverse-transcriptase polymerase chain reaction (RT-PCR) [7]. All data including demographic, comorbidities, clinical, laboratory data, therapeutic approach as well as the outcome of each patient were obtained from an electronic medical database. Laboratory data included complete blood count, the concentration of inflammatory markers (C-reactive protein (CRP), interleukin 6 (IL-6), ferritin, procalcitonin (PCT), liver enzyme activity, D-dimer, and parameters of kidney function. The estimated glomerular filtration rate (eGFR) was calculated using the Modification of Diet in Renal Disease (MDRD) equation, and CKD was defined as eGFR <60 ml/min /1.73 m2 if a history of kidney disease was obtained from medical records [8]. According to the baseline kidney function, patients were divided into three groups: eGFR <30 mL/min/1.73 m2 (severe CKD), eGFR 30-60 mL/ min/1.73 m2 (moderate CKD), and eGFR >60 mL/min/1.73 m2 (preserved kidney function).
The severity of COVID-19 at admission was assessed according to the blood oxygen saturation (SpO2) and clinical status was considered as symptomatic stable with SpO2> 95%, symptomatic unstable with two levels of SpO2 91-95% or SpO2 ≤ 90%, and critical with acute respiratory distress syndrome (ARDS). Data regarding the drugs used for COVID-19 treatment, including lowweight molecular heparin, dexamethasone, tocilizumab (TCZ), remdesivir (RDV), and antibiotics, as well as drug-related side effects, were collected throughout the hospitalization. Decisions on the therapeutic approach were made individually for each patient and based on the multidisciplinary agreement of the nephrologist, infectologist, and pulmonologist.
Statistical Analysis
Statistical analysis was carried out using SPSS software (version 21.0). The mean and standard deviation (SD) or median and interquartile range, depending on the distribution of the quantitative variables studied, were used as measures of central tendency and dispersion. Comparison between mean values of groups was performed by Student’s t-test for variables with normal distribution and Mann Whitney U test and Kruskal - Wallis ANOVA for continuous and ordinal variables. Multivariate logistic regression model were performed with death within four weeks after the start of hospitalization as the dependent variable. P values less than 0.05 were considered statistically significant.
Results
AKI
We pooled 269 in-hospital patients between March 2020, and April 2021. Five patients were excluded (three cases with chronic kidney disease (one case receiving regular maintenance dialysis, one with membranous glomerulonephritis, one with nephrotic syndrome), one case diagnosed with pericardial effusion after admission, one case missing the core medical record. Baseline characteristics of these patients are shown in (Table 1). The median age of the patients was 64.2 years, and 59.85% of them were male. Most of these patients presented with dyspnea (96.65%), cough (92.19%), and fever (87.73%). Of all the patients studied were discharged 69.88%, 16.73% died during hospitalization, and 13.38% were transferred to another institution.
Table 1:Characteristics of COVID-19 patients (AKI study) admitted in the hospital stratified by baseline eGFR.
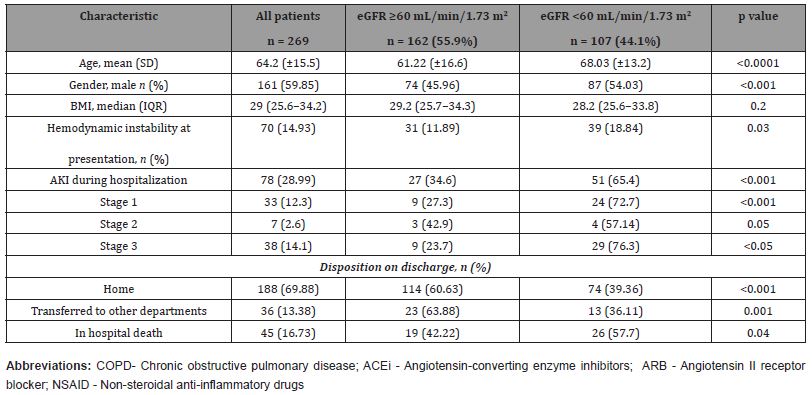
On admission, eGFR was <60 mL/min/1.73 m2 in 44.1% (n = 107) of the patients. Patients with an eGFR <60 mL/min/1.73 m2 at presentation were significantly older (mean age 68.03 vs. 61.22 years; p <0.0001), more likely to be males (54.03 vs. 45.96%), and have hypertension, diabetes, hyperlipidemia, and were higher smoker among other preexisting comorbidities. The incidence of AKI during hospitalization was 28.99% (n=78) and was significantly higher in patients who presented with eGFR <60 mL/min/1.73 m2 (65.38 vs.34.61%). 24 pts., 4 pts., and 29 pts. of these patients had AKI stages 1, 2 and 3, respectively. Eleven out of 32 patients with documented chronic kidney disease (CKD) prior to admission developed AKI during the hospitalization. Overall, the in-hospital mortality was 16.73% in COVID-19 patients. The inhospital mortality was significantly higher in patients with an eGFR <60 mL/min/1.73 m2 (26 pts.) then in patients with an eGFR ≥60 mL/min/1.73m2 (19 pts.) at presentation. (Table 1).
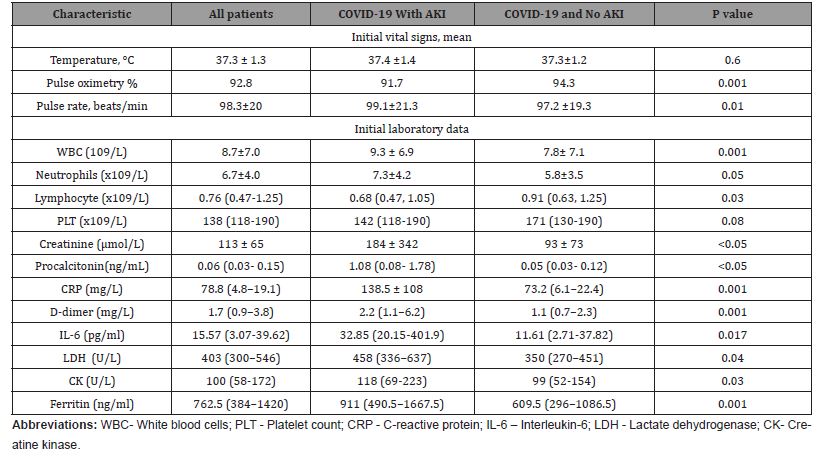
(Table 2) shows the results of clinical and laboratory tests of the patients performed on admission. Compared with the patients without AKI, the patients with AKI had higher white blood cells (WBC) and neutrophil counts, higher levels of D-dimer, C-reactive proteins (CRP), procalcitonin, interleukine-6 (IL-6), creatine kinase, and had low pulse oxygen saturation at admission, they appeared to have mild symptoms of dyspnea, possibly as a result of gradual deterioration of the condition to allow for adaption and compensation. (Table 2) Clinical and laboratory findings of the hospitalized patients (AKI study) positive for COVID-19
Table 2:Clinical and laboratory findings of the hospitalized patients (AKI study) positive for COVID-19
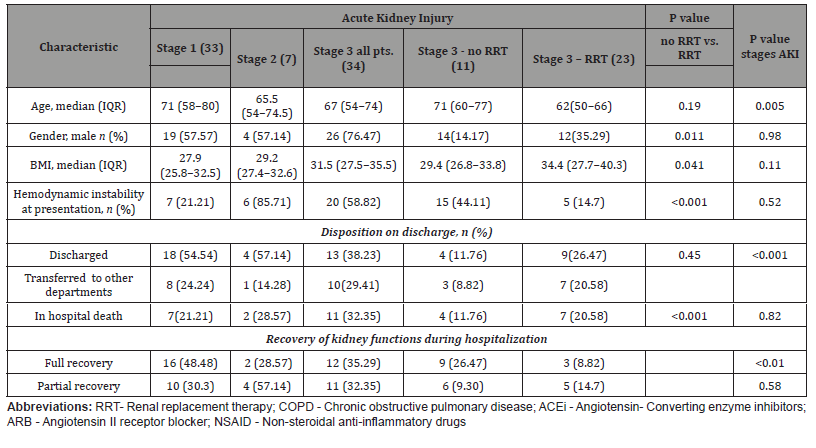
The number of patients requiring renal replacement therapy (RRT) during the hospitalization was 23 (Table 3). This represented 8.5% of all patients and 29.48% of those with AKI. All the patients who required RRT by definition had stage 3 AKI. The modalities of RRT were continuous RRT in 3 patients (13.04% of all those who required RRT) and intermittent hemodialysis in 20 patients (86.9%). One patient required both modalities of RRT. Unfortunately, 30.43% of the patients who were treated with RRT (7 out of 23 patients) died during the hospitalization. Of those with stage 3 AKI who were not dialyzed, 36.4% (4 out of 11) died. Kidney function recovered sufficiently in 9 patients treated with RRT and no longer required dialysis upon discharge. Table 3 SARS CoV-2 patients stratified by stages of AKI and demand for dialysis.
Table 3:SARS CoV-2 patients stratified by stages of AKI and demand for dialysis.
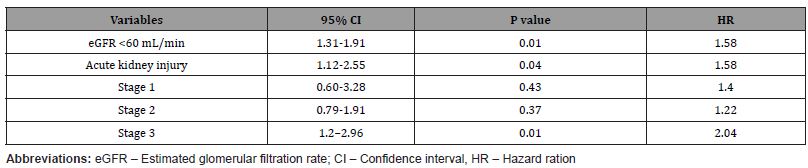
In the multivariable model, adapted to age, gender, and comorbidities, eGFR <60 ml/min, and AKI (stage 3) are associated with a higher risk of hospital mortality during the follow-up period (Table 4).
Table 4:Association of kidney disease with in-hospital mortality in COVID-19 patients .
CKD
Detailed baseline characteristics of patients according to the renal function at hospital admission are summarized in (Table 5). Of all 1022 adult patients included in the study, patients with preserved kidney function (eGFR >60mL/min) were significantly younger in comparison to the patients with moderate (eGFR 30-60 ml/min) and severe kidney failure (eGFR <30mL/min) with a mean age of 57.1 ± 16.5 years and male predominance (53.5%).
In comparison to the patients with preserved kidney function, patients with moderate and severe kidney failure were significantly older and showed a more serious course of COVID-19 on admission, defined by a higher prevalence of SO2 ≤90%. Comorbid conditions like chronic obstructive pulmonary disease, diabetes, and cardiovascular diseases (hypertension, coronary disease, and heart failure) as well as cerebrovascular diseases and malignancies were more common in patients with kidney failure than in patients with preserved kidney function. Furthermore, patients with kidney failure were more likely to be treated with concomitant medications. Inflammatory markers were significantly lower in patients with preserved kidney function than in patients with moderate and severe kidney failure (Table 5). Patients with kidney failure were more commonly treated with the IL-6 inhibitor tocilizumab (TCZ), dexamethasone, and low molecular weight heparin (LMWH) compared to the patients with preserved kidney function. Remdesivir (RDV) use was significantly lower in patients with CKD. Among patients with severe kidney failure, four patients were treated with RDV (off-label), three were treated concomitantly with RDV and TCZ, and two were treated with dexamethasone- all of them were classified as category 5 on the ordinal scale. Furthermore, patients with severe renal failure more commonly needed antibiotic treatment (Table 5).
Table 5:Baseline characteristics of the CKD patients divided toward the renal function.
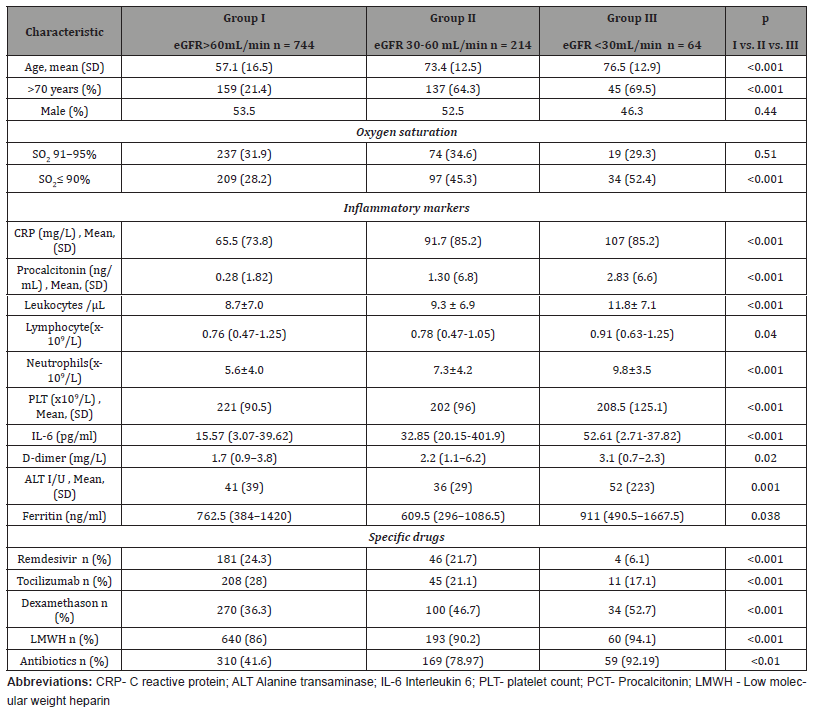
The probability that patient will experience 28-day hospital mortality and the need for mechanical ventilation was significantly increased with deterioration of kidney function. Clinical improvement the 14th, 21st, and 28th day of follow-up were more likely in patients with preserved kidney function compared to the patients with moderate and severe kidney failure, as well as in patients with moderate compared to severe kidney failure (Table 6).
Table 6:Outcomes of CKD patients divided toward the renal function
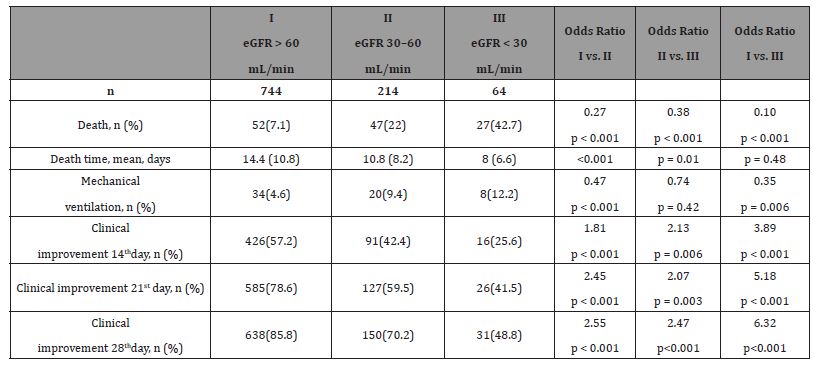
One of four patients with severe kidney failure who were treated with RDV died of sepsis. In the remaining three, no safety problems or deterioration of renal function were observed. On contrary, two of them experienced clinical improvement, 21 and 28 days after admission respectively.
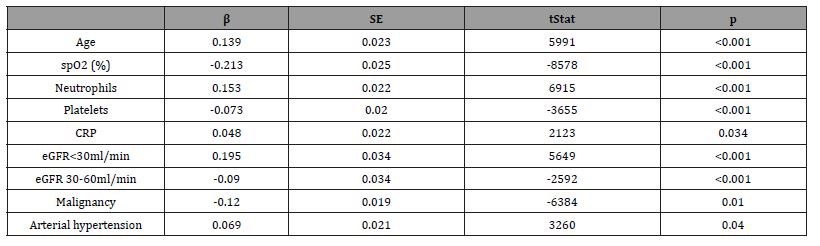
Increased age, higher neutrophil count and CRP concentration, as well as lower baseline SpO2, decreased platelet counts and eGFR were identified as independent predictors of four weeks (28-day) mortality of CKD patients in logistic regression analysis (Table 7). In-hospital treatment, BMI, D-dimer, alanine aminotransferase (ALT), and PCT were not independently associated with 28-day mortality.
Table 7:Independent predictors of 4-weeks mortality among CKD patients
Discussion
AKI
In this observational, retrospective study of COVID-19 patients, we found significant rates of patients who developed in-hospital AKI. Current evidence suggests that in COVID-19 patients, there is a higher prevalence of AKI in patients with more severe forms of COVID-19. AKI develops early during hospitalization and is a result of an interplay of virus-mediated injury and a dysregulated inflammatory response [9].The need for dialysis is considered to be a negative survival prognostic factor. Knowledge of AKI can lead to better optimization and prognostication of patients with COVID-19. Acute kidney injury is common in critically ill patients with COVID-19, affecting approximately 20-40% of patients admitted to hospital and is considered as a marker of disease severity and a negative prognostic factor for survival. AKI appears to result from the interaction of multiple variables, via various basic pathophysiological mechanisms [10].
Compared to studies of AKI in COVID-19 patients from other countries, we found our incidence to be is similar to that in an Italian study (27.8%) [11]. As compared with the above-cited study, the incidence of stage 3 AKI was highest in our cohort. It is not possible to determine with certainty the causes of this variation. However, we did remark a higher prevalence of comorbid conditions in our group. We found that among the 269 patients included in this study, the incidence of AKI was substantially higher than the overall incidence of 0.5%-7% reported in previous studies (1) and was comparable to that in critically ill patients hospitalized with other illnesses [12]. The high incidence of AKI observed in this study was presumably attributed to several reasons. First, most of the COVID-19 patients included in this study had severe disease. Although many patients had low pulse oxygen saturation at admission, they appeared to have mild symptoms of dyspnea, possibly as a result of gradual deterioration of the condition to allow for adaption and compensation. The rapid deterioration after admission in many patients also suggests the severe condition of these patients. Second, the methods that we used to diagnose and stage AKI might cause overestimation of the incidence and severity of AKI. In the early stage of the COVID-19 outbreak, the medical resources were overwhelmed, and many patients did not have a baseline serum creatinine test prior to admission or in the previous year so that we could only estimate the baseline serum creatinine according to the patient’s sex, weight, medical history, and other related parameters. The incidence of AKI was determined based on baseline values and serum creatinine levels within 7 days of admission. In order to reduce the bias of the estimation, we used the eGFR method for AKI diagnosis in these patients [9], by which the incidence of AKI was 28.6%, respectively.
We found that older age, multiple pre-existing comorbidities, and increased white blood cell count, and low lymphocyte count, were all risk factors for AKI in patients with COVID-19. Markers of acute inflammation (ferritin and CRP), muscle injury (creatinine kinase), increased levels of PCT, and D-dimer levels were significantly higher in patients with a low eGFR at presentation and in those with AKI suggesting causality by a dysregulated immune/ inflammatory response, the so-called “cytokine” storm.
The prevalence of several pre-existing comorbidities, including chronic lung diseases and dyslipidemia did not differ significantly due to the relatively small number of cases of AKI in this study. Lymphopenia indicates that decreased immunity might occur in patients with COVID-19, and increased levels of PCT and CRP suggest that some patients may have secondary bacterial infections that caused excessive inflammatory responses. Previous studies [1,13] have shown that patients with COVID-19 have significantly increased D-dimer levels, multiple organ damage, and electrolyte disturbances. Our study showed that these abnormalities were more pronounced in patients with AKI. The occurrence and development of AKI often lead to the imbalance of blood volume and electrolytes, accumulation of metabolites, and aggravation of multiple organ dysfunction, thus creating a vicious cycle. The majority of patients with stage 1 AKI recovered and were discharged, but those who progressed into stage 2/3 AKI had a high mortality rate, possibly due to the untimely treatment. Early intervention of AKI is thus important in these patients, and continuous renal replacement should be considered to prevent the progression of AKI.
In our cohort, 23 of the 78 patients with AKI required RRT. Among those who received RRT, 7 (20.58%) died versus 4 (11.76%) in the stage 3 AKI group who did not undergo RRT (p<0.001). Thus, there was no significant improvement in survival, and the average mortality remained relatively high in both the RRT and non-RRT groups. These findings should prompt the early involvement of a palliative care team to define goals of care in patients with COVID-19 disease and stage 3 AKI. In our experience, ultrafiltration was not well tolerated. It often increased pressor requirements and did little to improve the ventilatory status. We observed a high incidence of hypercoagulability and frequent clotting of the dialysis filters, lines, and catheters. Thus, the patients required higher than the usual doses of heparin.
CKD
Patients with CKD are prone to SARS-CoV-2 infection due to the frequent hospital visits. Furthermore, they have an increased risk of COVID-19 progression to a severe or critical form of the disease, primarily due to a damaged immune system [14,15]. In the current study, nearly 20% of hospitalized COVID-19 patients were diagnosed with CKD, and 18% of them were diagnosed with severe CKD. Besides severe COVID-19 clinical presentation at hospital admission, pre-existing kidney disease was also independently related to higher in-hospital mortality, specifically in those with serious renal failure, which is consistent with the results of previous studies [16-20]. Furthermore, a large international study that used the results of an international database assessed risk factors for the serious clinical presentation of COVID-19 and determined that CKD is linked with the highest risk for severe disease presentation and high mortality rate in COVID-19 [21].
Hypoxemia related to the severe clinical presentation of COVID-19 was linked with poor prognosis specifically in patients with kidney failure in our study. The present study identified older age as a strong independent predictor of in-hospital mortality, which is consistent with previous studies related to CKD and COVID-19 [23]. Our study did not prove gender as an influencing factor for clinical presentation and in-hospital mortality in patients with CKD. In patients with severe renal failure, the mortality rate was almost equal among women and men. Therefore, the results differ from results of other studies which identified the male gender to be associated with severe clinical course and higher mortality in COVID-19 [23], but further studies are needed to clarify these gender differences.
In the current study, patients with CKD showed significantly higher white blood cell and neutrophil count, higher levels of IL-6, PCT, and CRP, as well as higher D-dimer levels at hospital admission compared to patients without kidney dysfunction. These parameters are increased proportionally related to the stage of kidney failure and they represent the manifestation of proinflammatory condition in COVID-19. Higher neutrophil count, higher concentrations of CRP, as well as decreased platelet count, were independently associated with higher hospital mortality in patients with CKD as previously confirmed in other studies [24,25].
Between comorbidities, hypertension, and malignancy were independent predictors of four weeks survival, while, interestingly, diabetes and COPD did not prove to be independently associated with survival of patients with CKD and COVID-19. The prognostic impact of comorbid conditions has been considered in many studies, and the results vary depending on the population analyzed the model size, and the character of the study. Park et al. found that both, hypertension and diabetes were risk factors for mortality during COVID-19 [26]. The same results have been shown among Indian patients by Gupta et al. [27].
Related to COVID-19 treatment, dexamethasone and TCZ were more commonly used, especially in patients with CKD probably due to the severe clinical presentation in this patient group. However, we failed to show their prognostic impact on the in-hospital outcome. RDV use was significantly less frequent in patients with CKD, probably in order to prevent the nephrotoxic effect of this drug. Nevertheless, four patients received RDV as off-label therapy, primarily due to a very severe form of clinical presentation at hospital admission. Similarly, Pettit et al. used RDV for treatment of a small number of 20 ESRD patients with COVID-19 and reported that treatment was relatively safe with the possible benefit that overbears the theoretical risk of kidney toxicity [28].
The present study was limited for several reasons, primarily due to its retrospective design. Analyses were based on available electronic health data with possible input errors and a lack of information regarding the CKD and AKI etiology. Laboratory tests were also lacking in some patients, and that was the reason why some parameters were not assessed as prognostic factors. Since treatment with remdesivir and tocilizumab is not indicated in patients with serious renal failure (eGFR <30 mL /min), and on the other hand, avoiding such treatment may affect the final in-hospital outcome, the predictive value of CKD on the outcome of COVID-19 patients may be overestimated. Second, we did not explore the impact of treatment on AKI, because our treatments were based on WHO’s and (ADQI) standardized protocols [29,30], in which the antiviral drugs, antibacterial drugs, and hormones have little effect on renal function. Third, the urgency in data collection and the short follow-up time of the patients (the shortest hospital stay of the patients was only 14 days) may affect the final prognostic evaluation of the patients to cause bias in survival time analysis. We especially emphasize the fact that little data were available regarding urine output, a defining characteristic of AKI.
Conclusion
The kidney is a primary target organ of SARS-CoV-2 and the incidence of AKI is high in hospitalized patients with COVID-19. Deterioration of kidney function aggravates other organ damage. Pre-existing cardiovascular and renal diseases are potential risk factors for AKI in patients with COVID-19. In the other hand, the reported frequency of CKD amongst hospitalized patients with COVID-19 varies widely. CKD among hospitalized patients is associated with poor prognosis, increased length of hospitalization, and increased health care costs. Also, our patients experienced a high incidence of AKI and high mortality, especially in AKI stage 3. Unfortunately, RRT provided little survival benefit. However, the advantage of this study is that the patient population was rather large for Bosnian circumstances and pretty heterogeneous, which increases the usability of the findings. Finally, CKD and AKI were common in pre-vaccination waves among hospitalized COVID-19 patients and were independent risk factors for death despite improvements in treatment.
Acknowledgements
None
Conflict of interest
The authors report no conflicts of interest in this work.
References
- Huang C, Wang Y, Li X, Ren L, Zhao J, et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395(10223): 497-506.
- Chan L, Chaudhary K, Saha A, Kinsuk Chauhan, Akhil Vaid, et al. (2020) Acute kidney injury in hospitalized patients with COVID-19. medRxiv 2020.
- Hassanein M, Radhakrishnan Y, Sedor J, Vachharajani T, Vachharajani VT, et al. (2020) COVID-19 and the kidney. Cleve Clin J Med. 1; 87(10): 619-631.
- Kes P (2021) Acute kidney injury in patients with covid-19: a challenge for nephrologists. Acta Med Croatica. 2021;75: 3-27.
- Zarębska-Michaluk D, Jaroszewicz J, Rogalska M (2021) Impact of Kidney Failure on the Severity of COVID-19. J Clin Med. 10(9): 2042.
- Khwaja A (2012) KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 120(4): c179-184.
- World Health Organization (2020) Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected: Interim Guidance; Version 1.2; World Health Organization: Geneva, Switzerland.
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, et al. (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med. 150: 604-612.
- Batlle D, Soler MJ, Sparks MA, Hiremath S, South AM, et al. (2020) Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 31(7): 1380-1383.
- Izzedine H, Jhaveri KD (2021) Acute kidney injury in patients with COVID-19: an update on the pathophysiology. Nephrol Dial Transplant 36: 224-226.
- Fanelli V, Fiorentino M, Cantaluppi V, Gesualdo L, Stallone G, et al. (2020) Acute kidney injury in SARS-CoV-2 infected patients. Crit Care 24(1): 155.
- Yang X, Yu Y, Xu J (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a singlecentered,retrospective, observational study [J]. Lancet Respir Med 8(5):475-81.
- Xiao G, Hu H, Wu F, Sha T, Zeng Z, et al. (2021) Acute kidney injury in patients hospitalized with COVID-19 in Wuhan, China: a single-center retrospective observational study. Nan Fang Yi Ke Da Xue Xue Bao 25; 41(2):157-163.
- Cheng Y, Luo R, Wang K, Meng Zhang , Zhixiang Wang, et al. (2020) kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 97: 829-838.
- Cai R, Zhang J, Zhu Y, Lin Liu, Yueming Liu, et al. (2021) Mortality in chronic kidney disease patients with COVID-19: A systematic review and meta-analysis. Int Urol Nephrol 53(8):1623-1629.
- Ozturk S, Turgutalp K, Arici M, Ali Riza Odabas, Mehmet Riza Altiparmak, et al. (2020) Mortality analysis of COVID-19 infection in chronic kidney disease, hemodialysis and renal transplant patients compared with patients without kidney disease: A nationwide analysis from Turkey. Nephrol Dial Transplant. (4)35: 2083-2095.
- Flythe JE, Assimon MM, Tugman MJ, Emily H Chang, Shruti Gupta, et al. (2021) STOP-COVID Investigators. Characteristics and Outcomes of Individuals with Pre-existing Kidney Disease and COVID-19 Admitted to Intensive Care Units in the United States. Am J Kidney Dis. 77: 190-203.
- Coca A, Burballa C, Centellas-Pérez FJ, a Jose Perez Saez, Elena Bustamante-Munguira, et al. (2020) Outcomes of COVID-19 among Hospitalized Patients with Non-dialysis CKD Front Med. (3)7: 615312.
- Yang D, Xiao Y, Chen J, Y Chen, P Luo, et al. (2020) COVID-19 and chronic renal disease: Clinical characteristics and prognosis. QJM(1)13: 799-805.
- Mirjalili H, Dastgheib SA, Shaker SH, Reza Bahrami, Mahta Mazaheri, et al. (2021) Proportion and mortality of Iranian diabetes mellitus, chronic kidney disease, hypertension and cardiovascular disease patients with COVID-19: A meta-analysis. J Diabetes Metab Disord. 2021 Feb 26;20(1): 905-917.
- Clark A, Jit M, Warren-Gash C (2020) Centre for the Mathematical Modelling of Infectious Diseases COVID-19 working group. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: A modelling study. Lancet Glob Health 8: e1003-e1017.
- Popadic V, Klasnja S, Milic N, Nina Rajovic, Aleksandra Aleksi,c et al. (2021) Predictors of Mortality in Critically Ill COVID-19 Patients Demanding High Oxygen Flow: A Thin Line between Inflammation, Cytokine Storm, and Coagulopathy. Oxid Med Cell Longev. 2021: 6648199.
- Vogel MJ, Mustroph J, Staudner ST, Simon B Leininger, Ute Hubauer ,et al. (2021) Kidney injury molecule-1: potential biomarker of acute kidney injury and disease severity in patients with COVID-19. J Nephrol. 34(4):1007-1018.
- Liu W, Tao ZW, Wang L, Ming li yuan, Kui Liu, et al. (2020) Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin. Med J 133(9): 1032-1038.
- Valeri AM, Robbins-Juarez SY, Stevens JS, Wooin Ahn, Maya k Roa, et al. (2020) Presentation and Outcomes of Patients with ESKD and COVID-19. J Am Soc Nephrol 31:1409-1415.
- Park BE, Lee JH, Park HK, Hong Nyun kim, Se Yong Jang, et al. (2021) Impact of Cardiovascular Risk Factors and Cardiovascular Diseases on Outcomes in Patients Hospitalized with COVID-19 in Daegu Metropolitan City. J Korean Med Sci. 36(2): e15.
- Gupta A, Nayan N, Nair R, Krishna Kumar, Aditya Joshi, et al. (2021) Diabetes Mellitus and Hypertension Increase Risk of Death in Novel Corona Virus Patients Irrespective of Age: a Prospective Observational Study of Co-morbidities and COVID-19 from India. SN Compr Clin Med 3(4): 937-944.
- Pettit NN, Pisano J, Nguyen CT, Alison K lew, Anirudha Hazra, et al. (2020) Remdesivir Use in the Setting of Severe Renal Impairment: A Theoretical Concern or Real Risk? Clin Infect Dis. ciaa 6;73(11): e3990-e3995.
- Russo E, Esposito P, Taramasso L, Laura Magnasco, Michela Saio, et al. GECOVID working group. (2021) kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy. J Nephrol 34:173-183.
- Emami A, Javanmardi F, Pirbonyeh N, Akbari A (2020) Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19:A Systematic Review and Meta-Analysis. Arch Acad Emerg Med. 8: 24;8(1): e35.
-
Rebic D*, Hamzic-Mehmedbasic A, Granov N, Dzubur A, Odobasic M and Hasanspahic S. Acute and Chronic Kidney Disease and Their in-Hospital Outcomes During COVID-19 Pandemic Waves in the Pre-Vaccination Period. Annals of Urology & Nephrology. 3(3): 2023. AUN.MS.ID.000563.
-
Coronavirus disease 19; Acute kidney injury, Chronic kidney disease; Hospital mortality
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






