 Review article
Review article
The Implications of Pyroptosis in Conditions Affecting the Genitourinary Tract
Samer Younes, Department of Pharmacy, Tartous University, Syria
Received Date:March 25, 2024; Published Date:April 05, 2024
Abstract
Pyroptosis, a unique form of programmed cell death distinct from apoptosis and necrosis, is thought to be closely associated with the pathogenesis of various diseases. Although there has been considerable attention given to the relationship between pyroptosis and urinary diseases, a comprehensive review on this subject is currently lacking. The objective of this study is to address this gap by investigating the involvement of pyroptosis in the development and progression of both benign urinary diseases and urinary malignancies. Through this thorough examination, it has been determined that pyroptosis plays a significant role in the pathogenesis of urinary diseases. In summary, this review not only offers valuable insights for future research directions but also proposes innovative approaches for utilizing pyroptosis as a powerful tool in combating urinary diseases.
Keywords: Pyroptosis; acute kidney injury (AKI); diabetic nephropathy (DN); prostatic hyperplasia (BPH); genitourinary tract
Abbreviations:ASC: Caspase-recruitment domain
ROS: Reactive oxygen species
IL-18: Interleukin-18
IL-1β: Interleukin-1β
LPS: Lipopolysaccharide
TAK1: TGF-β-activated kinase-1
GSDMA: Gasdermin A
GSDMB: Gasdermin B
GSDMC: Gasdermin C
GSDMD: Gasdermin D
GSDME: Gasdermin E
GZMB: Granzyme B
GZMA: Granzyme A
USF2: Upstream stimulatory factor 2
THBS1: Thrombospondin-1
NLRP1: NOD-like receptor 1
AIM2: Absent in melanoma 2
NLRP3: NOD-like receptor 3
HMGB1: High mobility group box 1
ERS: Endoplasmic reticulum stress
TLR2: Toll-like receptor 2
ROCK1: Rho-associated coiled-coil containing protein kinase-1
TXNIP: Thioredoxin–interacting protein miR-93
MicroRNA-93 PRDX3: Peroxiredoxin 3
STAT3: Transcription 3
HK2: Hexokinase 2
LDHA: Lactate dehydrogenase A
ENO2: Enolase 2
USP24 :Ubiquitin-specifc peptidase 24
IGFBP3: Insulin-like growth factor-binding protein 3
Introduction
Pyroptosis, a form of programmed cell death that has been recently discovered, is classified as an inflammatory programmed cell death (PCD) [1]. Its initial description dates back to 1992, but the term “pyroptosis” was coined in 2001 when it was observed that macrophages infected with bacteria underwent rapid lytic cell death, which was found to be dependent on caspase-1 activity [2]. Recent research has indicated that macrophages not only regulate pyroptosis but also play a crucial role in the development of acute kidney injury (AKI), diabetic nephropathy (DN), and renal fibrosis. Pyroptosis is characterized by the formation of pores in the cell membrane, cell swelling, and the release of inflammatory intracellular contents [3]. The release of inflammatory factors, such as interleukin-1β (IL-1β) and interleukin-18 (IL-18), during cell lysis amplifies the inflammatory effects and activates immune responses [4]. The discovery of the gasdermin D (GSDMD) protein has provided insights into the underlying mechanism of pyroptosis. Shi et al. demonstrated that caspase-1/11/4/5 can induce pyroptosis by cleaving GSDMD and releasing its N-terminal domain [5].
In addition to GSDMD, the gasdermin family comprises five other members. In humans, the gasdermin family includes GSDMA, GSDMB, GSDMC, GSDMD, GSDME/DFNA5, and PVJK/DFNB59. In mice, there are five gasdermin members, namely GSDMA, GSDMC, GSDMD, GSDME, and PJVK/DFNB59, with the absence of GSDMB. All gasdermins, except DFNB59, possess two conserved domains: an N-terminal effector domain and a C-terminal inhibitory domain. Under normal circumstances, moderate pyroptosis plays a significant role in the host’s defense against pathogen infections. However, when pyroptosis becomes excessive, it can lead to uncontrolled inflammatory responses, extensive cell death, and severe tissue damage, which in turn can result in the development of inflammatory or autoimmune diseases. As a form of cell death that triggers inflammation, pyroptosis presents a promising avenue for harnessing the anti-tumor immune response and potentially eliminating cancer. Numerous studies have highlighted the pivotal role of pyroptosis in various types of cancer, such as breast cancer, gastric cancer, and lung cancer [6].
In this article, we aim to provide a comprehensive overview of the different signaling pathways involved in pyroptosis, allowing for a deeper understanding of the molecular mechanisms underlying this process. Furthermore, we delve into the specific role of pyroptosis in urinary diseases and propose potential directions for future research in this area. The conventional pyroptosis pathway is regulated by caspase-1. Inflammasomes are composed of patternrecognition receptors (PRRs), also known as inflammasome sensors, apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), and inactive pro-caspase-1. PRRs have the ability to identify pathogen-associated molecular patterns and danger-associated molecular patterns (PAMPs and DAMPs) [7]. NLRs, such as NLRP1, NLRP3, and NLRC4, AIM2, and pyrin are included in the PRRs [8]. NLRs typically consist of a leucine-rich repeat (LRR), a nucleotide-binding oligomerization domain (NACHT/NOD), and a caspase-recruitment domain (CARD) or pyrin domain (PYD) and are categorized into NLRPs or NLRCs based on the presence of a PYD or CARD at their N-terminus [9].
A PYD is essential for interaction with ASC. The NOD is involved in ATP-dependent activation of the signal [10]. The LRR is responsible for recognizing ligands and autoinhibition. The CARD is involved in recruiting pro-caspase-1. Upon receiving a stimulating signal, inflammasome sensors recruit pro-caspase-1 (which contains a CARD) either directly through homotypic binding of CARD or indirectly through the PYD via ASC, which has a PYD and a CARD. Subsequently, caspase-1 activation occurs through self-cleavage [11]. Activated caspase-1 not only cleaves inactive IL-1β and IL-18 precursors but also cleaves GSDMD to generate GSDMD-NT and GSDMD-CT. GSDMD-N creates pores in the plasma membrane, resulting in cell swelling and pyroptosis [12]. Most gram-negative bacteria induce the non-canonical inflammasome pathway. In humans, this nonclassical signaling pathway is triggered by caspase-4 and caspase-5, while in mice, it is mediated by caspase-11. These caspases are activated through direct interaction with lipopolysaccharide (LPS) [13]. Once activated, caspase-4/5/11 cleaves GSDMD, leading to the promotion of pyroptosis.
However, these caspases are unable to cleave pro-IL-18/pro-IL- 1β but can cleave GSDMD, resulting in K+ efflux and activation of the NLRP3/caspase-1 pathway. This cascade ultimately leads to the maturation and release of interleukin-18 (IL-18) and interleukin-1β (IL-1β) [14]. The apoptotic caspase-mediated pathway, along with the inflammatory caspase-1/4/5/11, can also trigger pyroptosis. Chemotherapeutic drugs have the ability to induce caspase-3 to cleave GSDME, resulting in the formation of GSDME-N termini, which in turn cause pyroptosis [15]. Moreover, pathogenic Yersinia has been observed to inhibit TGFβ-activated kinase-1 (TAK1) through the Yersinia effector protein YopJ, leading to caspase- 8-related cleavage of GSDMD and the subsequent initiation of pyroptosis [16]. Interestingly, caspase-8 induces GSDMC cleavage, thereby activating a non-canonical pyroptosis pathway in cancer cells [17] (Figure 1).
On the other hand, the granzyme-mediated pathway involves the serine protease Granzyme A (GzmA), which has long been recognized as a mediator of cell death within the granzyme family. Zhou et al. discovered that GZMA, derived from cytotoxic T lymphocytes, cleaves GSDMB to induce pyroptosis [18]. In 2020, it was reported that CAR-T cells release granzyme B (GzmB), which activates caspase-3 and subsequently triggers the caspase-3/ GSDME-mediated pyroptotic pathway, leading to pyroptosis [19]. Additionally, Zhang et al. found that GzmB directly cleaves GSDME, inducing pyroptosis and enhancing anti-tumor immunity while inhibiting tumor growth [20] (Figure 1).
Pyroptosis in Benign Urinary Diseases
Pyroptosis, a form of programmed cell death, has been found to play a significant role in the development of various benign urinary diseases. One such disease is interstitial cystitis (IC), also known as bladder pain syndrome (BPS) [21]. IC is a chronic pain disorder that primarily affects the bladder, pelvis, or abdomen [22]. Studies have shown that the NLRP3 inflammasome, a multiprotein complex involved in the activation of inflammatory responses, is a crucial player in the development of IC. Elevated expression levels of NLRP3, caspase-1, and GSDMD have been observed in patients with IC [23]. Wang et al. discovered that the NLRP3/GSDMD-N pathway is activated and contributes to the development of IC. Additionally, they found that aster tataricus extract (ATE) can serve as an inhibitor of NLRP3, offering a potential treatment option for IC. The identification of the NLRP3/caspase-1/GSDMD-N pathway as a novel mechanism provides a promising direction for further research on IC [24]. In the case of benign prostatic hyperplasia (BPH), a condition characterized by the nonmalignant overgrowth of prostatic tissue surrounding the urethra, pyroptosis also appears to be involved.
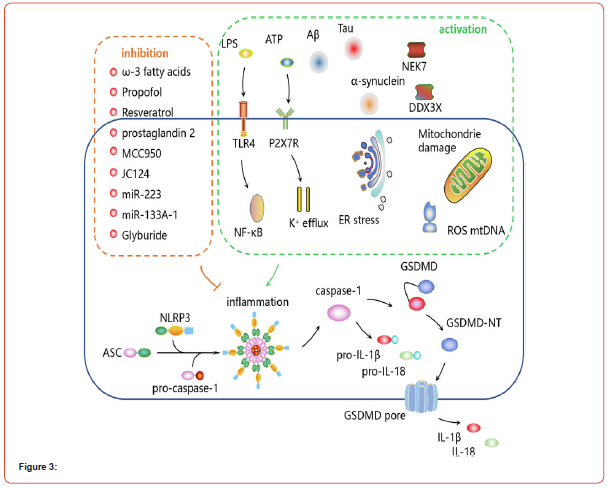
BPH leads to the constriction of the urethral opening, resulting in lower urinary tract symptoms (LUTS) such as urgency, frequency, nocturia, incomplete bladder emptying, and a weak urine stream [25]. Inflammation has been identified as a key factor in the development of BPH. Studies have shown elevated expression levels of NLRP1, caspase-1, IL-18, and IL-1β in BPH. This suggests that the NLRP1/caspase-1 pathway is activated and contributes to the pathogenesis of BPH. Further research is needed to fully understand the role of pyroptosis in BPH and explore potential therapeutic interventions targeting this pathway [26]. Jiang and colleagues discovered that peroxiredoxin 3 (PRDX3) inhibits autophagy flux and triggers pyroptosis, leading to inflammatory reactions and promoting prostate tissue overgrowth. Recent findings suggest that the levels of AIM2 mRNA are higher in benign prostatic hyperplasia (BPH) tissue compared to normal prostate tissue. AIM2 recruits ASC and pro-caspase-1 to form the AIM2 inflammasome, resulting in cell swelling and pyroptosis [27]. These investigations have aided in identifying potential therapeutic targets for BPH. The signaling pathways that regulate pyroptosis in BPH are illustrated in Figure 3.
Pyroptosis in Acute Kidney Injury (AKI)
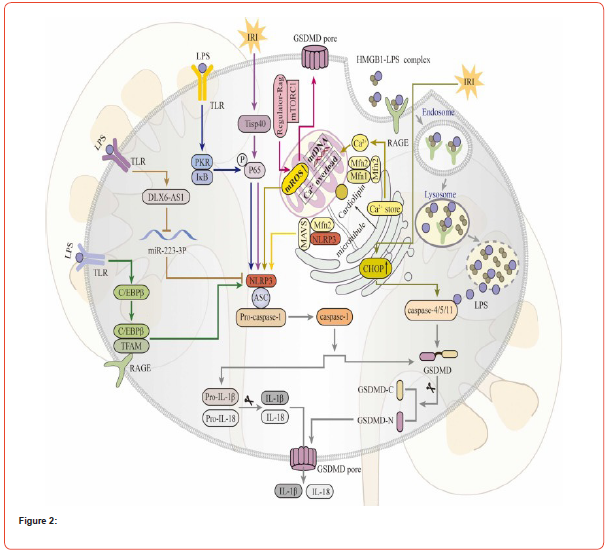
Acute kidney injury (AKI) is characterized by a sudden rise in serum creatinine, a reduction in urine output, or both. Recent studies have highlighted the involvement of pyroptosis in AKI. Sun et al. observed high expression levels of thrombospondin-1 (THBS1) and upstream stimulatory factor 2 (USF2) in patients with sepsis-induced AKI. USF2 upregulates THBS1 expression, activating the TGF-β/Smad3/NLRP3/caspase-1 pathway and promoting pyroptosis, worsening sepsis-induced AKI. Miao et al. noted a significant increase in GSDMD expression in both cisplatininduced and ischemia-reperfusion (I/R) AKI models [28]. Knocking out caspase-11 or GSDMD reduced kidney damage in mice with cisplatin-induced AKI. A 2020 study revealed elevated levels of high mobility group box 1 (HMGB1), IL-1β, IL-18, NLRP3, and GSDMD proteins in an AKI model. Hence, it is proposed that the HMGB1/ NLRP3/GSDMD pathway plays a crucial role in AKI pathogenesis. Additionally, Li et al. demonstrated that the ROS/NLRP3/caspase-1/ GSDMD pathway mediates contrast-induced AKI (CI-AKI) through pyroptosis, and treatment with baicalin reduced inflammation and oxidative stress levels.
Furthermore, research has indicated that macrophage-derived exosomal miRNAs have significant implications in AKI [29]. The suppression of caspase-3 resulted in the prevention of GSDME-N cleavage, leading to the reduction of cisplatin-induced pyroptosis and kidney dysfunction. As a result, the caspase-3/GSDME-induced pyroptosis is a significant factor in the development of acute kidney injury (AKI). Juan et al. identified that the exosomal miR- 93/thioredoxin-interacting protein (TXNIP) signaling pathway is crucial in the advancement of sepsis-induced AKI, with M1 exosomes promoting pyroptosis and M2 exosomes inhibiting it [30]. It is widely recognized that Rho-associated coiled-coil containing protein kinase-1 (ROCK1) plays a vital role in various pathological processes, including pyroptosis, inflammation, and endoplasmic reticulum stress (ERS). Wang et al. demonstrated that ROCK1 regulates LPS-induced kidney cell pyroptosis through Toll-like receptor 2 (TLR2)-mediated ERS, thereby hastening the progression of sepsis-induced AKI [31]. The signaling pathways that control pyroptosis in AKI are illustrated in Figure 2.
Pyroptosis in Diabetic Nephropathy
Diabetic nephropathy (DN), also known as diabetic kidney disease (DKD), is a common and severe long-term microvascular complication that arises from damage to the renal glomeruli and tubules. Extensive evidence suggests that chronic inflammation contributes to the development of DN [32]. The involvement of pyroptosis signaling pathways in the progression of DN has become a focal point for researchers and healthcare professionals [33]. In 2020, it was revealed that the TXNIP/NLRP3 axis serves as a critical pathway in the regulation of DN through pyroptosis. Additionally, Ke et al. discovered that the endoplasmic reticulum stress-related factor IRE1α upregulates TXNIP/NLRP3 inflammasome-induced pyroptosis in DN rats [34]. Li et al. made an important discovery regarding the NLRP3/caspase-1/GSDMD signaling pathway in diabetic nephropathy (DN). They found that this pathway was significantly upregulated, leading to a dramatic increase in the secretion of IL-1β and IL-18 in DN mice [35]. Furthermore, they confirmed that SYR, a specific treatment, effectively inhibited the NLRP3/caspase-1/GSDMD pyroptosis pathway by upregulating NRF2 signaling in DN [36].
In another study by Li et al., it was observed that the expression of p-NF-κB, ASC, cleaved-IL-1β, NLRP3, cleaved-caspase-1, and GSDMD-N was elevated in a DN mouse model [37]. Additionally, they confirmed that geniposide (GE) could inhibit the development of DN through the APMK/SIRT1/NF-κB pathway [38]. This suggests that the APMK/SIRT1/NF-κB axis could potentially serve as a new signaling pathway for the treatment of DN. Moreover, the activation of the NLRP3 inflammasome has been implicated in the pathogenesis of DN. Wang et al. demonstrated that under high glucose conditions, the expression of NLRC4, IL-1β, and IL- 18 was increased, leading to pyroptosis in renal tubular epithelial cells [39]. Additionally, Komada et al. showed that the activation of the AIM2 inflammasome by DNA from necrotic cells contributes to chronic kidney injury and pyroptosis [40]. Furthermore, Cheng et al. provided evidence that caspase-11/4- and GSDMD-mediated pyroptosis were activated in a DN mouse model and were involved in the development of DN [41]. These findings collectively confirm the significant roles of pyroptosis and inflammasomes in renal injury, ultimately impacting the pathogenesis of DN.
Pyroptosis in Urinary Tract Cancers
Urinary tract cancers, particularly bladder cancer, are prevalent malignancies [42]. Recent research has highlighted the significant role of pyroptosis in the development and progression of bladder cancer. For instance, He et al. discovered that GSDMB interacts with STAT3, leading to increased phosphorylation of STAT3. This, in turn, upregulates the expression of key glycolytic enzymes such as HK2, LDHA, ENO2, and IGFBP3, promoting glycolysis and cancer cell proliferation in bladder cancer cells [43]. Furthermore, their study revealed that USP24 binds to GSDMB, preventing its degradation in bladder cancer cells [44]. Consequently, the USP24/GSDMB/ STAT3 axis emerges as a potential targetable signaling pathway for bladder cancer therapy. Chen et al. demonstrated through Kaplan- Meier curves that high levels of GSDMB and CASP6 are associated with improved prognoses in bladder cancer patients [45]. They also observed that tumors with elevated GSDMB and CASP6 expression were characterized by immune-inflammation, while those with low levels were immune-desert tumors. Additionally, they elucidated the crucial roles of GSDMB and CASP6 in immune infiltration [46].
El-Gamal et al.’s findings indicated that GSDMD expression is significantly higher in muscle-invasive bladder cancer (MIBC) compared to non-muscle-invasive bladder cancer (NMIBC) and control groups [47]. This suggests the involvement of GSDMD in the pathogenesis of bladder cancer and muscle invasion, with potential implications for predicting local tumor recurrence based on tissue expression levels [48]. Moreover, Peng et al. discovered that CD147 promotes cell proliferation in bladder cancer by upregulating GSDMD expression [49]. Pyroptosis, a form of programmed cell death, has also been implicated in the development of prostate cancer (PCa). The caspase-1 pathway, a classical pyroptosis pathway, plays a significant role in PCa [50]. NLRP3, a protein involved in physiological and pathological processes, including tumor progression, has been found to be elevated in PCa tissues and cell lines. This elevation is positively correlated with the expression of caspase-1 [51]. The NLRP3 inflammasome, activated by NLRP3, has been shown to promote tumor growth in PCa by activating caspase-1.
Additionally, NLRP12, another protein, has been found to be significantly higher in PCa tissue compared to adjacent benign tissue. NLRP12 is believed to play a crucial role in activating NF- κB and IL-1β signaling, contributing to the pathogenesis and progression of PCa [52]. It has been observed that NLRP12 can upregulate caspase-1, IL-1β, and IL-18, thereby promoting the occurrence and progression of PCa. Furthermore, studies have demonstrated the involvement of lipopolysaccharide (LPS) in the proliferation, migration, and invasion of PCa cells. LPS activates the caspase-4/5/11 pathway, leading to pyroptosis induction. However, the role of LPS-mediated pyroptosis in PCa is still under investigation. Moving on to renal cell carcinoma (RCC), it accounts for a small percentage of malignant diseases in adults. RCC is the seventh most common cancer in men and the ninth most common in women. The most prevalent type of RCC is clear cell RCC (ccRCC), followed by papillary RCC and chromophobe RCC. In recent years, the relationship between pyroptosis and the development of RCC has been extensively studied.
Cui et al. conducted a study and discovered that the expression of GSDMB was significantly higher in ccRCC tissues compared to the surrounding normal tissues. Furthermore, they confirmed that the upregulation of GSDMB was strongly associated with immune infiltrates and poor survival in ccRCC . These findings suggest that GSDMB has the potential to serve as a biomarker for poor prognosis and a potential target for immune therapy in ccRCC. Liver X receptors (LXRs), specifically nuclear receptor subfamily 1, group H, member 2 (NR1H2 or LXRB) and nuclear receptor subfamily 1, group H, member 3 (NR1H3 or LXRA), belong to the nuclear receptor superfamily and are expressed in various cells. Wang et al. conducted a study and found that the expression levels of NLRP3, a key component of the NLRP3 inflammasome, were significantly lower in ccRCC tissue compared to normal kidney tissue. They also discovered that LXRα promoted tumor metastasis by downregulating the NLRP3 inflammasome in ccRCC [53].
Additionally, the inhibition of bromodomain-containing 4 (BRD4) has been shown to prevent cell proliferation and epithelialmesenchymal transition (EMT), playing an anti-tumor role in RCC. This is achieved by activating the NF-κB-NLRP3-caspase-1 pyroptosis signaling pathway [54]. Zhang et al. found that the expression of most pyroptosis regulatory genes is positively correlated and plays a significant prognostic role in ccRCC [55]. AIM2, a protein involved in the innate immune response, has been found to play a crucial role in the development of various tumors. Recent studies have shown that AIM2 is highly expressed in ccRCC and promotes tumor development through immune activation pathways Tang et al. conducted a study and discovered that a long non-coding RNA called FOXD2 adjacent opposite strand RNA 1 (FOXD2-AS1) affects the expression of GSDMB and NLRP1. Interestingly, they also found that downregulating the expression of FOXD2-AS1 reduced the proliferation and migration of ccRCC cells [56-64].
Conclusion
Pyroptosis is a recently discovered type of cell death that is controlled by gasdermin proteins, typically activated by caspases. It plays a critical role in the onset, progression, and advancement of urological conditions. Further comprehensive exploration of pyroptosis in urological disorders will enhance our comprehension of diagnosing and treating urinary ailments. There is an urgent need for additional research to conduct more clinical trials and investigate the potential therapeutic applications of pyroptosis in urinary diseases.
References
- Xia X, Wang X, Cheng Z, Qin W, Lei L, et al. (2019) The role of pyroptosis in cancer: pro-cancer or pro-“host”? Cell Death Dis 10(9): 650.
- Rao Z, Zhu Y, Yang P, Chen Z, Xia Y, et al. (2022) Pyroptosis in inflammatory diseases and cancer. Theranostics 12(9): 4310-4329.
- Wang Y, Zhang H, Chen Q, Jiao F, Shi C, et al. (2020) TNF-α/HMGB1 inflammation signaling pathway regulates pyroptosis during liver failure and acute kidney injury. Cell Prolif 53(6): e12829.
- Fink SL, Cookson BT (2007) Pyroptosis and host cell death responses during Salmonella infection. Cell Microbiol 9(11): 2562-2570.
- Shi J, Zhao Y, Wang K, Shi X, Wang Y, et al. (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526(7575): 660-665.
- Shi J, Gao W, Shao F (2017) Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem Sci 42(4): 245-254.
- Thi HTH, Hong S (2017) Inflammasome as a therapeutic target for cancer prevention and treatment. J Cancer Prev 22(2): 62-73.
- Strowig T, Henao-Mejia J, Elinav E, Flavell R (2012) Inflammasomes in health and disease. Nature 481(7381): 278-286.
- Wu J, Fernandes-Alnemri T, Alnemri ES (2010) Involvement of the AIM2, NLRC4, and NLRP3 inflammasomes in caspase-1 activation by Listeria monocytogenes. J Clin Immunol 30(5): 693-702.
- Yu P, Zhang X, Liu N, Tang L, Peng C, et al. (2021) Pyroptosis: mechanisms and diseases. Signal Transduct Target Ther 6(1): 128.
- Ramos-Junior ES, Morandini AC (2017) Gasdermin: a new player to the infammasome game. Biomed J 40(6): 313-316.
- Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, et al. (2016) GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. Embo J 35(16): 1766-1778.
- Shi J, Zhao Y, Wang Y, Gao W, Ding J, et al. (2014) Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514(7521): 187-192.
- Wang Y, Gao W, Shi X, Ding J, Liu W, et al. (2017) Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547(7661): 99-103.
- Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, et al. (2018) Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A 115(46): E10888-e10897.
- Hou J, Zhao R, Xia W, Chang C, You Y, et al. (2020) PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat Cell Biol 22(10): 1264-1275.
- Zhou Z, He H, Wang K, Shi X, Wang Y, et al. (2020) Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368(6494): eaaz7548.
- Liu Y, Fang Y, Chen X, Wang Z, Liang X, et al. (2020) Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci Immunol 5(43): eaax7969.
- Zhang Z, Zhang Y, Xia S, Kong Q, Li S, et al. (2020) Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579(7799): 415-420.
- Daniels AM, Schulte AR, Herndon CM (2018) Interstitial cystitis: an update on the disease process and treatment. J Pain Palliat Care Pharmacother 32(1): 49-58.
- Tudrej KB, Piecha T, Kozłowska-Wojciechowska M (2019) Role of NLRP3 inflammasome in the development of bladder pain syndrome interstitial cystitis. Ther Adv Urol 11: 1756287218818030.
- Deng W, Yang Z, Yue H, Ou Y, Hu W, et al. (2020) Disulfiram suppresses NLRP3 inflammasome activation to treat peritoneal and gouty inflammation. Free Radic Biol Med 152: 8-17.
- Wang X, Fan L, Yin H, Zhou Y, Tang X, et al. (2020) Protective effect of Aster tataricus extract on NLRP3-mediated pyroptosis of bladder urothelial cells. J Cell Mol Med 24(22): 13336-13345.
- McVary KT (2006) BPH: epidemiology and comorbidities. Am J Manag Care 12(5 Suppl): S122-128.
- Kashyap M, Pore S, Wang Z, Gingrich J, Yoshimura N, et al. (2015) Inflammasomes are important mediators of prostatic inflammation associated with BPH. J Inflamm (Lond) 12: 37.
- Ponomareva L, Liu H, Duan X, Dickerson E, Shen H, et al. (2013) AIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancer. Mol Cancer Res 11(10): 1193-1202.
- Miao N, Yin F, Xie H, Wang Y, Xu Y, et al. (2019) The cleavage of gasdermin D by caspase-11 promotes tubular epithelial cell pyroptosis and urinary IL-18 excretion in acute kidney injury. Kidney Int 96(5): 1105-1120.
- Xia W, Li Y, Wu M, Jin Q, Wang Q, et al. (2021) Gasdermin E deficiency attenuates acute kidney injury by inhibiting pyroptosis and inflammation. Cell Death Dis 12(2): 139.
- Liu Y, Minze LJ, Mumma L, Li X, Ghobrial R, et al. (2016) Mouse macrophage polarity and ROCK1 activity depend on RhoA and non-apoptotic Caspase 3. Exp Cell Res 341(2): 225-236.
- Su D, Guan L, Gao Q, Li Q, Shi C, et al. (2017) ROCK1/p53/NOXA signaling mediates cardiomyocyte apoptosis in response to high glucose in vitro and vivo. Biochim Biophys Acta Mol Basis Dis 1863(4): 936-946.
- Wang QL, Xing W, Yu C, Gao M, Deng L (2021) ROCK1 regulates sepsis-induced acute kidney injury via TLR2-mediated endoplasmic reticulum stress/pyroptosis axis. Mol Immunol 138: 99-109.
- Papadopoulou-Marketou N, Paschou SA, Marketos N, Adamidi S, Adamidis A, et al. (2018) Diabetic nephropathy in type 1 diabetes. Minerva Med 109(3): 218-228.
- Moreno JA, Gomez-Guerrero C, Mas S, Sanz A, Lorenzo O, et al. (2018) Targeting inflammation in diabetic nephropathy: a tale of hope. Expert Opin Investig Drugs 27(11): 917-930.
- Ke R, Wang Y, Hong S, Xiao L (2020) Endoplasmic reticulum stress related factor IRE1α regulates TXNIP/NLRP3-mediated pyroptosis in diabetic nephropathy. Exp Cell Res 396(2): 112293.
- Li G, Liu C, Yang L, Feng L, Zhang S, et al. (2023) Syringaresinol protects against diabetic nephropathy by inhibiting pyroptosis via NRF2-mediated antioxidant pathway. Cell Biol Toxicol 39(3): 621-639.
- Li F, Chen Y, Li Y, Huang M, Zhao W (2020) Geniposide alleviates diabetic nephropathy of mice through AMPK/SIRT1/NF-κB pathway. Eur J Pharmacol 886: 173449.
- Wang Y, Gou R, Yu L, Wang L, Yang Z, et al. (2021) Activation of the NLRC4 inflammasome in renal tubular epithelial cell injury in diabetic nephropathy. Exp Ther Med 22(2): 814.
- Komada T, Chung H, Lau A, Platnich J, Beck P, et al. (2018) Macrophage uptake of necrotic cell DNA activates the AIM2 inflammasome to regulate a proinflammatory phenotype in CKD. J Am Soc Nephrol 29(4): 1165-1181.
- Cheng Q, Pan J, Zhou ZL, Yin F, Xie HY, et al. (2021) Caspase-11/4 and gasdermin D-mediated pyroptosis contributes to podocyte injury in mouse diabetic nephropathy. Acta Pharmacol Sin 42(6): 954-963.
- Dobruch J, Oszczudłowski M (2021) Bladder cancer: current challenges and future directions. Medicina (Kaunas) 57(8): 749.
- He H, Yi L, Zhang B, Yan B, Xial M, et al. (2021) USP24-GSDMB complex promotes bladder cancer proliferation via activation of the STAT3 pathway. Int J Biol Sci 17(10): 2417-2429.
- Chen X, Chen H, Yao H, Zhao K, Zhang Y, et al. (2021) Turning up the heat on nonimmunoreactive tumors: pyroptosis influences the tumor immune microenvironment in bladder cancer. Oncogene 40(45): 6381-6393.
- El-Gamal R, Abdelrahim M, El-Sherbiny M, Enan E, Nablaway M (2022) Gasdermin D: a potential mediator and prognostic marker of bladder cancer. Front Mol Biosci 9: 972087.
- Peng J, Jiang H, Guo J, Huang J, Yuan Q, et al (2020) CD147 expression is associated with tumor proliferation in bladder cancer via GSDMD. Biomed Res Int 2020: 7638975.
- Nguyen-Nielsen M, Borre M (2016) Diagnostic and therapeutic strategies for prostate cancer. Semin Nucl Med 46(6): 484-490.
- Xu Z, Wang H, Qin Z, Zhao F, Zhou L, et al. (2021) NLRP3 inflammasome promoted the malignant progression of prostate cancer via the activation of caspase-1. Cell Death Discov 7(1): 399.
- Karan D, Tawfik O, Dubey S (2017) Expression analysis of inflammasome sensors and implication of NLRP12 inflammasome in prostate cancer. Sci Rep 7(1): 4378.
- Xing WY, Zhang ZH, Xu S, Hong Q, Tian Q, et al. (2020) Calcitriol inhibits lipopolysaccharide- induced proliferation, migration and invasion of prostate cancer cells through suppressing STAT3 signal activation. Int Immunopharmacol 82: 106346.
- Rini BI, Campbell SC, Escudier B (2009) Renal cell carcinoma. Lancet 373(9669): 1119-1132.
- Warren AY, Harrison D (2018) WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: standards and controversies. World J Urol 36(12): 1913-1926.
- Cui Y, Zhou Z, Chai Y, Zhang Y (2021) Upregulated GSDMB in clear cell renal cell carcinoma is associated with immune infiltrates and poor prognosis. J Immunol Res 2021: 7753553.
- Bobin-Dubigeon C, Chauvin A, Brillaud-Meflah V, Boiffard F, Joalland MP, et al. (2017) Liver X receptor (LXR)-regulated genes of cholesterol trafficking and breast cancer severity. Anticancer Res 37(10): 5495-5498.
- Wang K, Xu T, Ruan H, Xiao H, Liu J, et al. (2019) LXRα promotes cell metastasis by regulating the NLRP3 inflammasome in renal cell carcinoma. Cell Death Dis 10(3): 159.
- Tan YF, Wang M, Chen ZY, Wang L, Liu Z (2020) Inhibition of BRD4 prevents proliferation and epithelial-mesenchymal transition in renal cell carcinoma via NLRP3 inflammasome-induced pyroptosis. Cell Death Dis 11(14): 239.
- Zhang Y, Chen X, Fu Q, Wang F, Zhou X, et al. (2021) Comprehensive analysis of pyroptosis regulators and tumor immune microenvironment in clear cell renal cell carcinoma. Cancer Cell Int 21(1): 667.
- Zhang X, Wei X, Wang Y, Wang S, Ji C, et al. (2021) Pyroptosis regulators and tumor microenvironment infiltration characterization in clear cell renal cell carcinoma. Front Oncol 11: 774279.
- Tang X, Zhang A, Feng Y, Su Y, Wang X, et al. (2021) A novel pyroptosis-related lncrnas signature for predicting the prognosis of kidney renal clear cell carcinoma and its associations with immunity. J Oncol 2021: 9997185.
-
Samer Younes*. The Implications of Pyroptosis in Conditions Affecting the Genitourinary Tract. Annals of Urology & Nephrology. 4(3): 2024. AUN.MS.ID.000590.
-
Pyroptosis; acute kidney injury (AKI); diabetic nephropathy (DN); prostatic hyperplasia (BPH); genitourinary tract; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






