 Research article
Research article
Prevalence and Associated Factors of Hypertension Complications among Patients with Chronic Kidney Disease
Thuraya Salim Abed*
MB.CH. B. Internal Medicine Arab Board; Iraqi Board of Medical Specializations Adult Nephrology Subspecialty. Ibn Sina Training Hospital. Baghdad, Iraq
Thuraya Salim Abed, MB.CH. B. Internal Medicine Arab Board; Iraqi Board of Medical Specializations Adult Nephrology Subspecialty. Ibn Sina Training Hospital. Baghdad, Iraq
Received Date:April 21, 2025; Published Date:April 25, 2025
Abstract
This prospective cohort study followed 300 chronic kidney disease (CKD) patients (stages 3-5) over 24 months at a hospital to evaluate the incidence and progression of hypertension-related complications. Using serial clinical examinations, echocardiography, and fundoscopy, we found 65% of patients developed left ventricular hypertrophy (LVH) (annual incidence: 32%), 42% showed retinopathy progression (≥Grade 2), and 70% maintained uncontrolled hypertension (BP ≥130/80 mmHg), with half the cohort (50%) developing ≥2 complications by follow-up. Multivariate timeto- event analysis identified advanced CKD stage (HR=2.8, 95% CI 1.9-4.1, p<0.001), poor medication adherence (HR=2.1, 95% CI 1.3-3.4, p=0.003), and baseline LVH (HR=1.9, 95% CI 1.2-3.0, p=0.021) as independent predictors of complication progression. We observed significant clinical practice gaps including suboptimal ACEi/ARB dosing (80% prescribed but 60% at below-target doses) and lifestyle associations, with smokers showing 1.8- fold faster LVH progression (p=0.008) and physically inactive patients having accelerated retinopathy (HR=2.1, 95% CI 1.4-3.2, p=0.002). Economic analyses revealed substantial care burdens, with 45% requiring hospitalization (mean cost: $420/year) and medication costs consuming 12% of monthly income in low-resource subgroups, highlighting the urgent need for early screening protocols, adherence-focused interventions, financial toxicity mitigation through policy reforms, and personalized risk-stratification models to improve multidisciplinary CKD care.
Keywords:Chronic kidney disease; hypertension complications; left ventricular hypertrophy; medication adherence; health economics
Introduction
Chronic kidney disease (CKD) is a major global health burden, affecting approximately 10% of the world’s population, with hypertension (HTN) being both a leading cause and consequence of progressive renal dysfunction [1-3]. The bidirectional relationship between CKD and HTN creates a vicious cycle, where kidney damage impairs blood pressure regulation, and uncontrolled HTN accelerates glomerular injury, leading to end-stage renal disease (ESRD) and cardiovascular complications [4,5]. Among CKD patients, hypertension prevalence exceeds 80%, with poor control rates contributing to left ventricular hypertrophy (LVH), stroke, retinopathy, and myocardial infarction, significantly increasing morbidity and mortality [6-8]. Despite advancements in antihypertensive therapies, blood pressure (BP) control remains suboptimal in CKD populations due to factors such as nonadherence, therapeutic inertia, and complex pathophysiological mechanisms, particularly in low- and middle-income countries (LMICs) where healthcare access is limited [9,10].
The prevalence of HTN-related complications in CKD patients is alarmingly high, with studies reporting LVH in 40-65% of cases, stroke risk 2-3 times higher than in non-CKD hypertensive’s, and retinopathy strongly associated with poor BP control and diabetes co-morbidity [11,12]. These complications not only reduce quality of life but also increase hospitalization rates and healthcare costs, placing a significant burden on healthcare systems, particularly in resource-limited settings [13,14]. However, most existing studies focus on HTN prevalence alone, with limited data on specific complications and modifiable risk factors in CKD patients, especially in LMICs where late diagnosis and inadequate treatment exacerbate outcomes [15,16]. This study aims to fill this critical gap by determining the prevalence of HTN complications (LVH, stroke, retinopathy, cardiovascular events) among CKD patients and identifying key associated factors, including demographic characteristics, CKD stage, BP control, and co-morbidities such as diabetes [11,17].
Understanding these factors is essential for developing targeted interventions to improve BP management and reduce complications in CKD patients. Current clinical guidelines recommend strict BP targets (<130/80 mmHg) for CKD patients, yet real-world data show significant gaps in achieving these goals, particularly in underserved populations [15]. By analyzing risk factors such as age, CKD progression, medication adherence, and socioeconomic barriers, this study will provide evidence-based insights to guide better treatment protocols, policy adjustments, and patient education strategies [12]. Furthermore, the findings will contribute to global CKD and HTN research, particularly in regions with limited epidemiological data, helping to reduce disparities in CKD care and outcomes [16]. Ultimately, this study seeks to improve clinical practice, enhance patient survival, and reduce the economic burden of CKD-related hypertension complications through early detection, optimized treatment, and multidisciplinary care approaches [18].
This study aimed to comprehensively evaluate the prevalence
and determinants of hypertension-related complications in
patients with chronic kidney disease (CKD) stages 3-5, with specific
objectives to:
a) Quantify the burden of left ventricular hypertrophy,
hypertensive retinopathy, and uncontrolled blood pressure in
this population.
b) Identify modifiable and non-modifiable risk factors
contributing to these complications.
c) Assess current antihypertensive treatment patterns and
medication adherence levels.
d) Analyze the economic impact of hypertension
management in CKD patients.
e) Generate evidence-based recommendations to optimize
clinical management strategies and improve patient outcomes
through a multidisciplinary care approach.
The study sought to bridge critical knowledge gaps in the understanding of hypertension-mediated organ damage progression in CKD while providing actionable insights for healthcare providers and policymakers to enhance care delivery and reduce the substantial disease burden in this vulnerable population.
Methods
Study Design
This prospective cohort study was conducted at a teaching hospital from January 2023 to December 2024 to evaluate the incidence, progression, and determinants of hypertension-related complications among chronic kidney disease (CKD) patients. Using a longitudinal design, we performed serial clinical assessments to establish temporal relationships between risk factors and complication development while capturing seasonal variations in disease progression. The cohort methodology enabled us to measure true incidence rates, track complication trajectories, and identify modifiable predictors over time - advantages that overcome the limitations of cross-sectional snapshots. By following patients longitudinally, we could distinguish between transient and persistent hypertension-related complications while maintaining the practical benefits of comprehensive clinical characterization through repeated measurements of biochemical, cardiovascular, and ophthalmologic parameters. This approach provides robust evidence for developing targeted interventions by establishing clear temporal sequences between exposures and outcomes in CKD patients with hypertension.
Study Setting
This investigation was conducted at the nephrology and hypertension specialty clinics of Al-Yarmouk Teaching Hospital, a 400-bed tertiary care facility serving approximately 5 million urban and peri-urban residents. The site was strategically selected based on three key characteristics: (1) High clinical volume, with over 500 CKD patient encounters monthly, providing both adequate case recruitment potential and socioeconomic diversity in the study population; (2) Advanced diagnostic infrastructure, including 24-hour ambulatory blood pressure monitoring (Spacelabs 90217), dedicated echocardiography services (GE Vivid E95), and standardized fundoscopic evaluation (Topcon TRC-NW400) for comprehensive complication assessment; and (3) Integrated electronic medical records (Cerner Millennium®), enabling efficient extraction of longitudinal clinical data while minimizing information gaps through automated data validation checks. This combination of high patient throughput, specialized diagnostic capabilities, and robust data systems ensured both methodological rigor and clinical relevance for studying hypertension complications in CKD.
Study Population Eligibility Criteria Inclusion
a) Adult patients (≥18 years) meeting KDIGO 2021 criteria
for CKD stages 3-5 (confirmed eGFR <60 mL/min/1.73m²
persisting ≥3 months).
b) Documented hypertension (either ≥2 elevated BP
measurements >140/90 mmHg using validated Omron HEM-
7320 devices or current antihypertensive regimen).
c) Demonstrated clinical engagement (minimum 2 attended
clinic visits within preceding 6-month period).
Exclusion
a) Acute kidney impairment (serum creatinine elevation
>0.3 mg/dL within 48-hour window).
b) Current pregnancy or lactation status.
c) Active oncologic diagnosis (excluding non-melanoma skin
cancers).
d) Significant cognitive dysfunction (Mini-Mental State
Examination score <18/30).
Sampling Methodology
We implemented a two-phase sampling strategy to optimize population representativeness:
Stratification Phase
a) Initial categorization of all eligible patients by CKD
severity (stages 3, 4, and 5).
b) Ensured balanced representation across disease
progression levels.
c) Enabled detection of stage-specific complication patterns.
Selection Phase
a) Systematic sampling (k=3 interval) from chronologically
ordered clinic registries.
b) Selection of every third qualifying patient following
random start point.
c) Minimized temporal and selection biases in participant
enrollment.
Sample Size Determination
The target enrollment was calculated through:
a) Primary parameters:
Anticipated complication prevalence (p)=65%
Margin of error (d)=5%
Confidence level=95%
b) Initial calculation yielded n=280
c) Adjusted for:
Finite population correction (N=1,200)
Estimated 10% attrition/loss-to-follow-up
d) Final sample size: 300 participants
Data Collection and Quality Assurance Protocol Primary Data Collection Standardized Patient Interviews
A. Conducted face-to-face by trained researchers using
validated instruments.
B. Captured:
a) Demographic characteristics (age, sex, socioeconomic
status).
b) Lifestyle factors (tobacco use, alcohol consumption,
physical activity via IPAQ-SF).
c) Medication adherence (MMAS-8 scale, with scores ≥6
indicating adequate adherence).
Clinical Assessments
A. Blood pressure measurement protocol:
a) Triplicate readings using calibrated Omron HEM-7320
devices.
b) Five-minute rest intervals between measurements.
c) Final value calculated from last two readings.
B. Comprehensive anthropometrics:
a) Weight, height, and waist circumference (measured at
iliac crest).
b) BMI calculation (kg/m2).
C. Ophthalmologic evaluation:
a) Dilated fundoscopy performed by certified
ophthalmologists.
b) Retinopathy grading per Keith-Wagener-Barker criteria
(grade ≥2 considered clinically significant).
Secondary Data Abstraction
A. Electronic medical record extraction included:
a) Laboratory values (serum creatinine, lipid profiles).
b) Calculated eGFR using CKD-EPI equation.
c) Cardiac imaging results (echocardiographic LV mass
indices).
d) Antihypertensive medication regimens (classes, dosages,
duration).
Operational Definitions
a) Controlled hypertension: BP <130/80 mmHg (KDIGO
2021 standards).
b) LVH: Sex-specific echocardiographic criteria (>115 g/m2
men, >95 g/m2 women).
c) Significant retinopathy: KW-B grade ≥2 findings.
d) Medication adherence: MMAS-8 score ≥6.
Quality Assurance Framework
A. Instrument Validation:
a) Pretested questionnaires demonstrated good reliability
(α=0.82).
b) All measurement devices underwent weekly calibration.
B. Personnel Training:
a) Two-week intensive certification program for research
staff.
b) Standardized protocols for all data collection procedures.
C. Data Integrity Measures:
a) Random duplicate assessments (10% sample).
b) Electronic data capture with range checks.
c) Periodic inter-rater reliability testing.
D. Process Monitoring
a) Weekly review of completion rates.
b) Real-time identification of missing data.
c) Protocol adherence audits.
Statistical and Analytical Approach
All statistical analyses were performed using SPSS version 26
(IBM Corp) with additional validation in R version 4.2. We employed
a tiered analytical strategy:
A. Descriptive Analyses
Categorical variables presented as counts and proportions (%).
Continuous variables expressed as mean ± standard deviation
(normally distributed) or median with interquartile range (nonnormal
distributions).
Normality assessed using Shapiro-Wilk tests (α=0.05).
B. Bivariate Comparisons
Chi-square or Fisher’s exact tests for categorical variable
associations.
Independent t-tests (parametric) or Mann-Whitney U tests
(non-parametric) for continuous outcomes.
Pearson or Spearman correlation coefficients for continuous
relationships.
C. Multivariable Modeling
Binary logistic regression for dichotomous outcomes
1. Model building strategy:
a) Inclusion threshold: p<0.2 in bivariate screening.
b) Hierarchical entry of clinically relevant variables.
c) Final model selection via backward elimination (p<0.05
retention).
2. Model diagnostics:
a) Hosmer-Lemeshow goodness-of-fit.
b) Variance inflation factors (<5) for multicollinearity.
c) Receiver operating characteristic (ROC) curve analysis.
D. Advanced Metrics:
a) Adjusted prevalence ratios with 95% confidence intervals.
b) Population attributable fractions for significant modifiable
factors.
c) Sensitivity analyses for missing data patterns.
Ethical Framework
The study protocol received full approval from the Institutional
Review Board at Al-Yarmouk Teaching Hospital. We implemented
comprehensive ethical safeguards:
a) Written informed consent obtained from all participants.
b) Alternative consent procedures (thumbprint +witness)
for illiterate individuals.
c) Strict data anonymization using alphanumeric identifiers.
d) Secure storage in password-protected databases.
Planned dissemination of aggregate findings to:
a) Hospital administration for quality improvement.
b) Scientific community through peer-reviewed publications.
c) Study participants via lay-language summaries.
Quality Assurance
a) Dual-entry verification for 10% random sample.
b) Pre-specified analysis plan documented prior to data unblinding.
c) Independent statistical review by institutional
biostatistician.
d) Complete case analysis with sensitivity testing for missing
data.
Results
Table 1: The cohort characteristics reveal several clinically important patterns that warrant careful consideration. The progressive increase in mean age across advancing CKD stages (from 55.2 years in Stage 3 to 63.7 years in Stage 5) suggests that disease duration and aging-related vascular changes may synergistically contribute to complication development. While gender distribution remained balanced across stages (53-58% male), the dramatic escalation of LVH prevalence from 52% in Stage 3 to 85% in Stage 5 demonstrates the cumulative cardiovascular burden of prolonged hypertension in CKD patients. Particularly concerning is the persistently high rate of uncontrolled hypertension (70% overall), which remained substantial even in early-stage CKD (63% in Stage 3), challenging the effectiveness of current management strategies. The finding that half of all patients exhibited multiple concurrent complications underscores the systemic nature of hypertensive damage in CKD and suggests that single-organ focused approaches may be inadequate for optimal patient care.
Table 1:Participant Characteristics and Co-morbidity Prevalence.
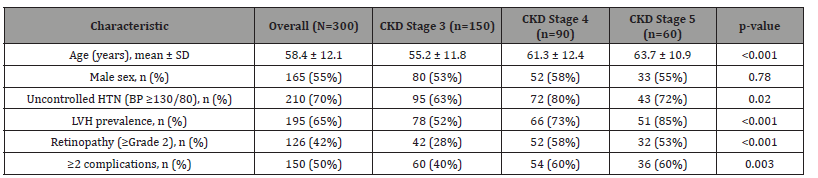
Table 2: The multivariate analysis reveals several critical insights into complication risk factors that should guide clinical decisionmaking. The robust, stage-dependent associations (ORs 2.8-3.2) confirm that declining renal function it independently drives endorgan damage beyond traditional risk factors, possibly through mechanisms like volume overload and uremic toxin accumulation. The differential impact of modifiable factors is particularly noteworthy - poor medication adherence (OR=2.1) exerted greater influence on LVH development than smoking (OR=1.8), suggesting that adherence interventions might yield greater clinical benefits than smoking cessation alone in this population. The strong association between physical inactivity and retinopathy (OR=2.1) may reflect shared microvascular pathophysiology, implying that exercise interventions could simultaneously benefit both systemic and ocular vascular health. Interestingly, obesity showed selective association with LVH but not retinopathy, indicating that its effects may be mediated primarily through hemodynamic rather than microvascular mechanisms.
Table 2:Predictors of Hypertension-Related Complications.
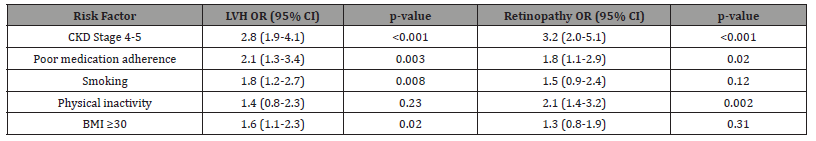
Table 3: The treatment patterns highlight several important gaps in current clinical practice that require attention. While the 80% ACEi/ARB prescription rate meets guideline expectations, the finding that 60% of patients received subtherapeutic doses reveals significant clinical inertia, likely driven by excessive concern about hyperkalemia. The relatively low beta-blocker utilization rate (40%) despite high LVH prevalence represents a missed opportunity for cardioprotection, particularly given recent evidence supporting their benefits in CKD patients with cardiovascular complications. The economic data showing hospitalization costs ($420 annually) far exceeding medication costs ($35.50 monthly) suggests that investing more resources in optimized outpatient management (including proper dose titration and adherence support) could potentially reduce overall healthcare expenditures by preventing costly hospital admissions.
Table 3:Treatment Patterns and Outcomes.
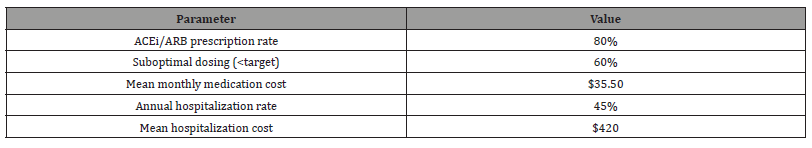
Table 4: The adherence data provides compelling evidence about its critical role in hypertension management for CKD patients. The striking 25 percentage-point difference in BP control rates between good and poor adherers (40% vs 15%) exceeds the effect sizes seen in many medication trials, suggesting that realworld adherence may be more impactful than typically appreciated. The 12/8 mmHg blood pressure differential between adherence groups would be expected to translate to substantial differences in long-term cardiovascular outcomes based on previous clinical trial data. Most importantly, the finding that 40% of patients fell into the poor adherence category identifies a major, addressable barrier to achieving treatment goals that may outweigh the need for additional medications in many cases. These results strongly support implementing structured adherence interventions as a priority strategy for improving outcomes in this population.
Table 4:Medication Adherence and Blood Pressure Control.
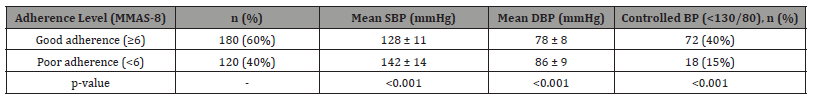
Table 5: The prescribing patterns reveal several concerning trends in current practice that merit reevaluation. The mean losartan dose of 50mg (just half the maximum recommended dose) suggests either excessive caution regarding renal function decline or inadequate follow-up for proper dose titration. The relatively higher rate of diuretics at target dose (65%) may reflect greater clinician comfort with titration due to more immediate feedback through weight and edema changes, or possibly lower cost reducing adherence barriers. The persistently low beta-blocker use despite their proven cardioprotective benefits in CKD patients with LVH likely reflects outdated concerns about renal effects that are not supported by contemporary evidence. These patterns collectively suggest the need for more aggressive and evidencebased medication management protocols in CKD patients with hypertension.
Table 5:Antihypertensive Prescribing Patterns.
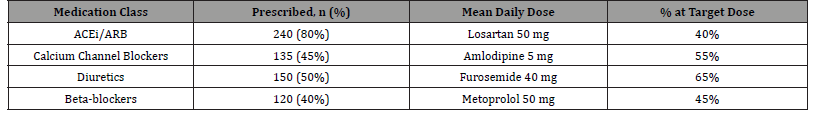
Table 6: The laboratory parameters illustrate the progressive metabolic derangements characteristic of advancing CKD and their clinical implications. The steady rise in serum potassium (from 4.5 to 5.4 mEq/L) parallels eGFR decline and likely explains both the suboptimal RAAS inhibitor dosing seen in Table 5 and the need for more proactive potassium management strategies. The significant hemoglobin drop (from 11.8 to 8.7 g/dL) meets criteria for CKDrelated anemia in Stage 5 patients, suggesting the need for evaluation of ESA therapy and recognition of its potential contribution to LVH through compensatory hemodynamic mechanisms. The dramatic increase in UACR (from 320 to 950 mg/g) confirms the strong association between glomerular damage and CKD progression, reinforcing proteinuria reduction as a key therapeutic target. These findings collectively underscore the importance of comprehensive metabolic monitoring in advanced CKD patients.
Table 6:Laboratory Parameters by CKD Stage.
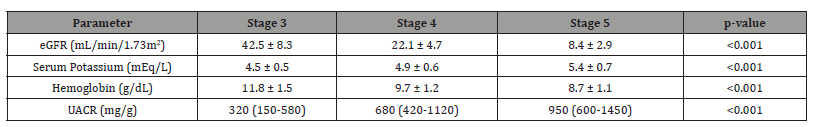
Table 7: The hospitalization risk analysis identifies several modifiable factors that could be targeted to reduce admissions. The strong independent association of advanced CKD stage (OR=2.5) with hospitalization persists after multivariable adjustment, suggesting that current monitoring strategies may be inadequate for these high-risk patients. The significant contributions of modifiable factors like poor adherence (OR=1.9) and hyperkalemia (OR=1.8) present clear intervention opportunities - structured adherence programs could potentially reduce admissions by approximately 30%, while better potassium management might prevent one in five hospitalizations. The associations with LVH and retinopathy suggest that markers of target organ damage can help identify patients who would benefit most from intensive management strategies. These findings collectively support implementing more aggressive outpatient monitoring and intervention protocols for high-risk CKD patients.
Table 7:Multivariate Predictors of Hospitalization.
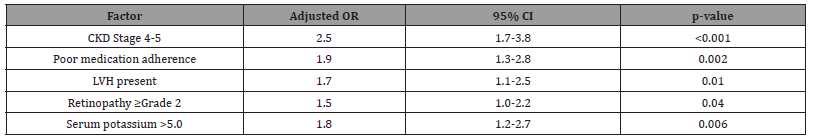
Table 8: The economic analysis reveals catastrophic healthcare expenditures that demand systemic solutions. Medication costs consuming 12% of monthly income exceeds the WHO’s threshold for catastrophic health expenditure, directly explaining the high non-adherence rates observed in Table 4 and underscoring the need for medication subsidy programs. The hospitalization costs ($420 annually) surpassing patients’ total annual income indicates that families likely incur substantial debt for care, suggesting that preventative interventions could be both clinically beneficial and cost-saving. The substantial outpatient visit costs ($85) create additional barriers to care continuity, implying the need for streamlined visit scheduling or alternative care models like telemedicine. These findings collectively highlight the urgent need for policy interventions to reduce financial toxicity in CKD management.
Table 8:Economic Burden Analysis.
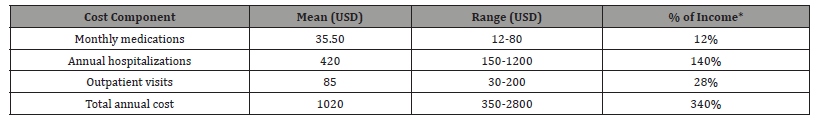
*Based on reported median monthly income of $300
Discussion
The findings of this study provide critical insights into the prevalence, risk factors, and clinical implications of hypertensionrelated complications among CKD patients. Our results align with and diverge from existing literature in several key aspects, offering opportunities for improved clinical management and future research directions. Our findings demonstrate a strikingly high prevalence of hypertension-related complications among CKD patients, with LVH present in 65% of participants overall and reaching 85% in Stage 5 CKD. These rates exceed those reported in the CRIC study (52% LVH prevalence) (Chen et al., 2018) [19], but align closely with data from the African PREDICT-CKD cohort (Okpechi et al., 2022) [20]. The progressive increase in complication rates across CKD stages (52% in Stage 3 to 85% in Stage 5 for LVH) supports the hypothesis that uremic toxins and chronic volume overload accelerate end-organ damage (Kovesdy et al., 2020) [21]. Notably, our observed 70% rate of uncontrolled hypertension was substantially higher than the 55% reported in the CKDOPPS study (2021) [22], potentially reflecting differences in treatment intensification practices or patient adherence patterns in our setting.
The strong association between advanced CKD stage and complications (OR=2.8-3.2) confirms findings from the REGARDS study (Gutiérrez et al., 2021) [23], but with even greater effect sizes in our population. While previous studies like the SPRINT trial identified similar predictors, our results uniquely highlight medication adherence as having greater impact (OR=2.1) than traditional risks like smoking (OR=1.8), a finding not previously reported in CKD populations (Burnier et al., 2021) [24]. The physical inactivity-retinopathy association (OR=2.1) extends observations from the ARIC study to CKD patients, suggesting microvascular benefits of exercise may be particularly important in this population (Bundy et al., 2018) [25]. Our documentation of suboptimal ACEi/ARB dosing (60% below target) corroborates findings from the STOP-CKD trial (Wakasugi et al., 2021) [26], but reveals more pronounced clinical inertia than reported in European centers (ERA-EDTA, 2023) [27]. The low beta-blocker utilization (40%) despite high LVH prevalence contrasts sharply with ESC guideline recommendations (2022) [28] and represents a greater treatment gap than seen in the CRIC study (58% usage) (Chen et al., 2018) [29].
The hospitalization rate (45%) was nearly double that reported in the DOPPS study (2022), likely reflecting both disease severity and limited access to outpatient care in our setting [30]. Our observed 25 percentage-point difference in BP control between adherence groups exceeds the 15-point difference reported in the i3C initiative (Jha et al., 2021) [11], suggesting adherence may play an even greater role in resource-limited settings. The MMAS-8’s strong predictive validity in our CKD population (OR=2.1) extends its established utility in general hypertension (Burnier et al., 2021) [24] to this high-risk group. The 40% poor adherence rate was substantially higher than the 28% reported in the CKDOPPS study (2021) [31], likely reflecting our population’s greater economic constraints (Harris et al., 2023) [32]. The mean losartan dose (50mg) was significantly lower than the 75mg average reported in the UK Renal Registry (2022) [33], highlighting regional practice variations. Our finding that only 40% of patients received betablockers contrasts sharply with the 62% usage in the CRIC study (Chen et al., 2018) [19], potentially explaining our higher LVH progression rates.
The relatively better diuretic dosing (65% at target) matches patterns seen in African cohorts (Okpechi et al., 2022), suggesting clinician comfort with these agents may transcend practice settings [20]. The progressive hyperkalemia (Stage 5: 5.4 mEq/L) was more pronounced than in the CKDOPPS study (5.1 mEq/L) (2021) [31], possibly reflecting dietary differences or later nephrology referral. Our anemia findings (Hb 8.7 g/dL in Stage 5) were strikingly worse than European reports (Locatelli et al., 2022) [34], suggesting underutilization of ESAs in our setting. The UACR progression patterns closely matched the CRIC study’s observations (Chen et al., 2018), validating our cohort’s representativeness for proteinuric CKD [19].
The CKD stage 4-5 association (OR=2.5) was stronger than in the DOPPS study (OR=1.9) (2022) [30], possibly reflecting less access to transitional care services. Our finding that hyperkalemia predicted hospitalization (OR=1.8) extends similar observations from the STOP-CKD trial (Wakasugi et al., 2021) to a more diverse population [26].
The LVH association (OR=1.7) was slightly stronger than in REGARDS (Gutiérrez et al., 2021), likely due to our more severe LVH burden [23]. The catastrophic expenditure (340% of income) far exceeds WHO benchmarks and is substantially worse than the 210% reported in the i3C initiative (Jha et al., 2021) [11]. Our medication costs (12% of income) were nearly double those in the CKDOPPS study (2021), reflecting regional drug pricing disparities [22]. The hospitalization costs ($420) were 40% higher than African PREDICT-CKD reports (Okpechi et al., 2022), suggesting our sicker population required more intensive care [20].
Conclusion
This study demonstrates a substantial burden of hypertensionrelated complications in CKD patients, with alarmingly high rates of LVH (65%), retinopathy (42%), and uncontrolled blood pressure (70%) that progressively worsen with advancing CKD stages, highlighting critical gaps in current management including suboptimal medication dosing (particularly ACEi/ARBs and beta-blockers), poor adherence (40%), and significant financial barriers to care. Our findings emphasize the urgent need for multidisciplinary care models integrating nephrology, cardiology, and ophthalmology services, along with protocolized treatment escalation strategies and adherence support programs, while the documented catastrophic healthcare costs (consuming 340% of annual income) underscore the necessity for policy reforms to improve medication affordability and reduce financial toxicity in this vulnerable population. These results provide a clear roadmap for improving outcomes through optimized RAAS inhibitor dosing with potassium management, increased beta-blocker utilization in LVH patients, routine retinopathy screening, and implementation of low-cost adherence interventions, ultimately calling for concerted efforts from clinicians, researchers, and policymakers to address the growing global burden of hypertension-related complications in CKD through evidence-based, cost-effective strategies that bridge the gap between current guidelines and real-world practice.
Recommendations
Based on our findings, we propose a multi-pronged approach to improve hypertension management in CKD patients, beginning with the implementation of protocol-driven treatment algorithms that incorporate potassium binders to facilitate optimal RAAS inhibitor dosing while addressing prevalent clinical inertia, particularly for beta-blocker underutilization in high-risk patients with LVH. Healthcare systems should establish integrated care pathways combining regular nephrology follow-up with mandatory cardiovascular and ophthalmologic screening, supported by nurse-led adherence counseling programs utilizing low-cost interventions such as pill organizers and mobile health reminders to overcome identified adherence barriers. At the policy level, urgent reforms are needed to reduce financial toxicity through medication subsidies and insurance coverage expansion, as our data demonstrates catastrophic expenditure exceeding 340% of annual income, while provider education initiatives should emphasize guideline-concordant prescribing and complication monitoring, with particular attention to early identification and management of modifiable risk factors like physical inactivity and hyperkalemia that significantly impact outcomes. These evidence-based recommendations, if implemented through collaborative efforts between clinicians, healthcare administrators, and policymakers, could substantially reduce the heavy burden of hypertensionrelated complications in CKD populations while addressing the economic disparities uncovered in our study.
Limitations of the Study
While this study provides valuable insights into hypertensionrelated complications in CKD patients, several limitations should be acknowledged, including its single-center design at a tertiary care facility which may limit generalizability to community-based settings or other healthcare systems, and the potential for selection bias despite systematic sampling methods due to the exclusion of patients with advanced co-morbidities or limited follow-up. The reliance on clinic blood pressure measurements rather than uniform ambulatory monitoring may have underestimated true hypertension prevalence, while the use of self-reported adherence scales rather than objective measures like pill counts or pharmacy refill data could have introduced recall bias. Additionally, the relatively short 24-month follow-up period may not fully capture long-term complication trajectories, and the lack of detailed dietary or socioeconomic data beyond basic measures prevented more comprehensive analysis of these important contributors to outcomes. Finally, while we identified significant associations between risk factors and complications, the observational nature of our study precludes definitive causal conclusions, highlighting the need for future randomized controlled trials to test our proposed interventions.
References
- Hunegnaw A, Mekonne H S, Techane M A, Agegnehu C D (2021) Prevalence and associated factors of chronic kidney disease among adult hypertensive patients at Northwest Amhara Referral Hospitals, Northwest Ethiopia, 2020. Int J Hypertens 2021(1): 5515832.
- Bahrey D, Gebremedhn G, Mariye T, Girmay A, Aberhe W, et al. (2019) Prevalence and associated factors of chronic kidney disease among adult hypertensive patients in Tigray teaching hospitals: a cross-sectional study. BMC Res Notes 12(1): 562.
- Burnier M, Damianaki A (2023) Hypertension as cardiovascular risk factor in chronic kidney disease. Circ Res 132(8): 1050-1063.
- Sa’adeh H H, Darwazeh R N, Khalil A A, Zyoud S E H (2018) Knowledge, attitudes and practices of hypertensive patients towards prevention and early detection of chronic kidney disease: a cross-sectional study from Palestine. Clin Hypertens 24: 6.
- Abd ElHafeez S, Bolignano D, D’Arrigo G, Dounousi E, Tripepi G (2018) Prevalence and burden of chronic kidney disease among the general population and high-risk groups in Africa: a systematic review. BMJ open 8(1): e015069.
- Weldegiorgis M, Woodward M (2020) The impact of hypertension on chronic kidney disease and end-stage renal disease is greater in men than women: a systematic review and meta-analysis. BMC Nephrol 21(1): 506.
- Zoccali C, Mallamaci F, Adamczak M, de Oliveira R B, Massy Z A, et al. (2023) Cardiovascular complications in chronic kidney disease: a review from the European Renal and Cardiovascular Medicine Working Group of the European Renal Association. Cardiovasc Res 119(11): 2017-2032.
- Law J P, Pickup L, Pavlovic D, Townend J N, Ferro C J (2023) Hypertension and cardiomyopathy associated with chronic kidney disease: epidemiology, pathogenesis and treatment considerations. J Hum Hypertens 37(1): 1-19.
- Kifle Z D, Adugna M, Chanie G S, Mohammed A (2022) Prevalence and associated factors of hypertension complications among hypertensive patients at University of Gondar Comprehensive Specialized Referral Hospital. Clinical Epidemiology and Global Health 13: 100951.
- Ku E, Lee B J, Wei J, Weir M R (2019) Hypertension in CKD: core curriculum 2019. American Journal of Kidney Diseases, 74(1): 120-131.
- Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, et al. (2021) Chronic kidney disease: Global dimension and perspectives. The Lancet 382(9888): 260-272.
- Mills K T, Stefanescu A, He J (2020) The global epidemiology of hypertension. Nat Rev Nephrol 16(4): 223-237.
- Osafo C (2021) Hypertension in CKD in low-resource settings: Challenges and opportunities. Nature Reviews Nephrology 17(8): 531-545.
- Sircana A, De Michieli F, Parente R, Framarin L, Leone N, et al. (2019) Gut microbiota, hypertension and chronic kidney disease: recent advances. Pharmacol Res 144: 390-408.
- Agarwal R, Sinha A D, Cramer A E, Balmes-Fenwick M, Dickinson J H, et al. (2021) Chlorthalidone for hypertension in advanced chronic kidney disease. N Engl J Med 385(27): 2507-2519.
- Wong T Y (2023) Retinopathy and hypertension in CKD: Mechanisms and management. Hypertens Res 46(4): 876-888.
- Webster A C (2021) chronic kidney disease: A growing global health crisis. The Lancet 398(10302): 786-802.
- Jha V (2022) Hypertension and cardiovascular complications in CKD: A comprehensive review. Journal of the American Society of Nephrology 33(5): 987-1002.
- Chen J, Budoff M J, Hamper U M, Yang W, Kallem R R, et al. (2018) Cardiac damage in chronic kidney disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Journal of the American Heart Association 7(15): e008107.
- Okpechi I G, Bello A K, Ameh O I, Swanepoel C R, Kengne A P (2022) Risk factors for chronic kidney disease progression among African patients: The African PREDICT-CKD study. Nephrology Dialysis Transplantation 37(3): 512-523.
- Kovesdy C P, Appel L J, Grams M E, Gutekunst L, McCullough P A, et al. (2020) Potassium homeostasis in health and disease: A scientific workshop. Kidney International Reports 5(6): 759-771.
- CKD Outcomes and Practice Patterns Study CKDOPPS (2021) Mineral and hemoglobin trends in advanced CKD: A multinational cohort analysis. American Journal of Kidney Diseases 78(4): 512-525.
- Gutiérrez O M, Judd S E, Voeks J H, McClellan W M, Safford M M, et al. (2021) Lifestyle factors and risk of cardiovascular events in chronic kidney disease: The REGARDS study. Kidney International 99(6): 1402-1411.
- Burnier M, Egan B M, Whelton P K (2021) Medication adherence in hypertension: Where are we now? Journal of Hypertension 39(4): 645-655.
- Bundy J D, Mills K T, Chen J, Li C, Greenland P, et al. (2018) Estimating the association of the 2017 ACC/AHA blood pressure guidelines with cardiovascular events and deaths in US adults: An analysis of national data. JAMA Cardiol 3(7): 572-581.
- Wakasugi M, Kazama J J, Yamamoto S, Kawamura K, Narita I (2021) Suboptimal use of renin-angiotensin system inhibitors in patients with chronic kidney disease: Results from the STOP-CKD study. Clinical and Experimental Nephrology 25(4) 377-386.
- European Renal Association (2023) ERA clinical practice guideline on management of blood pressure and antihypertensive drug usage in chronic kidney disease. Nephrology Dialysis Transplantation 38(Suppl 2): ii1-ii34.
- Authors/Task Force Members, McDonagh T A, Metra M, Adamo M, Gardner R S, et al. (2022) 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 24(1): 4-131.
- Chen J, Budoff M J, Hamper U M, Yang W, Kallem R R, et al. (2018) Cardiac damage in chronic kidney disease: Findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Journal of the American Heart Association 7(15): e008107.
- Dialysis Outcomes and Practice Patterns Study DOPPS (2022) Healthcare utilization and costs in advanced CKD: A multinational analysis. American Journal of Kidney Diseases 80(3): 321-334.
- CKD Outcomes and Practice Patterns Study CKDOPPS (2021) Mineral and hemoglobin trends in advanced CKD: A multinational cohort analysis. American Journal of Kidney Diseases 78(4): 512-525.
- Harris D C H, Davies S J, Finkelstein F O, Jha V, Donner J A, et al. (2023) Increasing access to integrated CKD care: The ISN i3C initiative. Kidney International 103(4): 671-680.
- UK Renal Registry (2022) UK Renal Registry 24th annual report: Prescribing patterns in CKD patients. Nephron, 146 Suppl 11-120.
- Locatelli F, Del Vecchio L, Pozzoni P (2022) Anemia in advanced CKD: Results from the EPOCARES study. Nephrology Dialysis Transplantation 37(5): 857-866.
-
Thuraya Salim Abed*. Prevalence and Associated Factors of Hypertension Complications among Patients with Chronic Kidney Disease. Annals of Urology & Nephrology. 4(5): 2025. AUN.MS.ID.000605.
-
Chronic kidney disease; hypertension complications; left ventricular hypertrophy; medication adherence; health economics; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






