 Research Article
Research Article
Etelcalcetide in Hemodialysis: Better Secondary Hyperparathyroidism Control and Gastrointestinal Tolerance in the Clinical Practice
Fátima Moreno Guzmán1, Vicent Esteve Simó1*, Irati Tapia González1, Gemma Martínez Calvo2, Alicia Madurga Hernández3, Verónica Duarte Gallego1, Anna Saurina Solé1, Monica Pou Potau1 and Manel Ramírez De Arellano Serna1
1Department of Nephrology, Consorci Sanitari de Terrassa, Spain
2Department of Pharmacy, Consorci Sanitari de Terrassa, Spain
3Department of Laboratory, Consorci Sanitari de Terrassa, Spain
Vicent Esteve Simó, Servei Nefrología Hospital de Terrassa Consorci Sanitari Terrassa, Spain
Received Date:November 23, 2023; Published Date:December 04, 2023
Abstract
Background: Etelcalcetide, a new calcimimetic agent for the treatment of secondary hyperparathyroidism (SHPT) in hemodialysis (HD)
patients has been recently approved. Etelcalcetide offers better SHPT control, higher therapeutic compliance, and lower side effects. However, its
use in clinical practice is not widespread.
Objectives: To assess effectiveness, safety, satisfaction degree and gastrointestinal tolerance of etelcalcetide for SHPT treatment in our HD
patients.
Methods: A prospective 16 weeks single-center study in HD patients treated with etelcalcetide for SHPT. Main mineral bone disorder (CKD-MBD)
biochemical parameters, satisfaction degree using a Visual Analogue Scale (VAS), Gastrointestinal Symptom Rating Scale (GSRS) and Gastrointestinal
Quality of Life Index (GIQLI) were analyzed.
Results: 65 HD patients.20 patients included (10 cinacalcet previously), 65% men. Mean age 52.4 ± 16.3 years. A significant decrease in Ca (9.3
± 0.1 vs 8.4 ± 0.1 mg/dl), P (5.9 ± 0.4 vs 4.9 ± 0.4 mg/dl), i-PTH (693 ± 384.1 vs 354.5 ± 235.5 pg/ml) and FGF-23 (3418.5 ± 3236.5 vs 1692.4 ± 1180.6
pg/ml) serum levels were observed at the end of study. Likewise, significant decrease in GSRS (8.7 ± 5.6 vs 5.7 ± 4.4), particularly in indigestion
syndrome (2.6 ± 1.8 vs 1.5 ± 1.3) as well as improvement in GIQLI (126.9 ± 10.7 vs 132.1 ± 9.5), particularly in treatment-related issues (3.2 ± 0.9
vs 3.8 ± 0.5), physical (3.2 ± 0.6 vs 3.4 ± 0.5) and emotional (3.3 ± 0.6 vs 3.7 ± 0.5) items were observed. Asymptomatic hypocalcaemia occurred
in 11.6%, with no dropouts. Compared with previous cinacalcet treatment, etelcalcetide was superior in adequate CKD-MBD control according to
national guidelines, adding improvements in both GSRS (4.2 ± 5.7 vs 1.9 ± 3.6) and GIQLI scores (7.8 ± 7.4 vs 2.4 ± 7.4) as well as VAS (8.7 ± 0.9 vs
6.1 ± 0.8) at the end of study.
Conclusion: Etelcalcetide was safe and effective in the SHPT treatment. Compared with traditional calcimimetics, etelcalcetide results in better
SHPT control and gastrointestinal tolerance respectively, without side effects. We will consider the use of etelcalcetide as the first calcimimetic
option for the SHPT treatment in our HD patients.
Keywords:Calcimimetics; secondary hyperparathyroidism; hemodialysis; gastrointestinal tolerance
Introduction
Chronic kidney disease (CKD) represents a major public health problem due to its high prevalence, its significant cardiovascular morbidity and mortality, and its socioeconomic cost [1]. The therapeutic goals are aimed at reducing and treating complications associated with CKD, such as anemia and secondary hyperparathyroidism (SHPT). SHPT secondary to CKD has been shown to be a direct cause of increased cardiovascular mortality in hemodialysis patients [2,3]. Hypocalcaemia, calcitriol deficiency, accumulation of phosphorus and fibroblast growth factor (FGF 23) in patients with CKD are a few of the many factors that stimulate the synthesis of parathyroid hormone (PTH) leading to various abnormalities: principally osseous, vascular and systemic abnormalities [4-6]. Over the years, a wide range of drugs for the control of SHPT have been marketed, including phosphorus binders, native vitamin D, and vitamin D analogues [7]. The advent of cinacalcet, a calcimimetic agent, represented a major change in the management of SHPT, achieving better results, particularly in patients for whom control was more difficult to achieve [8,9].
Its mechanism of action consists of increasing the sensitivity of the calcium-sensitive receptor located on the surface of the main cell of the parathyroid gland, thus reducing serum concentrations of PTH, calcium and phosphorus [10-13]. The main disadvantage of cinacalcet is poor gastrointestinal tolerance, mainly presenting as nausea and vomiting which lead to poor compliance with treatment [14-17]. Etelcalcetide, a new calcimimetic for the management of SHPT in hemodialysis (HD) patients, has recently become available. The principle advantage is its endovenous administration at the end of HD sessions, thus guaranteeing compliance with treatment and a smaller number of side effects. However, there are few publications about it use in daily clinical practice, and on its gastrointestinal effects [18-20]. This study aims to analyze the effects relating to control of changes in chronic kidney disease and mineral bone disorder (CKD-MBD), and to evaluate gastrointestinal tolerance after the administration of etelcalcetide in normal clinical practice in our hemodialysis-patient population.
Materials and Methods
A 16-week, prospective, single-center study was carried out including patients on the chronic HD program of our hospital, approved by the ethics committee (VES-ETE-2018-01) of our institution and conducted in accordance with the standards of the Helsinki Declaration. The inclusion criteria were patients on a hemodialysis program, affected by SHPT for a period greater than 2 months, being treated using cinacalcet previously or who had initiated treatment with etelcalcetide, and who had provided informed consent. The exclusion criteria were: hypocalcaemia adjusted by albumin <7.8 mg/dl; or those not giving consent. To facilitate hospital provision of care and following approval by the pharmacy committee of our hospital, an agreement was made to introduce etelcalcetide for the management of SHPT in our unit. Therefore, those patients having not previously received treatment, and meeting the requirements for initiation of calcimimetics for the control of SHPT, received treatment with etelcalcetide.
For those patients who previously received treatment with cinacalcet, it was replaced by etelcalcetide after one week of no treatment following the data sheet´s recommendations. The initial dose used in all cases was 2.5 mg administered at the end of each dialysis session, three times a week. A dose adjustment was subsequently made following the parameters recommended by the CKD-MBD Guidelines of the Spanish Nephrology Society (SEN) [21] based on the standard analytical controls and according to the standard clinical practice of each nephrologist. Both at the beginning and at the end of the study, the following data were collected for all patients included in the study: main sociodemographic variables and variables associated with renal disease (sex, age, etiology of kidney failure, time on HD), biochemical parameters related to CKD-MBD (serum levels of calcium (Ca), phosphorus (P), alkaline phosphatase (AP), magnesium (Mg), intact parathyroid hormone (i-PTH), 25 OH vitamin D3, albumin, fibroblast growth factor-23 (FGF-23) and hemodialysis characteristics (KTV according to 2nd-generation Daugirdas formula), dialysate calcium concentration, dry weight and interdialytic weight gain.
In reference to the standard medical treatment in relation to CKD-MBD, data was collected on the type of phosphorus binders (calcium-based binders, non-calcium-based binders, binders with added magnesium and ferric chelators), selective analogues of vitamin D receptors (AsRvD), native vitamin D, calcimimetics (average weekly dose of cinacalcet), proton pump inhibitors and antacids. Gastrointestinal tolerance was assessed by identifying gastrointestinal symptoms using the Gastrointestinal Symptom Rating Scale (GSRS) [22]. It is a specific, useful, and validated instrument for gastrointestinal disease that includes 15 points with a response scale of 0 (best result) to 3 (worst result), grouped into 5 blocks based on different gastrointestinal symptoms. The 5 symptom groups include: reflux, abdominal pain, diarrhea, indigestion, and constipation. As such, lower scores translate to better GI tolerance. The gastrointestinal quality of life was evaluated by the Gastrointestinal Quality of Life Index (GIQLI) test [23].
It is a validated and appropriate test for assessment of the quality of life related to health in patients with digestive complaints. It consists of 36 items with a response scale from 0 (worst result) to 4 (best result) that includes specific questions about digestive symptoms, physical ability, emotional aspects, social relationships, and treatment-related issues. In this case, a higher score relates to a better GI quality of life. Surveillance of the safety profile was carried out by collecting data on the main adverse effects: principally, the presence of hypocalcaemia (< 7.8 mg/dl) and its severity by means of electrocardiographic control tests at the start and end of the study. Other adverse effects evaluated were the presence of decompensated heart failure or acute pulmonary edema, evaluated using clinical assessment as well as dry weight and interdialytic weight gain. The degree of satisfaction was analyzed by means of a qualitative questionnaire, home-made designed, consisting of 4 questions. The first question evaluated the degree of satisfaction overall.
The remaining three questions evaluated treatment-related aspects: gastric tolerance, the significance in the control of CKDMBD, and the hospital dispensation form of calcimimetic. To evaluate these, in each of the questions, a visual analogue scale (VAS) was used with scores and visual representations ranging from 0 (worst score) to 10 (highest satisfaction score). Statistical analysis was carried out using SPSS version 24.0 (SPSS Inc., Chicago, IL, USA). Quantitative variables were expressed using the mean and standard deviation. The qualitative variables were expressed as a percentage. The comparison of quantitative data was carried out using the Wilcoxon test for non-parametric related variables, and the qualitative data was compared using the McNemar test; statistical significance for any comparison was set at a value of p < 0.05.
Results
We analyzed 65 patients who were prevalent on the chronic hemodialysis program at our unit. 20 patients with SHPT were included in the study. 65% were men with an average age of 52.4 ± 16.3 years, with a hemodialysis period of 74.9 ± 42.1 months. The main etiology of kidney failure was diabetic nephropathy, in 45% of cases. The average Charlson index was 7.8 ± 2.9. A total of 10 patients had previously received a calcimimetic treatment (cinacalcet) with an average dose of 264 mg/week (90-420) and a measured time of cinacalcet use of 24.2 ± 10.5 months. The final mean dose of etelcalcetide was 18 ± 7.5 mg/week (7.5-30). In our study, the observed equivalence rate for 30 mg cinacalcet was 2.5 mg/day of etelcalcetide.
Mineral Bone Metabolism
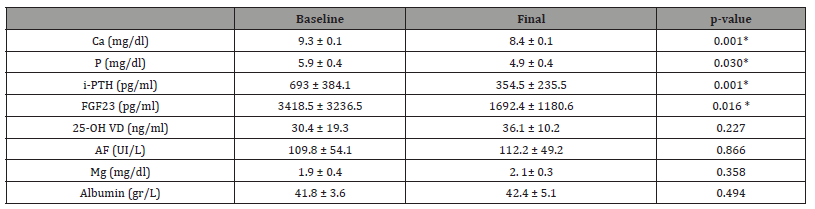
The main biochemical data relating to CKD-MBD are shown in Table 1. At the end of the study, we observed a significant decrease in serum Ca values (9.3 ± 0.1 vs 8.4 ± 0.1 mg/dl; p = 0.001), P (5.9 ± 0.4 vs 4.9 ± 0.4 mg/dl; p = 0.030), i-PTH (693 ± 384.1 vs 354.5 ± 235.5 pg/ml; p = 0.001), and FGF-23 (3418.5 ± 3236.5 vs 1692.4 ± 1180.6 pg/ml; p = 0.016). No significant differences were observed in the levels of 25-OH VD, alkaline phosphatase, magnesium and albumin. Similarly, following initiation of etelcalcetide we observed an increase in the percentage of patients within the optimal range as per the CKD-MBD guidelines from the SEN (Ca 42% vs 67%; P 18% vs 32%; i-PTH 8% vs 43%, respectively). 65% of patients used calcium dialysate concentrations of 2.5 meq/L; 30% used 3.0 meq/ L; and 5% used 3.5 meq/L. At the end of the study, we observed a 15% increase in the use of the calcium dialysate at 3.0 meq/L (30% vs 45%), without changes at the higher calcium dialysate concentrations. No relevant changes were observed in hemodialysis suitability parameters (KtV 1.41 ± 0.2 vs 1.42 ± 0.3, p = 0.821). No significant changes were observed in the percentage of calcium-based phosphorus binders (55% vs 60%), non-calcium-based (70% vs 60%), magnesium-based (5% vs 5%), ferric (10% vs 15%), calcitriol (10% vs 15%), calcifediol (50% vs 35%), and AsRvD (50% vs 40%) over the course of the study.
Table 1:Main CKD-MBD biochemical parameters. Baseline vs Final. Ca: calcium, P: phosphorus, iPTH -intact parathyroid hormone, FGF23: Fibroblast growth factor; 25-OH VD: 25 hydroxy vitamin D; Mg: Magnesium, AF: Alkaline phosphatase. (Mean and standard deviation). Statistical significance: *p<0.05.
Gastrointestinal Tolerance
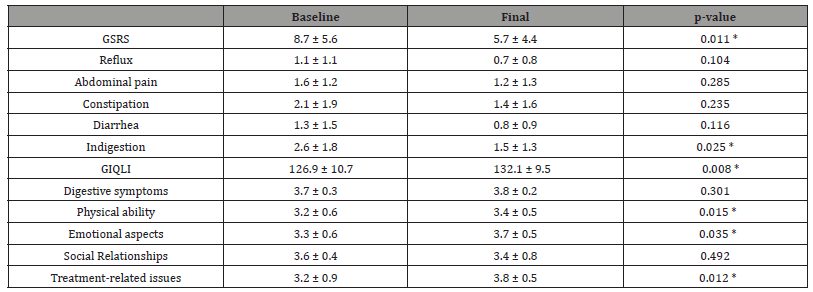
The results for gastrointestinal tolerance and quality of life are shown in Table 2. Overall, we observed a significant improvement in gastrointestinal symptoms (GSRS 8.7 ± 5.6 vs 5.7 ± 4.4; p = 0.011). On analysis of the different dimensions, we observed a lower score in all of them; although the differences were only significant in the indigestion section (2.6 ± 1.8 vs 1.5 ± 1.3; p = 0.025). Similarly, we achieved a significant increase in the gastrointestinal quality of life (GIQLI 126.9 ± 10.7 vs 132.1 ± 9.5; p = 0.008), observing an improvement in all dimensions of that test, with significant results in the sections of: physical ability (3.2 ± 0.6 vs 3.4 ± 0.5; p = 0.015), emotional aspects (3.3 ± 0.6 vs 3.7 ± 0.5; p = 0.035) and treatment-related issues (3.2 ± 0.9 vs 3.8 ± 0.5; p = 0.012). No changes were made in the administration of antacids (10%) or proton pump inhibitors (60%) over the course of the study.
Table 2:Gastrointestinal Symptom Rating Scale (GSRS) and Gastrointestinal Quality of Life Index (GIQLI): Global and specific symptoms or questions in the several dimensions are expressed (mean and standard deviation). Baseline vs final. Statistically significant *p<0.05.
Adverse Effects
11.6% of hypocalcaemias were observed at the end of the study: all were asymptomatic and etelcalcetide was not withdrawn in any case. No relevant changes were detected during electrocardiogram testing (QTc 467.9 ± 31.7 vs 454.1 ± 80.7 mm/s; p = 0.36), PR (165.5 ± 29.1 vs 165.5 ± 23.3 mm/s; p = 0.96), QRS (106.5 ± 23.9 vs 108.8 ± 25.4 mm/s; p = 0.24), dry weight (76.2 ± 19.9 vs 75.6 ± 19.5 kg; p = 0.238) and interdialytic weight gain (3.2 ± 1.2 vs 2.8 ± 0.9 kg; p = 0.156), episodes of decompensated heart failure or acute pulmonary edema.
Degree of satisfaction
Overall, VAS in patients was 8.7 ± 0.9. The VAS for gastric tolerance was 8.9 ± 1.3; for the hospital dispensation form of calcimimetic, the VAS was 9.5 ± 0.8; and for the significance control of CKD-MBD, it was 2.9 ± 1.7, respectively.
Etelcalcetide vs Cinacalcet
With the intention of highlighting the importance of the type of calcimimetic used for the aforementioned aspects, we carried out a comparative sub-analysis between patients who had previously taken cinacalcet (n=10) and those who initiated etelcalcetide (n=10). No significant differences in clinical or biochemical data relating to CKD-MBD were found between these groups. In the group with de novo initiation of etelcalcetide (with no previous treatment with cinacalcet), we observed a higher percentage of patients within the optimal calcium range (Ca 30 vs 60%), phosphors (P 35 vs 40%) and i-PTH (14 vs 43%) as recommended in CKD-MBD Guidelines set out by the SEN (Ca 8.5-9.5 mg/dl, P 3.5-5.5 mg/dl, i-PTH 150-300 pg/ml , avoid < 100 or > 500 pg/ml) (Figure 1). In relation to gastrointestinal symptoms and quality of life, this improvement in GSRS was particularly evident in relation to abdominal pain, indigestion, and constipation. The improvement in GIQLI for this group of patients was mainly related to the dimensions of physical ability, emotional aspects, and treatment-related issues (Figure 2). Globally, we observed a significant improvement, as per the difference (delta) of the mean values at the end of the study, in GSRS (-4.2 ± 1.8 vs -1.9 ± 1.1, p = 0.045) and GIQLI (7.8 ± 2.3 vs 2.4 ± 2.3, p = 0.009) in the previously cinacalcet group. Finally, those patients having previously received treatment with cinacalcet showed a VAS associated with the significance control of CKD-MBD (2.9 ± 0.6) like etelcalcetide. However, the overall VAS score was lower (6.1 ± 0.8), mainly in terms of gastric tolerance (5.4 ± 1.3) and hospital dispensation of cinacalcet (2.7 ± 0.6) with respect to etelcalcetide.
Discussion


In our study, the administration of etelcalcetide was safe and effective for the treatment of SHPT in our patients. In addition, compared to traditional calcimimetics, etelcalcetide achieved better control of SHPT with improved symptoms and gastrointestinal quality of life, without associated adverse effects. Over the years, a wide range of preparations has been marketed for the control of SHPT, with the greatest change in the management of SHPT occurring with the arrival of calcimimetics. Through its mechanism of action, which consists of increasing sensitivity of the calcium-sensitive receptor located on the surface of the main cell of the parathyroid gland, these drugs are able to reduce serum concentrations of PTH, calcium and phosphorus, achieving the best results in patients who have the greatest difficulties in controlling these. Etelcalcetide is a new calcimimetic for the management of SHPT for HD patients. Several studies, generally with declared conflicts of interest, demonstrate some superiority of etelcalcetide with respect to traditional calcimimetics for the control of SHPT [24-26]. However, very few publications are based on their use in daily clinical practice.
Xipell et al [27] conducted a 6-month prospective observational study in which they switched cinacalcet to etelcalcetide in a group of 29 patients with online haemodiafiltration (OL-HDF) program; they showed evidence of better effectiveness of etelcalcetide vs cinacalcet for the control of SHPT, especially in non-compliant patients.
Block et al carried out a double-blind randomized, etelcalcetide and cinacalcet vs placebo study in HD patients, over 26 weeks, which the main objective was to decrease PTH > 30% with respect to baseline levels. A greater reduction in both 30% and 50% target i-PTH concentrations was found in patients randomized to etelcalcetide as compared to cinacalcet. Our results, in patients with similar clinical characteristics and follow-up time, show similar data for the control of SHPT to those obtained by previously published studies. Similarly, our series also showed a significant decrease in FGF23 in patients treated with etelcalcetide, whose importance lies in the effects FGF23 has on vascular calcifications and its impact on cardiovascular morbidity and mortality [28,29].
However, a beneficial clinical effect of etelcalcetide on vascular calcifications in humans has not been demonstrated at present [30]. The improved control obtained with etelcalcetide could, in part, be explained by greater levels of compliance when administered intravenously in dialysis units, the longer half-life, and greater affinity for the calcium receptor located in the parathyroid gland [31,32]. The high morbidity and mortality of patients in HD brings with it a large pharmacological burden and the appearance of various different adverse effects. These may contribute to poor compliance with treatment, with the implications that this entails. The most relevant side effects of calcimimetics occur on a gastrointestinal level; mainly nausea and vomiting associated with high doses for the control of SHPT. Controlled administration post-hemodialysis, night-time administration or administration with food intake are some strategies used to minimize these adverse effects. The expected best gastrointestinal tolerance with endovenous administration of etelcalcetide has not been fully demonstrated.
While some observational studies reported a frequency of gastrointestinal side effects with cinacalcet that was lower with respect to etelcalcetide, the Block et al group, with a population size of 683 patients randomized to etelcalcetide vs cinacalcet during the first 8 weeks of treatment, showed no significant differences in relation to the presence of nausea or vomiting as self-reported by patients. In our study, we observed an improvement in the symptoms and gastrointestinal quality of life following administration of etelcalcetide, evaluated on the basis of approved and validated tests that are easy to apply and interpret in daily clinical practice. This gastrointestinal improvement was reflected in a greater degree of satisfaction as perceived by patients. The improvement in the digestive symptoms observed with etelcalcetide may be related, among others, to the non-daily administration pattern, to the lower dose of calcimimetics used for the control of SHPT, and to administration via an endovenous route, which would mean less interaction with other foods or prescribed drugs, thereby reducing presentations of uncomfortable gastrointestinal symptoms.
On the other hand, the improvement in the gastrointestinal quality of life could be attributed to both the reduced presentations of gastrointestinal symptoms and the lower levels of anxiety caused due to taking a drug that is not well tolerated by the patient. The improvement in the dimension relating to treatment aspects could be explained by the great convenience of post-dialysis administration, avoiding the inconvenience arising due to having to collect the preparations on a monthly basis from the hospital pharmacy of our center; being physically located at a distance from our dialysis unit and with fixed opening hours that do not always coincide with the usual HD sessions, this point should be highlighted for some patients with deteriorated quality of life associated with renal-replacement treatment. The most common adverse effect of calcimimetics is hypocalcaemia, tending to be somewhat more accentuated due to etelcalcetide with respect to cinacalcet, although generally cases are mild and asymptomatic.
In our study, hypocalcaemia occurred mainly in the initial stages of treatment, being always asymptomatic, avoiding the withdraw of etelcalcetide or make significant modifications to the characteristics of the HD, except for necessary dose adjustments and the slight increase in calcium dialysate concentrations in a patient population that usually prescribes calcium dialysate at the lower limit, or in terms of the prescribed medication. Some factors involved in its presentation may include increased treatment compliance due to intravenous administration by nursing staff at the dialysis unit; its longer half-life; and its greater affinity for the calcium receptor in the parathyroid gland. Additionally, our study provides safety data in the form of absence of electrocardiographic alterations, as well as the absence of other less-described adverse effects such as decompensated heart failure or acute pulmonary edema, indirectly assessed based on theoretical weight and interdialytic gain.
Based on our experience, we would recommend starting low dose etelcalcetide with subsequent progressive dose adjustment and close monitoring, especially in the early stages, of values for serum calcium, phosphorus and iPTH. One of the main points to be highlighted in our study is that it was carried out within the framework of standard clinical practice using a detailed working methodology, with no funding, and carrying out treatment modifications based on the recommendations of the CKD-MBD Guidelines from SEN, and according to the discretion of each physician. All these features allow us to provide real data based on the daily management of these patients. On the other hand, we believe that the evaluation of gastrointestinal symptoms and quality of life based on easy-touse validated scales, not used in previous studies, can provide more real and reliable data in relation to gastrointestinal symptoms after administration of calcimimetics. From the numerous limitations of our study, the following should be highlighted: the small number of patients; the non-randomized design of our study; and the short follow-up period.
Due to being carried out within standard clinical practice, the follow-up period as stipulated was established to match the usual analytical controls and standard follow-up periods of our unit. Moreover, etelcalcetide was initiated at 7,5 mg/ week according to our limited experience and in accordance with the data sheet of the drug, so we cannot rule out other results with doses adjusted to baseline i-PTH levels. Studies with a greater number of patients, different designs and with a longer duration would be necessary to confirm increased efficacy, safety and gastrointestinal tolerance following the use of etelcalcetide in the treatment of SHPT in HD patients. Therefore, our results should be interpreted with caution, mainly due to the limited number included in this subgroup of patients.
Conclusion
In conclusion, the use of etelcalcetide was safe and effective for the treatment of SHPT in our patients. Compared to traditional calcimimetics, etelcalcetide achieved improved compliance with treatment, better control of SHPT, and better gastrointestinal tolerance, with no associated side effects in our subgroup of patients. With our results, we will consider the use of etelcalcetide as the first option for calcimimetic treatment for controlling SHPT in our HD patients.
Declaration Of Conflicting Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
References
- Ortiz A, Covic A, Fliser D, Fouque D, Goldsmith D, et al. (2014) Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 383(9931): 1831-1843.
- Tentori F, Wang M, Bieber BA, Karaboyas A, Li Y, et al. (2015) Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 10(1): 98-109.
- Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, et al. (2004) Mineral Metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15(8): 2208-2218.
- Martínez-Castelao A, Górriz JL, Segura-de la Morena J, Cebollada J, Escalada J, et al. (2014) Consensus document for the detection and management of chronic kidney disease. Nefrologia 34(2): 243-262.
- Fraser WD (2009) Hyperparathyroidism. Lancet 374(9684): 145-158.
- Isakova T, Xie H, Yang W, Xie D, Anderson AH, et al. (2011) Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305(23): 2432-2439.
- Cunningham J, Locatelli F, Rodriguez M (2011) Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol 6(4): 913-921.
- Brown AJ, Dusso AS, Slatopolsky E (2002) Vitamin D analogues for secondary hyperparathyroidism. Nephrol Dial Transplant 10: 10-19.
- Lindberg JS, Culleton B, Wong G, Borah MF, Clark RV, et al. (2005) Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol 16(3): 800-807.
- Sumida K, Nakamura M, Ubara Y, Marui Y, Tanaka K, et al. (2013) Cinacalcet upregulates calcium-sensing receptors of parathyroid glands in hemodialysis patients. Am J Nephrol 37(5): 405-412.
- Komaba H, Nakanishi S, Fujimori A, Tanaka M, Shin J, et al. (2010) Cinacalcet effectively reduces parathyroid hormone secretion and gland volume regardless of pretreatment gland size in patients with secondary hyperparathyroidism. Clin J Am Soc Nephrol 5(12): 2305-2314.
- Bover J, Perez R, Molina M, Benavides B, Ariza F, et al. (2011) Cinacalcet treatment for secondary hyperparathyroidism in dialysis patients: an observational study in routine clinical practice. Nephron Clin Pract 118(2): c109-c121.
- Ureña P, Jacobson SH, Zitt E, Vervloet M, Malberti F, et al. (2009) Cinacalcet and achievement of the NKF/K-DOQI recommended target values for bone and mineral metabolism in real-world clinical practice--the ECHO observational study. Nephrol Dial Transplant 24(9): 2852-2859.
- Forni Ogna V, Pruijm M, Zweiacker C, Wuerzner G, Tousset E, et al. (2013) Clinical benefits of an adherence monitoring program in the management of secondary hyperparathyroidism with cinacalcet: results of a prospective randomized controlled study. Biomed Res Int 2013: 104892.
- Al-Hilali N, Hussain N, Kawy YA, Al-Azmi M (2011) A novel dose regimen of cinacalcet in the treatment of severe hyperparathyroidism in hemodialysis patients. Saudi J Kidney Dis Transpl 22(3): 448-455.
- Haq N, Chaaban A, Gebran N, Khan I, Abbachi F, et al. (2014) A prospective randomized pilot study on intermittent post-dialysis dosing of cinacalcet. Int Urol Nephrol 46(1): 113-119.
- Esteve Simo V, Moreno-Guzmán F, Martínez Calvo G, Fulquet Nicolas M, Pou Potau M, et al. (2015) Administration of calcimimetics after dialysis: same effectiveness, better gastrointestinal tolerability. Nefrologia 35(4): 403-409.
- Blair HA (2016) Etelcalcetide: First Global Approval. Drugs 76(18): 1787-1792.
- Cozzolino M, Galassi A, Conte F, Mangano M, Di Lullo L, et al. (2017) Treatment of secondary hyperparathyroidism: the clinical utility of etelcalcetide. Ther Clin Risk Manag 13: 679-689.
- Friedl C, Zitt E (2018) Role of etelcalcetide in the management of secondary hyperparathyroidism in hemodialysis patients: a review on current data and place in therapy. Drug Des Devel Ther 12: 1589-1598.
- Torregrosa JV, Bover J, Cannata Andía J, Lorenzo V, de Francisco AL, et al. (2011) Spanish Nephrology Society. Spanish Society of Nephrology recommendations for controlling mineral and bone disorder in chronic kidney disease patients (S.E.N.-M.B.D.). Nefrologia 1: 3-32.
- Kulich KR, Piqué JM, Vegazo O, Jiménez J, Zapardiel J, et al. (2005) Psychometric validation of translation to Spanish of the gastrointestinal symptoms rating scale (GSRS) and quality of life in reflux and dyspepsia (QOLRAD) in patients with gastroesophageal reflux disease. Rev Clin Esp 205(12): 588-594.
- Quintana JM, Cabriada J, López de Tejada I, Varona M, Oribe V, et al. (2001) Translation and validation of the gastrointestinal Quality of Life Index (GIQLI). Rev Esp Enferm Dig 93(11): 693-706.
- Block GA, Bushinsky DA, Cheng S, Cunningham J, Dehmel B, et al. (2017) Effect of Etelcalcetide vs Cinacalcet on Serum Parathyroid Hormone in Patients Receiving Hemodialysis with Secondary Hyperparathyroidism: A Randomized Clinical Trial. JAMA 317(2): 156-164.
- Block GA, Bushinsky DA, Cunningham J, Drueke TB, Ketteler M, et al. (2017) Effect of Etelcalcetide vs Placebo on Serum Parathyroid Hormone in Patients Receiving Hemodialysis with Secondary Hyperparathyroidism: Two Randomized Clinical Trials. JAMA 317(2): 146-155.
- Bushinsky DA, Chertow GM, Cheng S, Deng H, Kopyt N, et al. (2020) One-year safety and efficacy of intravenous etelcalcetide in patients on hemodialysis with secondary hyperparathyroidism. Nephrol Dial Transplant 35(10): 1769-1778.
- Xipell M, Montagud-Marrahi E, Rubio MV, Ojeda R, Arias-Guillén M, et al. (2019) Improved Control of Secondary Hyperparathyroidism in Hemodialysis Patients Switching from Oral Cinacalcet to Intravenous Etelcalcetide, Especially in Nonadherent Patients. Blood Purif 48(2): 106-114.
- Gutiérrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, et al. (2009) Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 119(19): 2545-2552.
- Nasrallah MM, El-Shehaby AR, Salem MM, Osman NA, El Sheikh E, et al. (2010) Fibroblast growth factor-23 (FGF-23) is independently correlated to aortic calcification in haemodialysis patients. Nephrol Dial Transplant 25(8): 2679-2685.
- Yu L, Tomlinson JE, Alexander ST, Hensley K, Han CY, et al. (2017) Etelcalcetide, A Novel Calcimimetic, Prevents Vascular Calcification in A Rat Model of Renal Insufficiency with Secondary Hyperparathyroidism. Calcif Tissue Int 101(6): 641-653.
- Fukagawa M, Yokoyama K, Shigematsu T, Akiba T, Fujii A, et al. (2017) A phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy and safety of etelcalcetide (ONO-5163/AMG 416), a novel intravenous calcimimetic, for secondary hyperparathyroidism in Japanese haemodialysis patients. Nephrol Dial Transplant 32(10): 1723-1730.
- Shigematsu T, Fukagawa M, Yokoyama K, Akiba T, Fujii A, et al. (2018) Long-term effects of etelcalcetide as intravenous calcimimetic therapy in hemodialysis patients with secondary hyperparathyroidism. Clin Exp Nephrol 22(2): 426-436.
-
Fátima Moreno Guzmán, Vicent Esteve Simó*, Irati Tapia González, Gemma Martínez Calvo, Alicia Madurga Hernández, Verónica Duarte Gallego, Anna Saurina Solé, Monica Pou Potau and Manel Ramírez De Arellano Serna. Etelcalcetide in Hemodialysis: Better Secondary Hyperparathyroidism Control and Gastrointestinal Tolerance in the Clinical Practice. Annals of Urology & Nephrology. 3(5): 2023. AUN.MS.ID.000571..
-
Regulatory dendritic cells, Kidney transplantation, Cell therapy, Specific immune microenvironment, Immunosuppressive agents, Kidney immunology, Genetic modification, Human leukocyte, Genetic modification, Drug intervention, Cytokine exposure, Immunosuppressant, Renal parenchyma
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






