 Case Report
Case Report
When Medication Fails: Choosing Palliative Care in Refractory Cases of Electrical Storm
Kushinga Bvute1*, Kai Yoshinaga2, Elise Hoy3
1Internal Medicine Residency Program, Florida Atlantic University, USA
2Charles E. Schmidt College of Medicine, Florida Atlantic University, USA
3Boonshoft School of Medicine, Wright State University, USA
Kushinga Bvute, Internal Medicine Residency Program, Florida Atlantic University, USA.
Received Date: March 02, 2022; Published Date:March 28, 2022
Abstract
Electrical storm is most commonly developed through ischemic progression that allows surreptitious isthmuses of tissue to propagate episodes of ventricular tachycardia or ventricular fibrillation. This is a case of an elderly man at a community hospital who presented with syncope and who developed progressively worsening ischemic damage to his heart that evolved into an electrical storm. Given his multiple comorbidities and the perioperative risks, he was not a candidate for ablative therapies, leaving medication the only option for his treatment course. Numerous antiarrhythmic regimens were attempted but were ultimately ineffective at reversing the progressive damage caused by the electrical storm. This case illustrates the challenges of treatment in cases where ablation is not an option, and the value of palliative care in providing patient-centered care.
Keywords:Electrical storm; Ablation; Palliative care
Abbreviations:ES: Electrical storm; AICD: Automatic Implantable Cardioverter-Defibrillator; VT: Ventricular tachycardia; VF: Ventricular fibrillation; CABG: Coronary artery bypass grafting; CKD: Chronic kidney disease
Introduction
An electrical storm (ES) is a state of cardiac electrical instability marked by multiple episodes of ventricular tachycardia (VT) or ventricular fibrillation (VF) within a relatively short period, typically 24 hours [1]. For patients with an automatic implantable cardioverter-defibrillator (AICD), ES refers to a discharge of three or more electrical therapies for sustained VT or VF in 24 hours [1,2]. While AICDs reduce sudden cardiac death risk and improve survival, 2-10% of patients with AICDs will experience ES each year, with subsequent mortality rates of up to 11% within three months and 38% within three years [3,4].
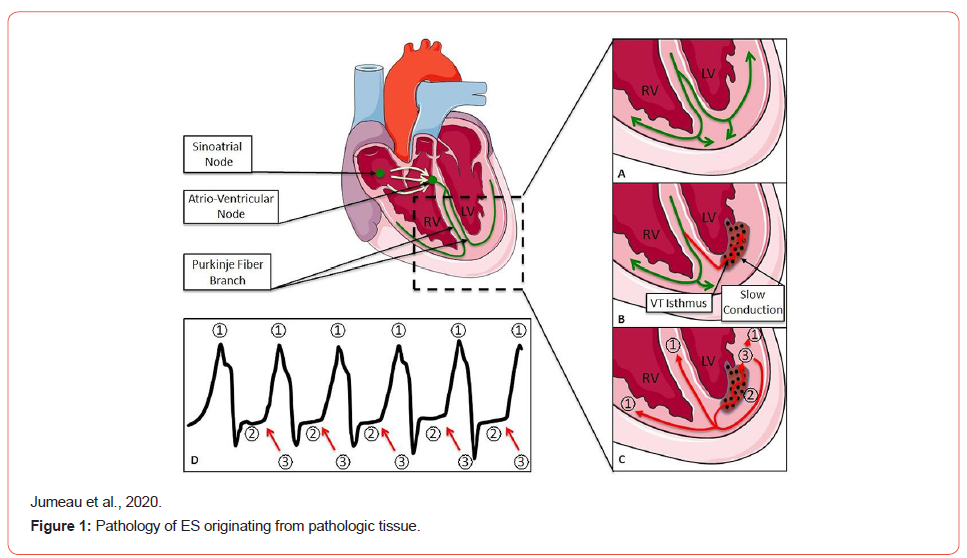
The ES develops when a vulnerable anatomic tissue (such as structural heart disease or scarring after an MI) is affected by a triggering event. Triggers include drug toxicities, electrolyte disturbances, new or worsening heart failure, thyrotoxicosis, and QT prolongation [5]. Risk factors for ES include markedly low ejection fraction and chronic kidney disease (CKD). Most episodes of ES require a re-entry mechanism (Figure 1), and 86-97% of episodes are typically monomorphic VT and rarely polymorphic VT, VF, or mixed VT/VF [6].
In the absence of prolonged QT or polymorphic VT, intravenous amiodarone is the antiarrhythmic drug of choice. Additionally, beta-blockers (preferably propranolol) reduce arrhythmogenic activity [1,7]. Since most ES originates primarily from the reentry mechanism, catheter ablation remains the gold standard for the treatment of ES. Ablation within 24 hours has a 94% success rate, 31% recurrence rate, and 9% mortality rate [8]. In addition, stellate ganglion blocks are an alternative for refractory cases [9]. However, in patients with advanced age, poor clinical status, and existing comorbidities, perioperative risks can be monumental and the extent of structural damage insurmountable. In such cases, including the case of our patient, a palliative approach may be suitable to meet goals of care and maintain patient autonomy. This case illustrates the challenges of managing medication-resistant ES in an elderly gentleman with continued AICD discharge who was not a candidate for invasive therapies.
Case Presentation
The patient was a 90-year-old man with a past medical history of coronary artery disease status post coronary artery bypass grafting (CABG), ischemic cardiomyopathy, and heart failure with 20% ejection fraction with AICD, distant history of pulmonary embolism, and CKD stage 4, who presented with sudden syncope while straining on the toilet. He had a similar syncopal episode 2 days prior to hospital admission. His home medications included amiodarone 200 mg daily, furosemide 20 mg daily, Lipitor 20 mg daily, metoprolol succinate 25 mg daily, Xarelto 2.5 mg bid, and potassium chloride 20 mg daily.
On arrival at the emergency department, the patient was awake, alert, and oriented. He had normal vitals: temperature of 36.3 C, heart rate of 60 bpm, respiratory rate of 17 bpm, blood pressure of 113/58 mmHg, and SpO2 100% on room air. His physical examination was unremarkable.
EKG showed dual chamber paced rhythm (Figure 2), and his chest X-ray was unremarkable. His blood work was significant for normocytic anaemia, thrombocytopenia, elevated ProBNP, high coagulation markers (in the setting of Xarelto), and acute-onchronic kidney injury (Table 1).
Table 1:Blood work on admission.

Table Abbreviations
GFR: Glomerular filtration rate
BUN: Blood urea nitrogen
AST: Aspartate aminotransferase
ALT: Alanine aminotransferase
CKMB: Creatine kinase-MB
T3: Triiodothyronine
T4: Thyroxine
TSH: Thyroid stimulating hormone
PT: Prothrombin time
INR: International normalized ratio
PTT: Partial thromboplastin time
HDL: High-density lipoprotein
LDL: Low-density lipoprotein
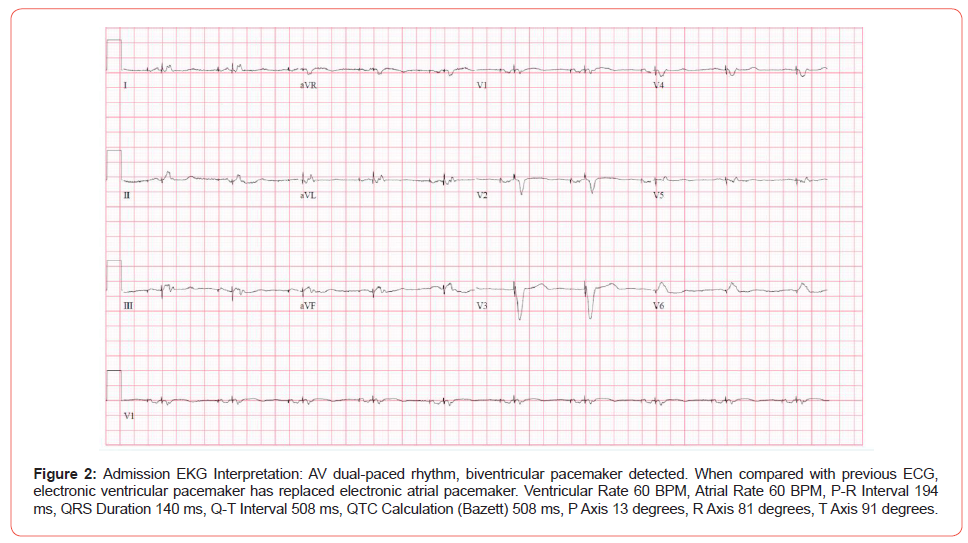
Differential Diagnosis
This 90-year-old male with a history of coronary artery disease status post CABG, ischemic cardiomyopathy, pulmonary embolism, CKD stage IV, heart failure with reduced ejection fraction 20-25% and a biventricular AICD, who presented with syncope had three possible diagnoses. The first possibility was cardiac syncope. This patient has a history of cardiac disease particularly heart failure with a reduced ejection fraction, which increases his risk for life threatening arrhythmias or malfunctioning of his biventricular AICD. The next possibility is vasovagal syncope. The patient had his first syncopal episode while defecating on the toilet. Although vasovagal syncope is the most common cause of syncope, the patient lacked the typical history of a slow progressive prodrome which includes light-headedness. Although the initial syncope was associated with defecation, subsequent episodes of sudden loss of consciousness at rest make vasovagal syncope unlikely. The last differential diagnosis was a pulmonary embolism. Although the patient had a prior history of pulmonary embolism, he had no dyspnoea or chest pain, and he had a low probability of venous thromboembolism based on the Wells criteria (no leg swelling, tachycardia, or signs suggesting deep venous thrombosis or immobilization). He was also compliant with his Xarelto, significantly reducing the probability of a pulmonary embolism. Interrogation of the patient’s device confirmed that the patient had runs of VT and VF during both syncopal episodes that required AICD shock to terminate the dysrhythmia. As such, his cardiac syncope was deemed to be due to these episodes of VF/VT.
Outcome
The patient was admitted and an electrophysiologist was consulted to reprogram the AICD to give him a VT therapy zone from 190 to 160 beats per minute and increase the VF zone to 210 beats per minute, a more aggressive measure to avoid prolonged episodes of VT and avoid shocks. Providers continued his home medications of amiodarone 200 mg daily and metoprolol succinate 25 mg daily but discontinued his diuretic and started mexiletine 150 mg bid.
Despite the new AICD programming and antiarrhythmics, he experienced presyncope on the following day during a brief episode of VT with a failed anti-tachycardia pacing sequence. He subsequently had sudden syncope at rest during 90 seconds of sustained VT. His AICD eventually fired, and he returned to consciousness before code responders performed cardiopulmonary resuscitation. He was transferred to the ICU where he was given a bolus of 150 mg IV amiodarone and 50 mg IV lidocaine and started on 0.5 mg/min of amiodarone infusion and 0.5 mg/min of lidocaine infusion. Mexiletine was discontinued and he started propranolol 10 mg every 6 hours. Despite aggressive reprogramming of the AICD, the patient had four separate runs of VT on his third hospital day, each resulting in defibrillation by his AICD. At this point, the patient met the diagnostic criteria for true ES and his EKG changes reflected new tissue damage (Figure 3). The choice of medications was limited given his poor renal function. The providers explained the long-term risks of high doses of amiodarone to the patient and family. The patient continued the amiodarone infusion at 0.5 mg/min, and a trial of amiodarone 400 mg bid was commenced. He started ranolazine 500 mg bid for synergistic effects and any possible ischemia, thereby reducing VT burden. Providers resumed mexiletine at 200 mg tide, increased propranolol to 20 mg every 6 hours, and added quinidine 300 mg bid.
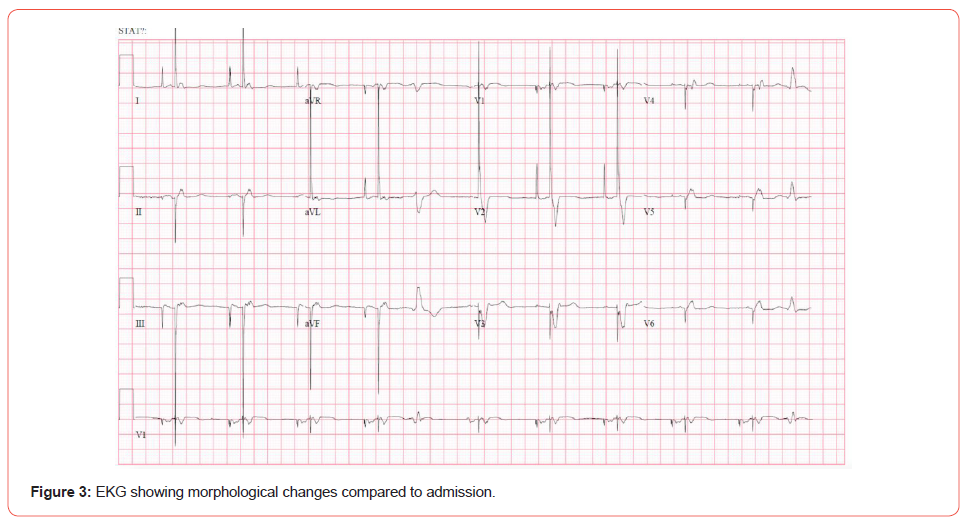
Since he continued to have ES, his prognosis was extremely poor and palliative care consultation was appropriate. Given his advanced age and poor renal function, ablation was considered unlikely to be beneficial as complication risk was high and his cardiac tissue was becoming progressively more damaged from the frequency of these arrhythmic events. After discussing goals of care with the family and expressing that he was not a candidate for ablation, the patient opted for aggressive medical management despite limited medication options. Unfortunately, he started experiencing adverse drug effects by the fifth hospital day, so providers discontinued the lidocaine infusion. He experienced repeated firing of his AICD for recurrent aberrant rhythms. Palliative care had another discussion with the patient regarding goals of care and had an extensive conversation on the challenges ahead since he continued to need defibrillation despite the medication. The patient and family understood that additional medical modalities were limited. The patient agreed to a hospice consultation. A joint family and patient decision were made to turn off his AICD. He became a “do not resuscitate” patient and a magnet was placed over the device to discontinue further defibrillation. He received hospice care for the remainder of his life. Shortly after, he experienced a run of VF from which he did not recover. He passed away peacefully.
Discussion
ES often represents part of the natural progression of advanced cardiac disease and may predict severe deterioration of the underlying disease [8]. ES in scarred tissue generates abnormal pathways through which surreptitious rhythm generation induces VF or VT. Our patient’s condition progressively worsened as he experienced recurrent defibrillations. Scar-mediated ventricular arrhythmias are commonly associated with re-entry mechanisms, which are generally amenable to catheter ablation; however, ablation carries significant perioperative risk for acute hemodynamic decompensation, especially in patients with markedly reduced ejection fraction, advanced age, and CKD [11,12]. Our patient with advanced heart failure and unstable VTs was at a high risk of hemodynamic collapse during an ablation procedure, and he was not a candidate for any invasive management due to his comorbidities. Finally, in terminally ill patients approaching the end of life, the continued function of an AICD can be futile, distressing for both the patient and his/her loved ones. In such patients, providers must have an in-depth discussion about goals of care with the patient and loved ones and recommend appropriate palliative options.
Acknowledgements
None.
Conflict of Interest
None.
References
- Kowey PR (1996) An overview of antiarrhythmic drug management of electrical storm. Can J Cardio 12 Suppl B: 3B.
- Pedersen CT, Kay GN, Kalman J, Borggrefe M, Della-Bella P (2014) EHRA/HRS/APHRS expert consensus on ventricular arrhythmias. Europace 16(9): 1257-1283.
- Exner D, Pinski S, Wyse G (2001) Electrical storm presages nonsudden death. The antiarrhythmics vs implantable defibrillators (AVID) trial. ACC Curr J 10(5): 73.
- Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ (2002) Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346(12): 877-883.
- Haegeli LM, Della-Bella P, Brunckhorst CB (2016) Management of a patient with electrical storm: role of epicardial catheter ablation. Circulation 133(7): 672-676.
- Bansch D, Bocker D, Brunn J, Weber M, Breithardt G (2000) Clusters of ventricular tachycardias signify impaired survival in patients with idiopathic dilated cardiomyopathy and implantable cardioverter defibrillators. J Am Coll Cardiol 36(2): 566-573.
- Chatzidou S, Kontogiannis C, Tsilimigras DI, Georgiopoulos G, Kosmopoulos M (2018) Propranolol Versus Metoprolol for Treatment of Electrical Storm in Patients with Implantable Cardioverter-Defibrillator. J Am Coll Cardiol 71(17): 1897-1906.
- Hendriks AA, Szili-Torok T (2018) Editor’s Choice-The treatment of electrical storm: an educational review. Eur Heart J Acute Cardiovasc Care 7(5): 478-483.
- Burjorjee JE, Milne B (2002). Propofol for electrical storm; a case report of cardioversion and suppression of ventricular tachycardia by propofol. Can J Anaesth 49(9): 973-977.
- Jumeau R, Ozsahin M, Schwitter J, Elicin O, Reichlin T (2020) Stereotactic Radiotherapy for the Management of Refractory Ventricular Tachycardia: Promise and Future Directions. Front Cardiovasc Med 7: 108.
- Santangeli P, Frankel DS, Tung R, Vaseghi M, Sauer WH (2017) Early Mortality After Catheter Ablation of Ventricular Tachycardia in Patients with Structural Heart Disease. J Am Coll Cardiol 69(17): 2105-2115.
- Santangeli P, Muser D, Zado ES, Magnani S, Khetpal S (2015) Acute hemodynamic decompensation during catheter ablation of scar-related ventricular tachycardia: incidence, predictors, and impact on mortality. Circ Arrhythm Electrophysiol 8(1): 68-75.
-
Kushinga Bvute, Kai Yoshinaga, Elise Hoy. When Medication Fails: Choosing Palliative Care in Refractory Cases of Electrical Storm. Anaest & Sur Open Access J. 3(3): 2022. ASOAJ.MS.ID.000561.
-
Ventricular tachycardia (VT), Automatic Implantable Cardioverter Defibrillator (AICD), Antiarrhythmic drug, Coronary Artery Bypass Grafting (CABG), Chest X-ray, Hemodynamic collapse.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






