 Review Article
Review Article
What Is the Prognostic Value of Sentinel Lymph Node Biopsy in Head and Neck Melanoma?
Jessica Dowling, Department of Surgery, Mater Dei Hospital, Malta
Received Date: October 28, 2024; Published Date: November 04, 2024
Abstract
Malignant melanoma is a rapidly progressing cancer. 20% of all malignant melanomas occur in the head and neck [1]. Melanomas in the head and neck have generally poorer prognosis than melanomas in other regions. Additionally, the neck has highly variable and challenging anatomy. Sentinel lymph node biopsy is an effective diagnostic tool that has been used in many cancers, less commonly in head and neck malignancy. Research showed variable results on the effectiveness and safety of sentinel lymph node biopsy vs safety and high recurrence rates of head and neck cutaneous melanomas. This review aims to assess recent literature regarding sentinel lymph node biopsy in head and neck cutaneous melanoma and its prognostic value with a secondary aim to create an improved local management strategy targeting this specific field.
Keywords: Sentinel lymph node biopsy; Head and neck cutaneous melanoma; Prognosis; Survival rate
Abbreviations: SLNB: Sentinel Lymph Node Biopsy; HNCM: Head and Neck Cutaneous Melanoma
Introduction
Malignant melanoma is a worldwide rapidly progressing cancer [2]. Primary cutaneous melanoma can be treated by surgery while more advanced cases require more aggressive therapy. The average lifetime risk for melanoma has reached 1 in 50 people in the Western world and an equally high mortality rate has also been observed [3].
According to Larson & Larson [1], almost 20% of all malignant melanomas occur in the head and neck, 85 -90% of which are cutaneous lesions [4]. The head and neck region carries poor prognosis for melanomas compared to other sites [5,6]. Of all head and neck melanomas, scalp and neck cancers have the highest mortality rate with a 10-year overall survival of only 60% [1]. Important structures and variable anatomy, namely lymphatic drainage of the head and neck create a challenge to management. The increased incidence and high mortality rate of HNCM requires early detection and treatment. SLNB has been increasingly utilized as a diagnostic and staging tool [6].
SLNB was initially described by Morton & al. in 1992 [7]. It is a safe and accurate procedure which has aided staging of cancers through analyzing the SLN with minimal morbidity [8]. The SLN is the first lymph node that receives tumor content and hence early detection leads to enhanced staging, expedited treatment and better survival rates [9]. A positive sentinel leads to complete dissection of all lymph nodes, a practice which has been widely used in breast and extremity melanoma cases to date.
With an increasing number of HNCM, up to 15-35% of all melanoma cases, research has been ongoing for carrying out SLNB in this area [10]. However, the subject is controversial owing to extensive lymphatic drainage in the neck, intricate anatomy, higher incidence of recurrence and lower SLN positivity [9]. Notwithstanding this, many studies exist to support opposite evidence, stating that SLNB in HNCM is equally safe, feasible and effective with regards to prognostication [11]. Locally, use of SLNB in HNCM is limited, mostly due to complex anatomy especially pattern of lymphatic drainage. This literature review is aimed at analysis of recent data, it being a good prognostic indicator of disease [6,9].
An overview of benefits and drawbacks of SLNB in HNCM is presented in Table 1.
Table 1: Benefits and drawbacks of SLNB in HNCM.
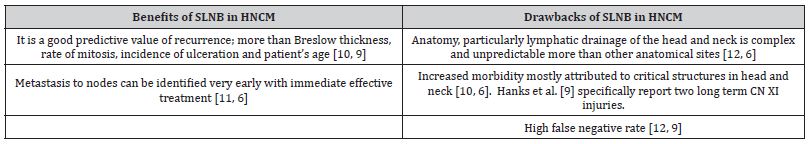
These arguments have led to different practice in various countries, including limited use of SLNB in HNCM locally, mostly related to specific consultant preference. This review is aimed to assess most recent literature regarding SLNB in HNCM and its prognostic value with a secondary aim to create an improved local management strategy targeting this specific field. The objective was formulated using PICO framework outlined below:
Table 2: Aim as per PICO Framework.

Search Strategy
An online search was conducted on Ovid and PubMed databases to answer the research question. A timeframe of 5 years was used and research in English language was considered. The 5-year timeframe was chosen to review the most recent evidence given the innovative nature and ongoing studies about the topic. The search results are summarized in Table 3.
Table 3: Search Strategy.
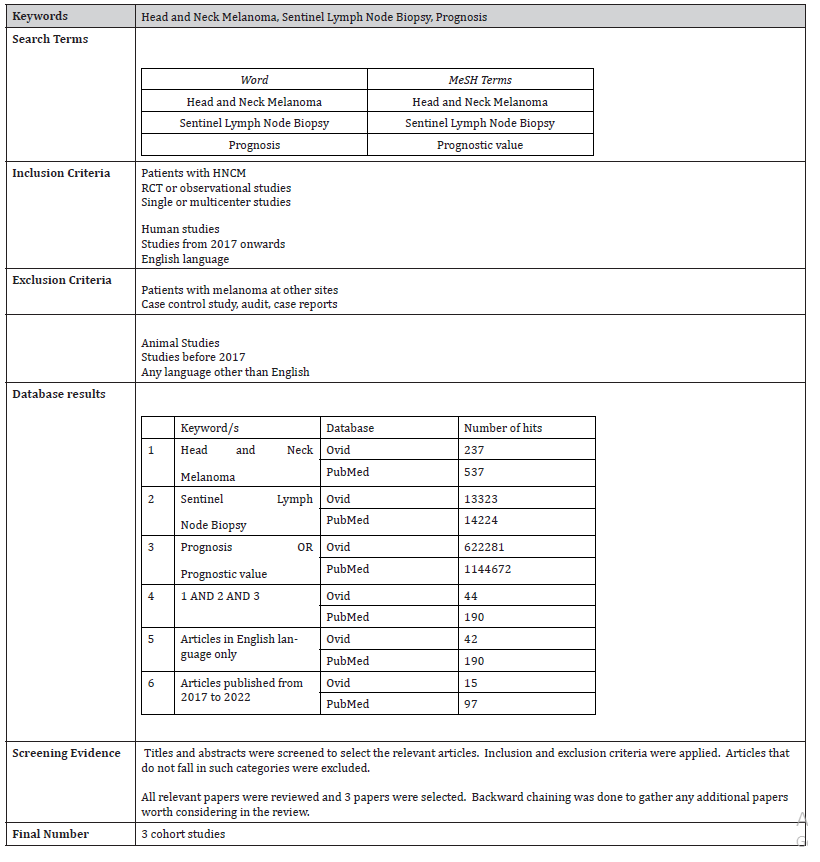
Studies retrieved included mostly retrospective observational studies from single or multicenter. Results were screened and those which did not match the search criteria were excluded. No randomized controlled trials were identified from the 15 final papers, possibly due to the current limited use of SLNB in HNCM on a global level.
3 observational studies will be considered for this literature review. Selection was based on satisfying as much conditions as possible out of the inclusion and exclusion criteria. Literature was evaluated using the Critical Appraisal Skill Programmed checklist [13] and graded by the Harbour & Miller hierarchy of evidence [14].
Literature Review
Three retrospective cohort studies will be presented in chronological order:
1) Sentinel lymph node biopsy in cutaneous head and neck melanoma by Evrard et al. [12].
2) Sentinel lymph node biopsy for melanoma of the head and neck: a multicenter study to examine safety, efficacy, and prognostic value by Passmore-Webb et al. [6].
3) Sentinel lymph node biopsy in head & neck melanoma: Long-term outcomes, prognostic value & accuracy by Hanks et al. [9].
Evrard et al. [12] describe Sentinel lymph node biopsy in cutaneous head and neck melanoma. The authors analyze a cohort of 124 patients treated at a single center between 2008 and 2012. The objective of the study was distinctly indicated, aiming to test how accurate and feasible SLNB is, the low morbidity it entails and its prognostic value. Outcomes sought are also indicated.
The main conclusion of this study is that SLNB is an excellent technique with good detection rate and negative predictive value. SLNB was found to be a powerful prognostic factor in HNCM and also an early detector of advanced stage cancer leading to improved oncological results.
The aim, methods and results of the study are showcased in Table 4.
Table 4: Aims, Methods and Results of Study 1 (Adapted from Evrard et al. [12]).
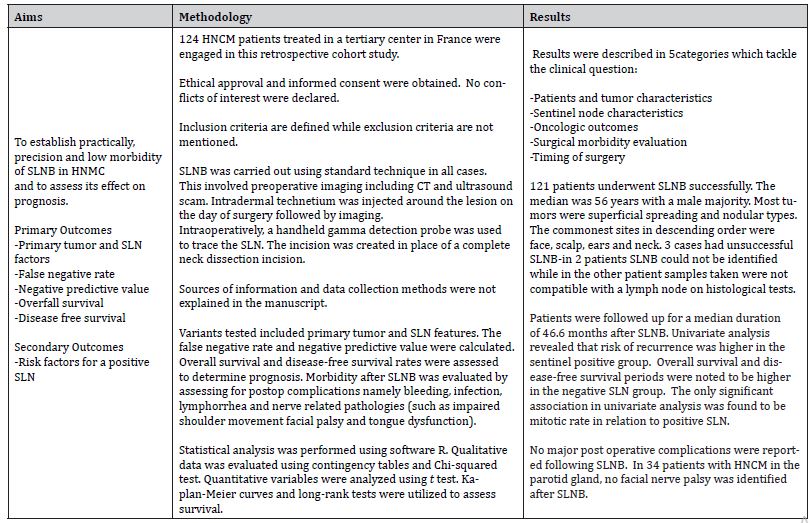
The objective of the study is well defined since the research question is clearly stated, hence guiding the design of the study to test the hypothesis. The data collection methods are not indicated; hence consistency of measurement tools is questionable resulting in decreased reliability. The small sample size and lack of power calculation challenges the cohort size indicating a higher risk of Type II error. The study population is in fact the smallest out of the 3 considered, possibly due to single-centered data.
Evrard et al. [12] declared no conflict of interest and state that they have obtained ethical approval. This means that good practice was enforced with low risk of observer bias in line with the Declaration of Helsinki [15].
The authors describe detailed inclusion criteria as follows:
Table 5: Inclusion criteria for Study 1 (Adapted from Evrard et al [12]).
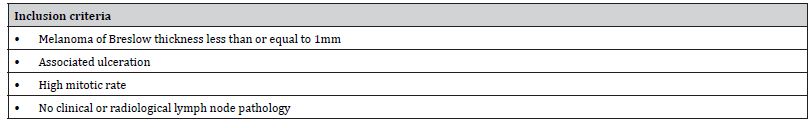
While one may assume that the exclusion criteria are comprised of factors not within the inclusion categories, it must be noted that there are no specific exclusion criteria thus increasing risk of confounding factors. Consecutive patients were recruited similar to Hanks et al. [9] study limiting the risk of selection bias.
Statistical significance was set at 5%. Adequate statistical tests were chosen for categorical and continuous variables, Chi-squared in the former and t test in the latter. The authors carried out a univariate analysis, but a multivariate analysis was not conducted. Hence it cannot be concluded if any significant results were due to covariance. This, in addition to missing exclusion criteria, leads to lack of the study’s content validity in view of decreased statistical rigor.
The study has a number of standardised factors including preoperative imaging using CT and ultrasound, intradermal injection of technetium followed by lymphoscintigraphy or SPECT and same incision in all cases. These common factors reduce the risk of observer bias. Surgeon’s experience was also considered and divided into two categories – surgeons who performed <30 surgeries and those who performed > 30. This classification was based on only one study by Morton et al. [16], presenting questionable reliability. Furthermore, the authors do not make any reference as to whether the same surgeon follows up the patient throughout the whole period of study, thus including an element of information bias.
Results were explained as text and figures. Univariate analysis showed that SLN and ulceration were statistically significant factors that influence recurrence (Figure 1).
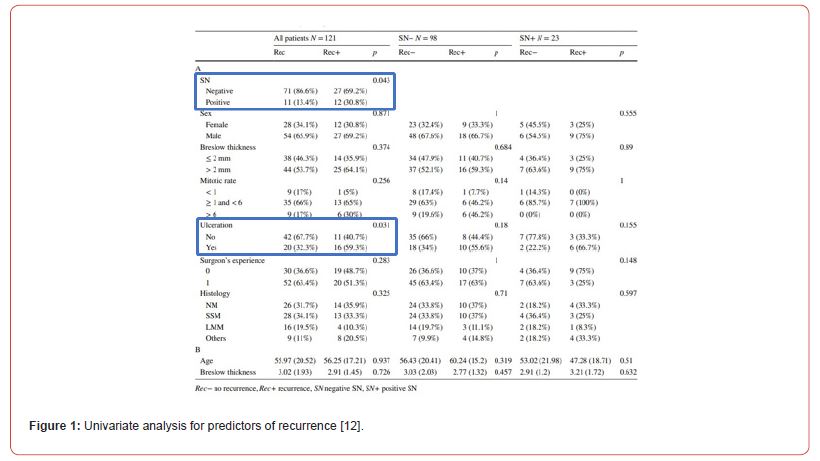
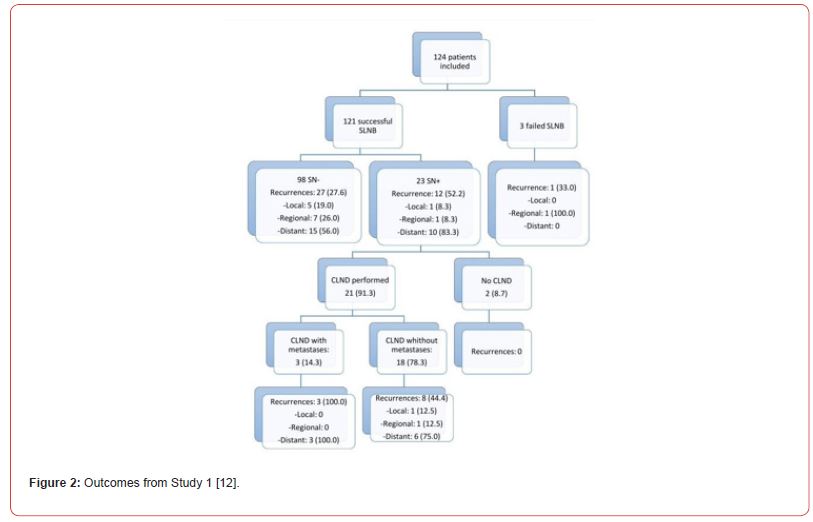
Patients that were not included until end of study were accounted for by detailed explanations. The authors gave a clear indication why 3 SLN (2.5%) failed and explained why this happened for each case. SLN was positive in 23 patients. Out of this number, 21 subjects underwent complete neck dissection while the other 2 were unfit due to underlying comorbidities and the nature of the lymph node, it being micro metastatic. These results are summarized in Figure 2.
Oncologic outcomes are a good indicator of prognosis. Patients were followed up for a median of 46.6 months. In the positive SLN group, 12 of 23 (52.2%) patients had recurrence within 19 months while the negative SLN group had recurrence in 27 of 98 (28.1%) patients. Distant metastasis was the commonest site of recurrence (83.3%) in both groups. This indicates that the majority of recurrences occurred in areas not previously sampled. 8 patients had regional recurrence in the absence of local recurrence showing a false negative rate of 7.1%. Hafström et al. [17] and Davis-Malesevich et al. [18] also report a low false negative rate, a confirmation of high sensitivity of the study.
SLNB has a good safety profile as no patients experienced any complications after biopsy. Safety profiles were also reported in other studies [11,19]. Furthermore, all 34 patients with HNCM in the parotid gland had no facial nerve weakness. This implies that SLNB is a very safe procedure with no post operative morbidity.
Cox proportional hazard models were an effective regression tool to analyze survival in relation to SLN status. Overall and disease-free survival rates were better in the negative SLN group, p < 0.001 and p = 0.013 respectively indicating SLNB as a strong prognostic marker.
In conclusion, this retrospective study would be graded as Harbour and Miller 2- given the small single Centre cohort, high risk of confounding primarily from inappropriate exclusion criteria and lack of complete statistical analysis due to absence of multivariate analysis. A larger study, possibly multicentered, with less confounding and more thorough statistical analysis will create more valid results that can be favorably transferable to the author’s local practice.
The retrospective cohort study by Passmore-Webb et al. [6] tackles Sentinel lymph node biopsy for melanoma of the head and neck: a multicenter study to examine safety, efficacy, and prognostic value. 143 patients treated at two specialist centers between May 2011 and September 2017 were considered. The study’s aims were to identify the accuracy, safety and prognostic significance of SLNB in HNCM.
The main results of the study showed that SLNB in HNCM has a high safety profile, gives accurate information for staging and provides good prognostication. It has a temporary morbidity rate of 2.1% and 0% permanent morbidity rate [6].
A summary of the study is presented in the table below.
The main rationale why Passmore-Webb et al. [6] chose this topic for research was explained in the introduction stating that melanoma has quadrupled its incidence since 1970. 22% of new melanoma cases occur in the head and neck and studies have been performed showing questionable safety of SLNB and aid in prognostication of HNCM. The study has a focused aim which enables authors to tackle the clinical question straightaway.
Data was collected using case notes from each author. The authors declared no conflict of interest and obtained necessary ethical approval, ensuring good research governance and limiting bias.
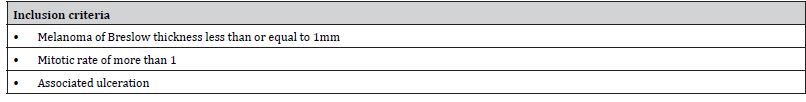
The inclusion criteria are set out as shown in Table 7, however exclusion criteria are not mentioned. It must be noted that initially 181 patients were recruited but 38 patients were eliminated due to short follow up of less than 1 year (27 patients) or failed lymphoscintigraphy, thus no SLNB (11 patients). This explanation is important as the authors account for all patients till the end of study. There is no mention whether all subjects were consecutive or otherwise. This factor along with no exclusion criteria creates a risk of selection bias in the recruitment process.
Table 6: Aims, Methods and Results of Study 2 (Adapted from Passmore-Webb et al. [6]).
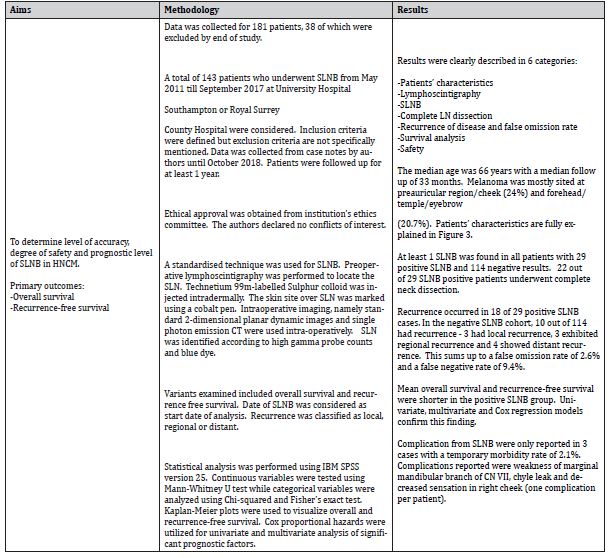
Table 7: Inclusion criteria for Study 2 (Adapted from Passmore-Webb et al. [6]).
The study is a multicentered retrospective study, the only one of the types from the 3 studies analyzed. A power calculation was not performed hence an adequate sample size was not calculated. This reduces validity and increases risk of Type II error.
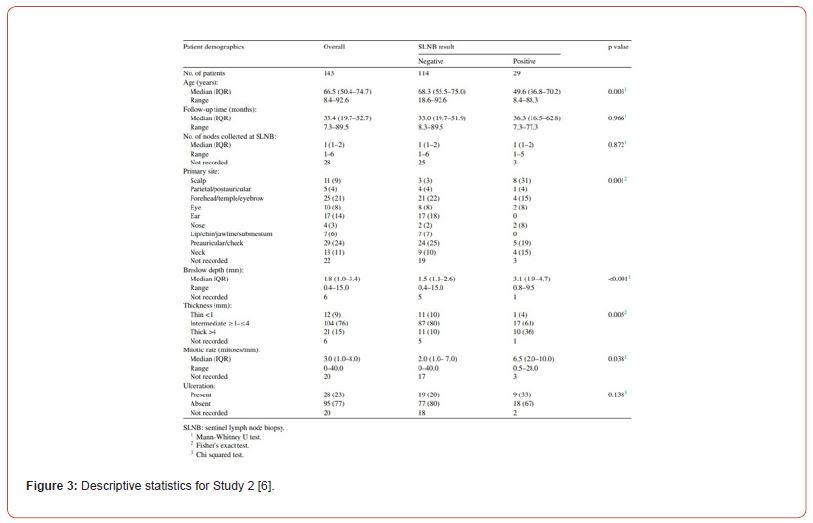
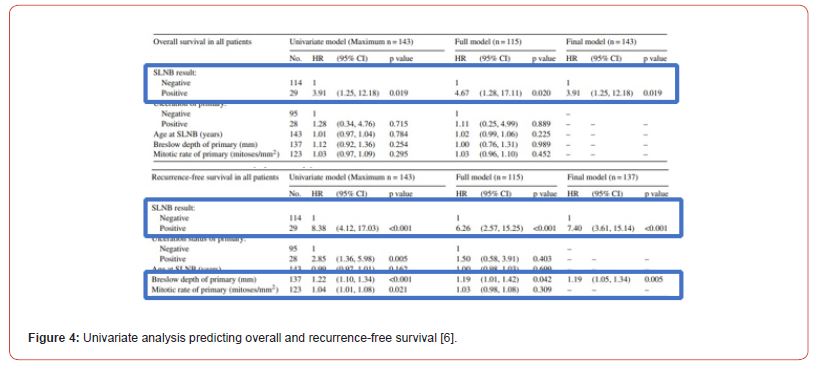
Statistical significance was set at 5%. The right statistical tests were used to assess relationship of variables and SLNB status-Mann Whitney U test for continuous variables, Chi Squared or Fisher exact test for categorical variables. Descriptive statistics are presented in Figure 3. Univariate and multivariate analysis were carried out with the main finding being that positive SLNB is a strong predictor of reduced overall survival (p = 0.019) and recurrence-free survival (p < 0.0001) as shown in Figure 4. Presence of ulceration (p = 0.005), Breslow thickness (p < 0.001) and mitotic rate (p = 0.021) are also statistically significant predictors of recurrence-free survival. By carrying out a multivariate analysis, authors reduce risk of confounding. Thorough statistical analysis increases rigor and internal validity of the study
A standardized technique is employed in all cases as explained in Table 6. However, the authors fail to mention surgeon’s grade or expertise and whether the same surgeon was following up patients throughout the whole study period. Different assessors might give rise to information bias while different surgeons’ expertise can result in variability in the sensitivity of the investigation.
The study demonstrates a high degree of accuracy as detection rate at operation reached 100% with at least 1 SLNB identified in all subjects. The authors compared this rate to MSLT-1 and Sunbelt studies with 97.5% (for non-head and neck melanoma) an 99.7% (for all sites) detection rate respectively [20, 21].
Follow up time was adequate to identify a total of 28 recurrences in a median 33 month follow up, this timeframe being the shortest as compared to the other 2 studies-46.6 months [12], 58.8 months [9].
False negatives were defined as patients with regional recurrence after negative SLNB with no local or distant recurrence. The false negative rate of 9.4% is comparable to rates in other studies-7.1% [12], 19.4% [9]. Passmore-Webb et al. [6] also compare this rate to other studies-9.5% [22], 14.8% [11]. By comparing this study to other studies, the authors increase transferability and external validity.
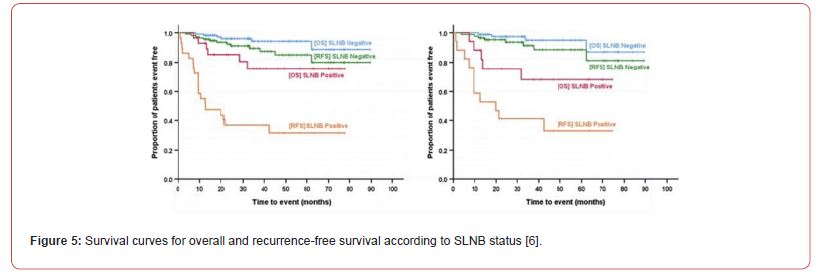
Survival rates are graphically represented using Kaplan-Meier curves in Figure 5, similar to graphs published by Evrard et al. [12] and Hanks et al. [9].
SLNB was also noted to have a high safety profile with a low rate of 2.1% of temporary morbidity. No permanent complications were identified.
Passmore-Webb et al. [6] make reference to the limitations of the study, namely low number of recurrences recorded, possibly due to the small cohort and short follow up time.
In summary, this retrospective multicenter cohort study is a level 2+ study with favorable reply to the research question. Despite the low risk of bias present, it has strong arguments and comparable data to other studies thus increasing external validity. A power calculation would be ideal to determine the correct sample size required. Like the other studies analyzed long term follow up including surveillance imaging is essential.
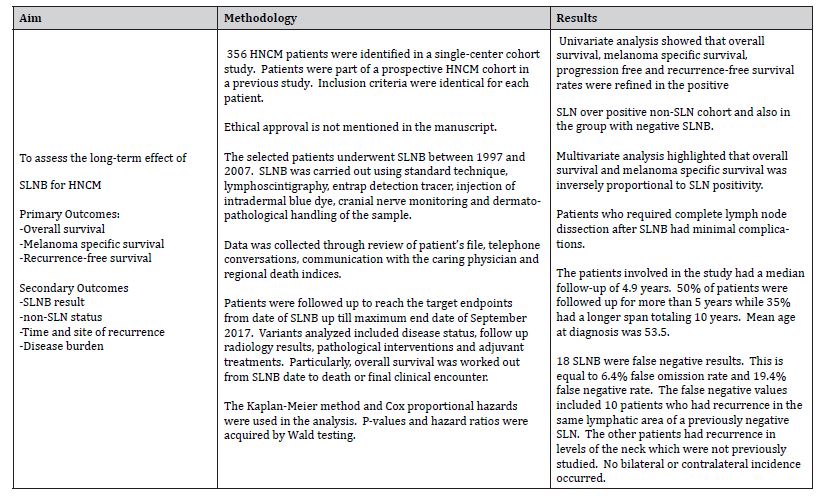
The retrospective cohort study by Hanks et al. [9] reviews Sentinel lymph node biopsy in head & neck melanoma: Long-term outcomes, prognostic value & accuracy. It comprises a single-center study with a 10year cohort of 356 patients over the period 1997- 2007, which data was previously incorporated in a prospective HNCM study by Erman et al. [11]. The aims were well defined and outcome measures were laid out to make sure that aims were fully accomplished.
The key finding from this study is that SLNB has a good prognostic value. Long-term follow up of patients is required due to delayed risk of recurrence, however this does not decrease the significance of SLNB as it is extremely prognostic for overall and melanoma specific survival rates.
The aim, methods and results are summarized in Table 8.
The selected data collection and analysis tools were adequate to reach the desired outcomes. Authors declared no conflict of interest at the beginning of the study and this is vital as they will be impartial thus limiting risk of bias. However, ethics approval was not mentioned in the text. Lack of ethical approval would result in a breach of the Declaration of Helsinki [15] and will reflect negatively on principles of governance. As a result, the study has higher probability of misconduct thus reducing scientific quality.
The inclusion and exclusion criteria are not clearly stated, resulting in decreased reliability. Absence of such criteria will yield inconsistent results. Despite this, the dataset has well defined characteristics as exhibited in Figure 6. Unselected consecutive patients were considered in the study. This limits selection bias as much as possible.
A power calculation is unfortunately not documented, however the cohort size used is the largest out of the three observational studies [12,6,9] The large cohort could be explained from the 10- year time span. However, data was only collected from a singlecenter thus increasing the risk of Type II error with a negative effect on validity and relevance. Hanks et al. [9] are very aware of this limitation and in fact emphasize that including more centers would have increased external validity resulting in more clinical significance.
Table 8: Aims, method and results of Study 3 (Adapted from Hanks et al. [9]).
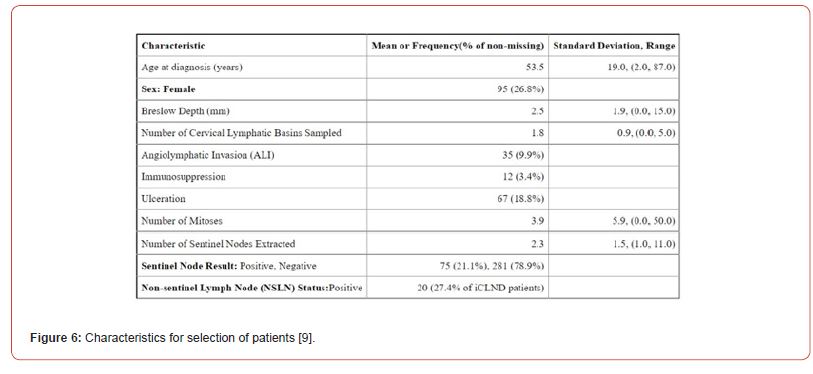
The fact that surgical technique was standardised reduces the risk of observer bias. Yet the proficiency of the surgeon carrying out the SLNB was never referred to in the study and this may affect sensitivity of the study. Also, there is no indication whether the same surgeon was following up the patient throughout the period of the study, thus resulting in information bias.
Results were clearly presented including visual representation through graphs as shown in Figure 7 below.
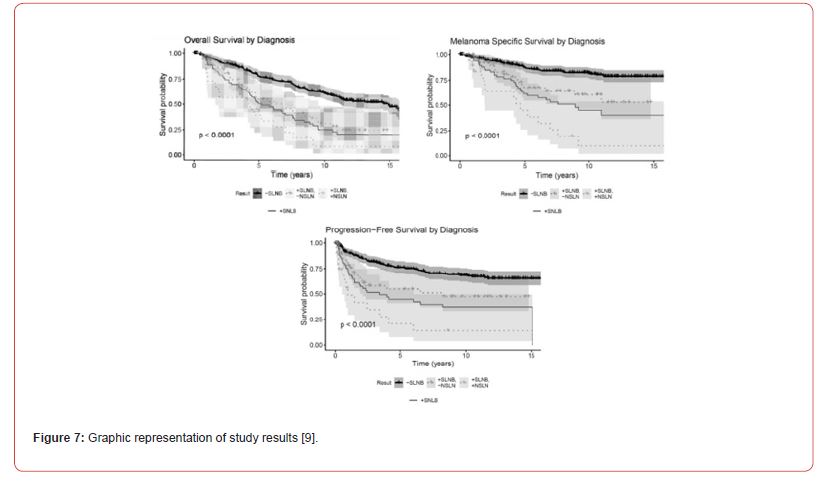
The Kaplan-Meier method and Cox proportional hazards analysis were used to estimate time-to event targets. Correlation between the patient, SLNB and tumor feature was evaluated. These tools are an effective way to investigate the effect of multiple factors on survival simultaneously.
Both univariate and multivariate analysis were carried out to find patterns between multiple factors being examined. A number of variables including overall survival, melanoma specific survival, progression free and regional free survival were significantly lower in negative SLN over the positive SLN group. Each modality of survival was significantly longer in SLNB negative patients as displayed in Figure 8.
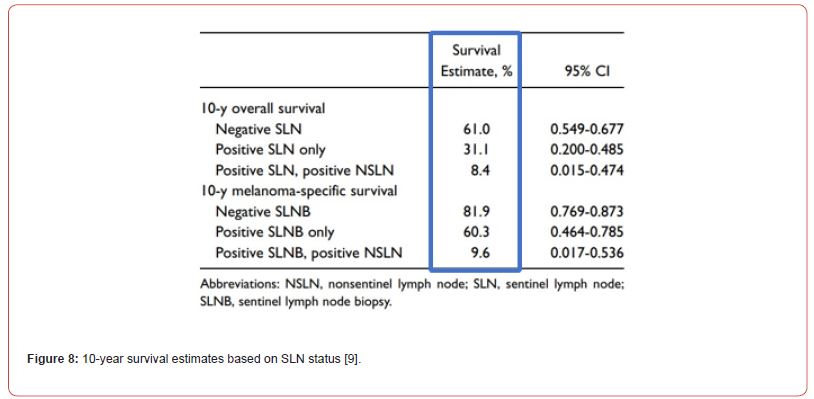
Since the dataset is from a previous study by Erman et al. [11], advances were developed in SLNB, increased use of cranial nerve monitoring intra operatively, enhanced imaging and management since publishing. These improvements must be considered when analyzing results as they might improve further the prognostic outcome of SLNB in HNCM.
Patients were followed up with 4.9 years median follow up. Follow up was more than 5 years in more than half of the patients and over 10 years in 35% of the cohort. This follow up time was sufficient enough to detect recurrence in 18 false negative patients. 10 patients had recurrence in the same lymph nodes, including 1 patient which recurred in both previously as well as new levels of the neck. The other 8 patients had recurrence only in sites that were not previously investigated.
In conclusion, the study presents a positive reply to the research question, stating that SLNB improves the prognostication of HNCM. Additionally long-term follow up is required in view of risk of delayed recurrence, mostly in lymph nodes not previously investigated. The study could be ranked as Harbour and Miller 2+ in view of low risk of bias. It provides favorable arguments to implement SLNB in HNCM at a local level. A larger centered study with more recent data subjects and precise notes on standardised surgeon proficiency should be included to improve the study.
Discussion
On reviewing literature, existing evidence states that SLNB is a good prognostic indicator of HNCM. The level of evidence in this review includes 2 studies classified as 2+ and 1 study graded as 2- as per Harbour & Miller [14], meaning that 2 out of the 3 studies exhibit low risk of confounding and bias.
The 3 studies show better survival rates in patients who have negative SLNB results. Also, they have low false negative rates as follows: 7.1% in Study 1 [12], 9.4% in Study 2 [12] and 19.4% in Study 3 [9]. The higher false negative rate in study 3 might be attributed to results obtained from an older study by Erman et al. [11] after which developments in SLNB might have occurred but were not accounted for.
The studies also display a high level of safety of SLNB in HNCM despite the variable anatomy in the region, hence refuting any arguments that SLNB in this area is not safe. In fact, Evrard et al. [12] reported no complications post-SLNB and Passmore-Webb et al. [6] only had 2.1% temporary morbidity rate with no permanent complications.
It must be noted that power calculation was not done and is a common limitation of all 3 studies. This might result in higher risk of Type II error. Also, 2 of 3 studies [12,9] are single-centered studies hence results are not generalizable to the whole population of individual countries.
Conclusion
Currently, evidence in favor of SLNB in HNCM is inconclusive. Despite the benefits of SLNB as a good prognostic marker and increased survival indices in SLNB negative patients, more rigorously conducted studies are essential before changing existing practice. Further studies with larger data sets after necessary power calculation are suggested to have more robust results.
More refined studies could include well defined inclusion and exclusion criteria, standardised surgeon experience and same surgeon follow up to limit risk of confounding. Additionally, a costbenefit analysis could also be considered in any future studies.
To conclude, the prognostic value of SLNB in HNCM has shown favorable results but further studies are required before implementing this management strategy on a local level. This will provide better definitive results for optimum patient care.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Larson D, Larson J (2010) Head and neck melanoma. Clinics in plastic surgery 37(1): 73-77.
- Liu Y, Sheikh MS (2014) Molecular pathogenesis and therapeutic management. Molecular and Cellular Pharmacology 6(3): 228.
- Rastrelli M, Tropea S, Rossi C, Alaibac M (2014) Melanoma: epidemiology, risk factors, pathogenisis, diagnosis and classification. In Vivo 28(6): 1005-1011.
- López F, Juan P Rodrigo, Antonio Cardesa, Asterios Triantafyllou, Kenneth O Devaney, et al. (2016) Update on primary head and neck mucosal melanomas. Head & Neck 38(1): 147-155.
- Kienstra M, Padhya T (2005) Head and neck melanoma. Cancer Control 12(4).
- Passmore-Webb B, B Gurney, H M Yuen, J Sloane, J Lee, et al. (2019) Sentinel lymph node biopsy for melanoma of the head and neck: a multicentre study to examine safety, efficacy, and prognostic value. British Journal of Oral and Maxillofacial Surgery 57(9): 891-897.
- Morton DL, D R Wen, J H Wong, J S Economou, L A Cagle, et al. (1992) Technical details of intraoperative lymphatic mapping for early-stage melonoma. Arch Surg 127(4): 392-399.
- Moncayo V, Alazraki A, Alazraki N, Aarsvold J (2017) Sentinel lymph node biopsy procedures. Seminars in Nuclear Medicine 47(6): 595-617.
- Hanks JE, Kevin J Kovatch, S Ahmed Ali, Emily Roberts, Alison B Durham, et al. (2020) Sentinel lymph node biopsy in head and neck melanoma: long-term outcomes, prognostic value, accuracy, and safety. Otolaryngology - Head and Neck Surgery 162(4): 520-529.
- Giudice G, et al. (2014) Sentinel lymph node biopsy in head and neck melanoma. Il Giornale di chirurgia 35(5-6): 149-155.
- Erman A, Ryan M Collar, Kent A Griffith, Lori Lowe, Michael S Sabel, et al. (2012) Sentinel lymph node biopsy is accurate and prognostic in head and neck melanoma. Cancer 118(4): 1040-1047.
- Evrard D, E Routier, C Mateus, G Tomasic, J Lombroso, et al. (2018) Sentinel lymph node biopsy in cutaneous head and neck melanoma. European Archives of Oto-Rhino-Laryngology 275(5): 1271-1279.
- Critical Appraisal Skills Programme (CASP) (2013) CASP Checklist (Online) Oxford.
- Harbour R, Miller J (2001) A new system for grading recommendations in evidence-based guidelines. British Medical Journal 323(7308): 334-346.
- World Medical Association (2013) World Medical Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 310(20): 2191-2194.
- Morton DL, Wen DR, Foshag LJ, Essner R, Cochran A (1993) Intraoperative lymphatic mapping and selective cervical lymphadenectomy for early-stage melanomas of the head and neck. Journal of Clinical Oncology 11(9): 1751-1756.
- Hafström A, Romell A, Ingvar C, Wahlberg P, Greiffi L (2016) Sentinel lymph node biopsy staging for cutaneous malignant melano,ma of the head and neck. Acta Oto-laryngologica 136(3): 312-318.
- Davis-Malesevich M, Ryan Goepfert, Mark Kubik, Dianna B Roberts, Jeffery N Myers, et al. (2015) Recurrence of cutaneous melanoma of the head and neck after negative sentinel lymph node biopsy. Head & Neck 37(8): 1116-1121.
- Schmalbach C, Bradford C (2015) Is sentinel lymph node biopsy the standard of care for cutaneous head and neck melanoma?. Laryngoscope 125(1): 153-160.
- McMasters K (2001) The sunbelt melanoma trial. Annals of Surgical Oncology 8(9 Supp): 41S - 43S.
- Morton DL, Cochran AJ, Thompson JF, Elashoff R, Essner R, et al. (2005) Sentinel node biopsy for early-stage melanoma: accuracy and mobidity in MSLT-1, an international multicentre trial. Annals of Surgery 242(3): 302-313.
- Kelly J, Fogarty K, Redmond HP (2009) A definitive role for sentinel lymph node mapping with biopsy for cutaneous melanoma of the head and neck. The Surgeon 7(6): 336-339.
-
Jessica Dowling*. What Is the Prognostic Value of Sentinel Lymph Node Biopsy in Head and Neck Melanoma?. Anaest & Sur Open Access J. 5(4): 2024. ASOAJ.MS.ID.000618.
-
Sentinel lymph node biopsy, Head and neck cutaneous melanoma, Prognosis, Survival rate, Head and neck cutaneous melanoma, diagnostic, Lymph nodes, Multicenter, Cancer
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






