 Review Article
Review Article
Ventricular Assist Devices Insertion, Overview and Anesthetic Considerations
Nabil A Mageed*, Ibrahim I Abd El Baser and Hani I Taman
Department of Anesthesia and Surgical Intensive Care, Faculty of Medicine, Mansoura University, Egypt
Nabil A Mageed, Department of Anesthesia and Surgical Intensive Care, Mansoura University, Egypt.
Received Date: March 21, 2020; Published Date: April 09, 2020
Abstract
Ventricular assist devices (VAD) represent a revolution for the management of severe heart failure. Their insertion requires the use of cardiopulmonary bypass. They are used either permanently for long term treatment of refractory heart failure or temporally as a bridge until cardiac transplantation and until cardiac recovery from reversible cardiomyopathy. Insertion of VAD is a risky surgery with high incidence of complication such as bleeding, cardiac tamponade, renal failure and device failure. Anesthetic management of patients with heart failure undergoing VAD insertion requires full review of the patient critical condition, understanding VAD physiology, extensive hemodynamic monitoring and a harmony between cardiac anesthesia and surgical teams. Marinating and protection the right ventricular function is highly important for the continuation of VAD function. The aim of this review is to put new insights on anesthetic management of VAD insertion and to show their different types and their physiology.
Keywords: Heart Failure; Ventricular assist devices; Anesthesia; Insertion
Introduction
Heart failure (HF) is a chronic disease with progressive deterioration occurring over years or decades. The increase in the incidence of HF can be attributed to high survival after cardiac insults like myocardial infarction, improvement of drug treatment of circulatory disorders, and population aging [1]. Although the great advances in medical therapy and management, HF is still a leading cause of high incidence of hospitalization, readmission and mortality [2].
Stages of Heart Failure
The American College of Cardiology and American Heart Association have designed a conceptual classification to help understanding the disease progression in four stages. Stage A represents a patient who are at high risk for HF but has no organic heart disease or symptoms of HF, for example, patients with hypertension, atherosclerosis disease, or diabetes mellitus. Stage B includes patients with organic heart disease but without overt clinical presentation, such as myocardial infarction and left ventricular remodeling. Stage C are those patients who have organic heart disease with previous or current symptoms of HF, such as shortness of breath and fatigue. Stage D represents patients with end-stage HF and sever symptoms at rest despite maximal medical therapy [3,4].
In stage D, different management approach should be employed including end-of-life care, heart transplant, permanent circulatory mechanical support, or drugs [5]. Medical treatment for chronic heart failure includes angiotensin-converting enzyme inhibitors (ACEIs), beta blockers, proper use of diuretics to relieve volume overload, and digoxin which may help to improve resistant symptoms and reduce hospital, anesthesia and surgical readmission rates. For all patients with wide QRS more than 150 milliseconds, biventricular pacing should be considered and for some patients with QRS of 120 to 150 milliseconds according the condition.
More recently, sinus-node inhibitor ivabradine, and LCZ696, which combines angiotensin II inhibition with a neprilysin inhibitor, have been demonstrated to hold promise for HF patients [6-9]. However for patients with end-stage heart failure the only effective treatment is surgical intervention. Heart transplant is the gold standard for treatment of end-stage HF. The left ventricular assist device is an increasingly used option for patients in Stage D HF to prolong life in lieu of heart transplant. Ventricular assist devices (VADs) are mechanical systems that reduce the workload of the heart, permitting the ventricle to rest, whilst maintaining cardiac output and perfusion of vital organs. They have subsequently gained popularity in both acute and chronic heart failure as potentially lifesaving treatment modalities [10].
Development of INTERMACS
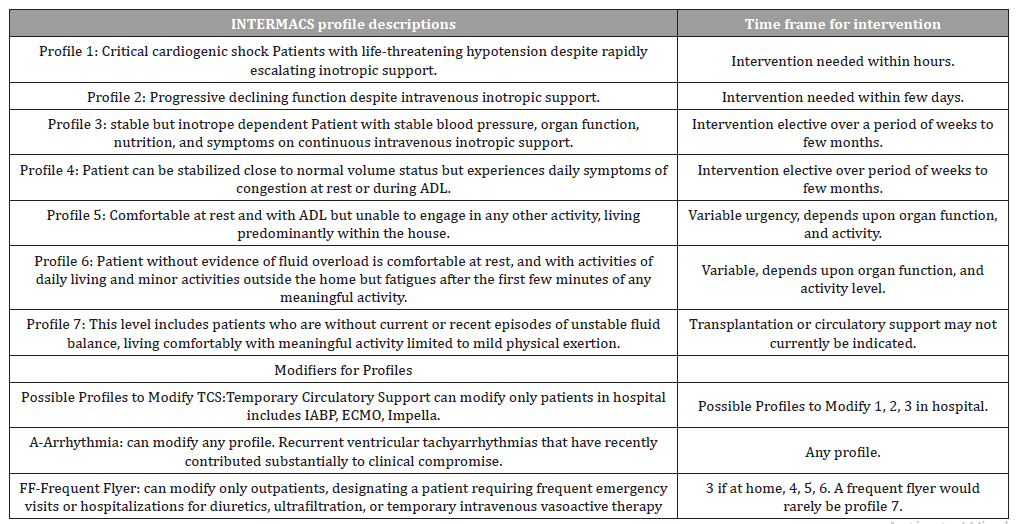
The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) was designed to facilitate communication with other colleagues regarding any patient with failing response to optimal medical therapy and needs further discussion of possible implantation of mechanical circulatory support devices (MCDs). Also is used for adjustment of pre-operative risk and clarification of target populations for future devices. INTERMACS has been acquiring data on most patients with implanted MCDs under the sponsorship of the National Heart, Lung, and Blood Institute (NHLBI) [11]. These profiles should facilitate collection of outcome data, help to address the varied needs of advanced heart failure patients and hoped to increase clarity of clinical profiling which will illuminate the progress of other surgery, other devices and all treatments for the advanced stages of heart failure [12,13].
Table 1:INTERMACS profile.
History and classification
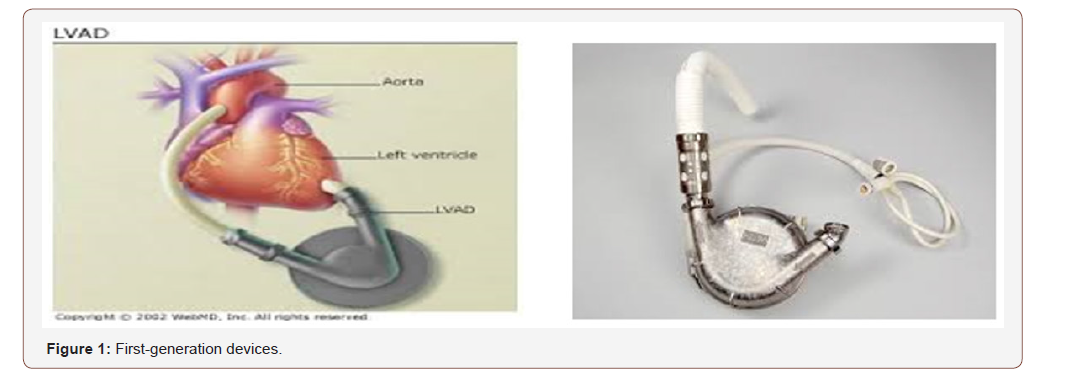
First-generation devices
These mimic normal cardiac function and circulation by providing pulsatile blood flow at physiological rates. The energy source used to eject blood from the pumping chamber may be pneumatic, hydraulic, or by mechanical pusher plate [14,15]. These mechanisms produce audible pump operation. Durability beyond 2 years is rare and operations for device exchange carry high risk (Figure 1).
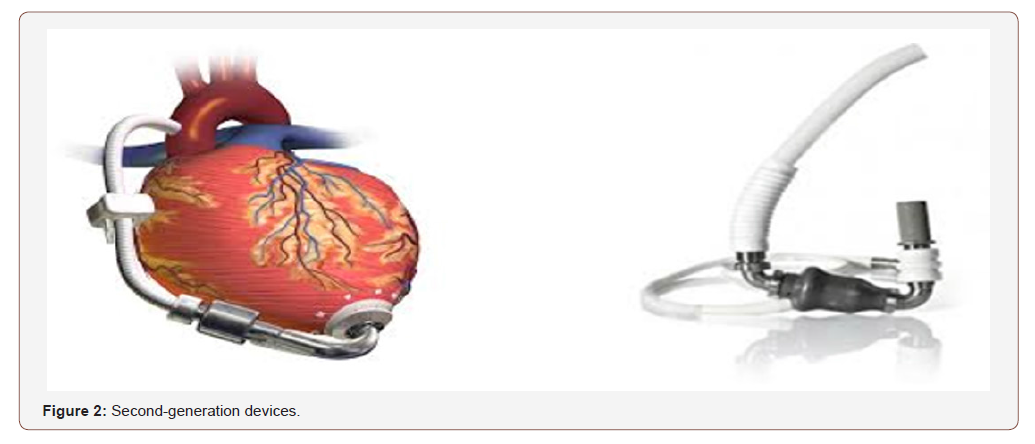
Second-generation devices
Flow is continuous and non-pulsatile, which reduces the pump size and the need for external venting. The term second generation describes rotary pumps, typically with axial blood flow, which have an internal rotor within the blood path that is suspended by contact bearings. These bearings are generally mechanical (ball, ruby, or fluid design) [16,17]. Spinning of the internal rotor and flow generation is by magnetic coupling of the rotor magnet to an external rotor (Figure 2).
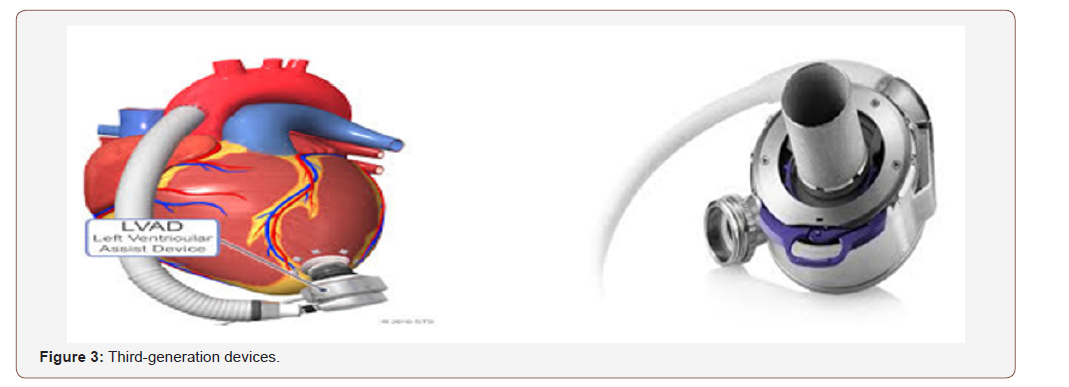
Third-generation devices
These rotary pumps have non-contact bearings and utilize centrifugal blood flow, incorporating either magnetic or hydrodynamic levitation of an internal impeller. Impeller rotation achieves blood flow through magnetic coupling to the pump motor [18,19]. Greater blood flow can be achieved around the impeller, which reduces thrombus formation and the intensity of antithrombotic therapy required (Figure 3).
The following selected definitions are important to the understanding of how mechanical support devices operate:
RPM: the revolutions per minute (RPMs), which determine pump flow.
Flow: the continuous flow from the LVAD is created by a spinning impeller, which generates forward flow. The device flow = Rotor speed/(Pump inflow – Pump outflow).
Pump power: LVAD pump power is a measure of the current and voltage applied to the motor and varies directly with pump speed and flow.
Pulsatility index: (PI) corresponds to the magnitude of flow pulse through the pump [20,21].
Indications for a Left Ventricular Assist Device
Strong indications: Bridge to transplant, destination or bridge to recovery [22]. All must apply
a. NYHA IV for 60–90 days.
b. Maximal tolerated medical therapy and CRT/ICD if indicated.
c. Chronic inotrope dependence.
d. LVEF 25%, PCWP 20 mm Hg.
Moderate indications: More often destination than bridge to transplant or recovery [23]. All must apply
a. NYHA IV for 30 days.
b. Maximal tolerated medical therapy and CRT/ICD if indicated.
c. Intermittent inotrope dependence.
d. LVEF 25%.
Indication to enable heart transplant. Either must apply
a. PVR 5 Woods units, secondary to chronic HF and expected to reverse after LVAD.
b. GFR, 25–30 mL/min/1.73 m2, secondary to chronic HF and likely to improve after LVAD.
Contraindications for a Left Ventricular Assist Device
Some may be relative, especially as technology improves [24].
a. Acute cardiogenic shock or arrest with uncertain neurologic status.
b. Irreversible contraindication to heart transplant if destination or recovery is not the aim.
c. Non-systolic HF.
d. Co-existing illness with life expectancy < 2 years.
e. Terminal severe comorbidity, e.g. metastatic or advanced cancer, severe liver disease or severe lung disease.
f. Active uncontrolled systemic infection or significant risk of infection.
g. Active severe bleeding.
h. Right HF not secondary to left HF.
i. Moderate or severe aortic insufficiency that will not be corrected.
j. Anatomical considerations such as hypertrophic cardiomyopathy, large ventricular septal defect.
k. Psychosocial limitations, e.g. inability to comply with medical regimen or device and driveline maintenance or inability of patient or companion to maintain LVAD operation and interpret alarms [25].
Anesthesia for VAD Insertion
Preoperative considerations
Patient optimization targets platelet count >150 x 103 mm-1, serum albumin >33 gm litre-1, normal liver enzyme levels, estimated glomerular filtration rate > 50 ml kg-1 min-1, hematocrit >34%, mean pulmonary artery pressures <25 mm Hg, and low inotropic support. Neurological assessment is documented, via transient cessation of sedation if the patient is intubated. Premedication is avoided to prevent cardiac or respiratory depression [26].
Monitoring and vascular access
Standard cardiac monitoring, including five-channel electrocardiography, is used. Existing I.V. access should be changed if there is an infection risk and lines sent for culture. Central venous access is mandatory, both for pulmonary artery catheterization and fluid resuscitation, with meticulous attention to sterility. Mixed venous oxygen saturation assesses the adequacy of cardiac output, aiming for >65%. Transoesophageal echocardiography (TEE) is used throughout the perioperative and immediate postoperative period. Assessment of coagulation via thromboelastography (TEG) provides a baseline to compare with after operation [6].
Induction and maintenance
Induction must prevent haemodynamic decompensation from reduced preload and contractility and is best performed inside the operating theatre with titrated increments of induction agents and inotropic support. Inhalation or I.V. anesthesia is suitable, but nitrous oxide best avoided. External defibrillation pads are attached. Antibiotic prophylaxis is customized to the institution’s pathogenic flora and includes broad-spectrum gram positive bacterial and anti-fungal cover. Body warming devices are used to maintain normothermia.
Surgical access is commonly via median sternotomy, but left thoracotomy with lung isolation via a double-lumen endotracheal tube is an option. Bleeding is common so aprotinin anti-fibrinolysis and cell salvage are routinely used. Full heparinization is required, irrespective of preoperative coagulation [27].
Perioperative period
TEE is used to exclude valve lesions, shunts and intracardiac thrombi as discussed above. CPB is generally but not universally used for placement of inflow cannulae within the ventricle, but duration can be minimized by tunnelling the percutaneous lead and completing the outflow cannula anastomosis before instituting bypass.
Inflow cannulae are directed posteriorly towards the mitral valve to prevent obstruction. Before VAD activation, all air should be removed, and the heart filled with blood. Deairing is via backflow of blood from the aorta to a Luer lock connection on the outflow cap and by elevating the LV apex. The VAD is initiated at low speed with a cross-clamp on the outflow cannula and a needle vent. CPB flow is then reduced followed by removal of the graft cross-clamp. The LV should be full and off CPB before the pump speeds are increased to avoid entraining air into the system, which is further minimized by flooding the surgical field with saline or carbon dioxide [6].
Weaning from CPB
Atelectatic lung is expanded before weaning bypass to prevent Valsalva-induced hypotension. CPB weaning concerns are from RV failure, pulmonary hypertension, systemic vasodilatation, and bleeding. TEE is vital for deairing, RV assessment, and to check cannulae positions and flow [28]. RV dysfunction can be difficult to predict, thus monitoring and prophylaxis against right heart failure must be instituted in all cases before separation from bypass, using inhaled nitric oxide, I.V. nitrates and phosphodiesterase inhibitors. RV dysfunction results in chamber dilatation and leftward ventricular septal deviation on TEE, predicting imminent failure. Atrial or AV sequential pacing is useful to maintain adequate cardiac output. Protamine must be used to reverse heparinization but can worsen RV function and cause pulmonary hypertension [29].
Potential Complications Associated with LVAD
Thrombosis
Patients with LVADs have an increased risk of thrombosis because the LVAD is foreign material in constant contact with the bloodstream. Patients are maintained on anticoagulation (usually warfarin) and antiplatelet therapy (usually aspirin). Patients who develop a thrombus may present with signs of HF. In the case of thrombosis, additional medical treatment such as heparin, glycoprotein 2b/3a antagonists, or tissue plasminogen activator may be necessary [30,31].
Infection
The current LVAD design is not a completely closed system as there is an open driveline exit. Trauma to this percutaneous exit site is a common cause for infection. The patient’s condition can rapidly progress to sepsis and/or shock. Treatment involves establishing hemodynamic stability. Blood culture and imaging tests are necessary to evaluate for internal abscess or vegetation in or around the device. The patient should be treated with appropriate antibiotics and may require long term suppressive antibiotic therapy [32,33].
Gastrointestinal bleeding
The use of anticoagulation and antiplatelet therapy increases risk of bleeding, particularly in the gastrointestinal tract and brain. Also, chronic anticoagulation can lead to platelet dysfunction or acquired Von Willebrand disease. In severe cases, patients will demonstrate hypotension, hypovolemic shock, and rectal bleeding [34,35].
Stroke
Patients with LVADs are at increased risk for both ischemic and hemorrhagic stroke. Acute ischemic strokes result from thromboembolic events due to pump thrombosis, subtherapeutic anticoagulation, or a prothrombotic state associated with activation of the immune system. Alternatively, patients on longterm anticoagulation are at increased risk for hemorrhagic stroke. Hemorrhagic stroke can occur in these patients due to hemorrhagic transformation of an ischemic stroke, over anticoagulation, or infection [36].
Right HF
After LVAD implantation, there is increased output from the left ventricle that can lead to right ventricle failure (RVF). An echocardiogram and laboratory testing for liver enzymes are indicated if RVF is suspected. It can be treated with diuretics and modification of LVAD parameters. In severe cases, mechanical support of the right ventricle may be required with a biventricular assist device [37,38].
Ventricular dysrhythmias
LVAD-induced ventricular dysrhythmias can occur if the patient has a preload deficiency with decreased filling of the left ventricle due to hypovolemia. With diminished left ventricular filling, the inflow cannula can become drawn down into the cardiac ventricular tissue. The cannula’s contact with the cardiac tissue can irritate the area and trigger a ventricular dysrhythmia. These dysrhythmias tend to be short and are repeated until the hypovolemia is corrected. Ventricular dysrhythmias can lead to cardiac arrest. Advanced cardiac life support measures that include chest compressions can be used in patients with LVADs [39].
The future
Design improvements in current VAD systems include totally implantable devices with transcutaneous energy transmission systems, which eliminate the need for a driveline insertion site and also designs allowing more acceptable side-effect profiles for thromboembolism, infection, and device failure.
In addition, there are two total artificial hearts in clinical use, which involve explantation of the native heart and replacement with the device. These are the CardioWest total artificial heart (Syncardia Inc.) and AbioCor (Abiomed Corporation).
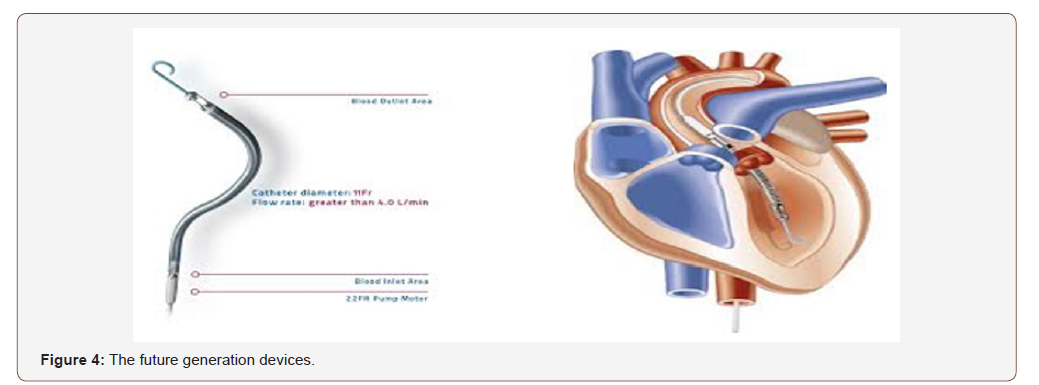
The latter organization also produces the Impella, one of a group of minimally invasive, catheter-based assist devices, which is inserted via the femoral artery, the tip of which crosses the aortic valve to rest in the LV and drive forward flow (Figure 4).
Finally, with more devices in production, there is a need for regulation. At present, all devices are regulated in accordance with rules set out by the INTERMACS controlling use in the USA.
Conclusion
Perioperative anesthetic management of patients undergoing VAD implantation represent a challenging mission for the cardiac anesthesia team. This require full cooperation between surgical and anesthetic teams. Extensive hemodynamic monitoring and cardiopulmonary bypass support are essential.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Liu L, Eisen HJ (2014) Epidemiology of heart failure and scope of the problem. Cardiol Clin 32(1): 1-8.
- DeVore D, Hammill G, Sharma P, Qualls G, Mentz J, et al. (2014) In-hospital worsening heart failure and associations with mortality, readmission, and healthcare utilization. J Am Heart Assoc 3(4): 211-218.
- Jessup M, Abraham T, Casey E, Feldman M, Francis S, et al. (2009) Focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults. Journal of the American College of Cardiology 53(15): 1343-1382.
- Yancy W, Jessup M, Bozkurt B, Butler J, Casey E, et al. (2013) ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 128(16): 118-214.
- Becnel F, Ventura O, Krim R (2017) Changing our approach to stage D heart failure. Prog Cardiovasc Dis 60(2): 205-214.
- Paul P, Kuppurao L (2012) Ventricular assist devices. Critical Care & Pain 12(3): 212-218.
- Jessup M (2014) Neprilysin inhibition, a novel therapy for heart failure. N Engl J Med 371(11): 1062-1066.
- Swedberg K, Komajda M, Bohm M, Borer J, Ford I, et al. (2010) Ivabradine and outcomes in chronic heart failure: a randomized placebo-controlled study. Lancet 376(9744): 875-885.
- Mc Murray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, et al. (2014) Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371(11): 993-1004.
- Slaughter MS, Rogers JG, Milano CA, Kitahara H, Gonoi W, et al. (2009) Advanced heart failure treated with continuous flow left ventricular assist device. N Engl J Med 361(23): 2241-2251.
- Limael E, Rodriguez L, Suarez E, Loebe M, Bruckner A (2013) Venticular assist devices therapy: new technology, new hope? Methodist Debakey Cardiovasc J 9(1): 32-37.
- Stevenson L, Pagani F, Young J, Jessup M, Miller L, et al. (2009) INTERMACS Profiles of Advanced Heart Failure: The Current Picture. J Heart Lung Transplant 28(6): 535-541.
- Kirklin JK, Naftel DC, Stevenson LW, Kormos RL, Pagani FD, et al. (2008) INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant 27(10): 1065-1072.
- Goldstein DJ, Oz MC, Rose EA (1998) Implantable left ventricular assist devices. N Engl J Med 339(21): 1522-1533.
- Frazier OH, Myers TJ, Radovancevic B (1998) The Heart Mate left ventricular assist system. Overview and 12-year experience. Tex Heart Inst J 25(4): 265-271.
- Burke DJ, Burke E, Parsaie F, Poirier V, Butler K, et al. (2001) The Heartmate II: design and development of a fully sealed axial flow left ventricular assist system. Artif Organs 25(5):380-385.
- Frazier OH, Delgado RM, Kar B, Patel V, Gregoric ID, et al. (2004) First clinical use of the redesigned HeartMate II left ventricular assist system in the United States: a case report. Tex Heart Inst J 31(2): 157-159.
- Nguyen DQ, Thourani VH (2010) Third-generation continuous flow left ventricular assist devices. Innovations (Phila) 5(4): 250-258.
- Hoshi H, Shinshi T, Takatani S (2006) Third-generation blood pumps with mechanical noncontact magnetic bearings. Artif Organs 30(5): 324-338.
- Slaughter MS (2010) Long-term continuous flow left ventricular assist device support and end-organ function: prospects for destination therapy. J Card Surg 25(4): 490-494.
- Uriel N, Morrison KA, Garan AR, Kato TS, Yuzefpolskaya M, et al. (2012) Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol 60(18): 1764-1775.
- Starrh L, Becker D (2018) Ventricular assist devices: The basics. The Journal for Nurse Practitioners 14(7): 538-544.
- (2018) INTERMACS
- Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, et al. (2007) Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 357(9): 885-896.
- Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, et al. (2001) Long-term mechanical left ventricular assistance for end stage heart failure. N Engl J Med 345(20): 1435-1443.
- Nicolosi AC, Pagel PS (2003) Perioperative considerations in the patient with a left ventricular assist device. Anesthesiology 98(2): 565-570.
- Mets B (2000) Anesthesia for left ventricular assist device placement. J Cardiothorac Vasc Anesth 14(3): 316-326.
- Chumnanvej S, Wood MJ, Mac Gillivray TE, Melo MFV (2007) Perioperative echocardiographic examination for ventricular assist device implantation. Anesth Analg 105(3): 583-601.
- Winterhalter M, Simon A, Fischer S, Meyer N, Chamtzidou N, et al. (2008) Comparison of inhaled iloprost and nitric oxide in patients with pulmonary hypertension during weaning from cardiopulmonary bypass in cardiac surgery: a prospective randomized trial. J Cardiothorac Vasc Anesth 22(3): 406-413.
- Nicholson D, Kaakeh Y (2018) Pharmacotherapy considerations for long-term management of patients with left ventricular assist devices. Am J Health Syst Pharm 75(11): 755-766.
- Baumann M, Kim B, Wieselthaler M (2015) Antithrombotic therapy for left ventricular assist devices in adults: A systematic review. J Thromb Haemost 13(6): 946-955.
- Topkara K, Kondareddy S, Malik F, Wang W, Mann L, et al. (2010) Infectious complications in patients with left ventricular assist device: Etiology and outcomes in the continuous-flow era. Ann Thorac Surg 90(4): 1270-1277.
- Nienaber J, Kusne S, Riaz T, Walker C, Baddour M, et al. (2013) Clinical manifestations and management of left ventricular assist device-associated infections. Clin Infect Dis 57(10): 1438-1448.
- Demirozu T, Radovancevic R, Hochman F, Gregoric D, Letsou V, et al. (2011) Arteriovenous malformation and gastrointestinal bleeding in patients with the Heart-Mate II left ventricular assist device. J Heart Lung Transplant 30(8): 849-853.
- Harvey L, Holley T, John R (2014) Gastrointestinal bleed after left ventricular assist device implantation: Incidence, management, and prevention. Ann Cardiothorac Surg 3(5): 475-479.
- Parikh S, Cool J, Karas G, Boehme K, Kamel H (2016) Stroke risk and mortality in patients with ventricular assist devices. Stroke 47(11): 2702-2706.
- Birati Y, Rame E (2014) Left ventricular assist device management and complications. Crit Care Clin 30(3): 607-627.
- Turner R (2019) Right ventricular failure after left ventricular assist device placement: The beginning of the end or just another challenge? J Cardiothorac Vasc Anesth 33(4): 1105-1121.
- Pedretti S, Cipriani M, Bonacina E, Vargiu S, Gil Ad V, et al. (2017) Refractory ventricular tachycardia caused by inflow cannula mechanical injury in a patient with left ventricular assist device: Catheter ablation and pathological findings. J Arrhythm 33(5): 494-496.
-
Nabil A Mageed, Ibrahim I Abd El Baser, Hani I Taman. Ventricular Assist Devices Insertion, Overview and Anesthetic Considerations. Anaest & Sur Open Access J. 1(4): 2020. ASOAJ.MS.ID.000520.
-
Heart Failure, Ventricular assist devices, Anesthesia, Insertion, Surgery, Chronic disease, Blood, High risk, Treatment
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






