 Case Report
Case Report
Use of VA-ECMO in Mechanical Mitral Valve thrombosis
Qian Du1, Jun Jin1, Si-hao Zheng1, Xu Li1, Lin Huang1, Chak-Kwan Tong1,2*
1Adult Intensive Care Unit, University of Hong Kong-Shenzhen Hospital, Shenzhen, China
2Department of Medicine, LKS University of Hong Kong, Hong Kong, China SAR
Chak-Kwan Tong, Adult Intensive Care Unit, University of Hong Kong-Shenzhen Hospital, Shenzhen, China and Department of Medicine, LKS University of Hong Kong, Hong Kong, China SAR.
Received Date: April 11, 2022; Published Date:April 29, 2022
Abstract
Use and choice of anticoagulation for patients with mechanical mitral valve are important parts of valvular heart disease care. Thromboembolism occurs if anticoagulant is not used correctly, which can result in life threatening condition. Peripheral VA-ECMO support is a life sustaining hemodynamic support, which allows bridging to definite treatment in such a life-threatening condition.
Introduction
After implant of prosthetic heart valve, there are many factors affecting the general well-being of the patient, including co-existing diseases, cardiac function, and development of valve-related complications. Good care of the valve and patient become important parts of patient care after prosthesis is implanted. Thrombosis of mechanical mitral valve is a life-threatening condition and timely treatment is necessary.
Case description
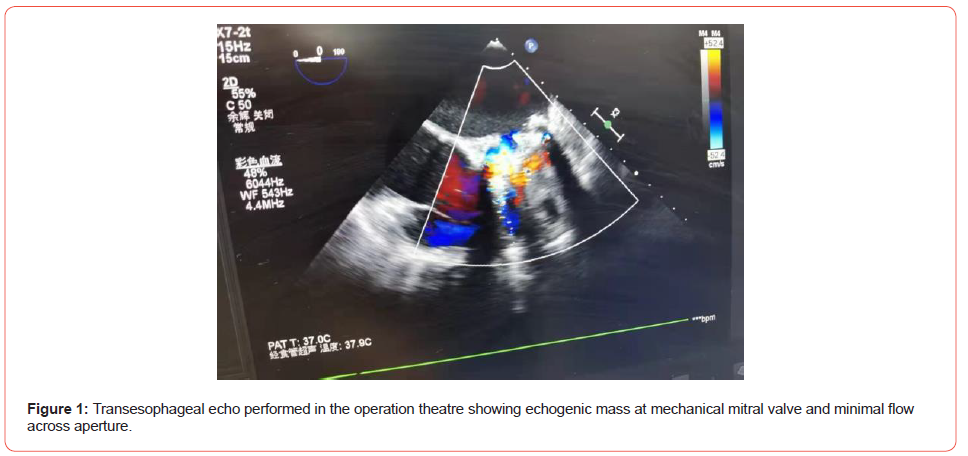
A 45 years old lady presented with generalized weakness for 3 days and chest discomfort for 1 day to the Accident & Emergency department of our hospital in Oct 2021. She had ischemic stroke in year 2016 and found to have chronic rheumatic heart disease. She underwent mitral valve replacement in the same year and had been taking oral warfarin (3.75mg daily) since then with therapeutic range achieved. By the end of year 2020, warfarin was changed to oral rivaroxaban (10mg) in another hospital, with good drug compliance. When she was seen at the A&E department, her Glasgow Coma Score was 15, blood pressure was 118 / 83mmHg, pulse 123bpm. Her peripheries were cold. She was tachypneic, signal of pulse oximetry was poor. She was given oxygen therapy, while further work up was performed. Within 30 minutes, her blood pressure dropped to 74/ 48mmHg. Bedside echocardiogram showed failed opening of the mechanical valve with minimal flow signal across the valve. (Figure 1) She was intubated and transferred to the Adult Intensive Care Unit (AICU) for further care.
Cardiac Surgeon was consulted for non-functioning mechanical mitral valve. Emergent mechanical valvular replacement was decided. Despite high dose of vasopressor, her blood pressure continued to deteriorate. While waiting for the surgical team and theatre staff to be ready, Intensivist instituted emergent peripheral veno-arterial membrane oxygenation (VA-ECMO) support in the AICU for the refractory cardiogenic shock. She developed cardiac arrest just before cannulation was started. Return of spontaneous circulation (ROSC) occurred after 4 minutes of cardiopulmonary resuscitation. She was sent to the operation theatre right after VAECMO was established.
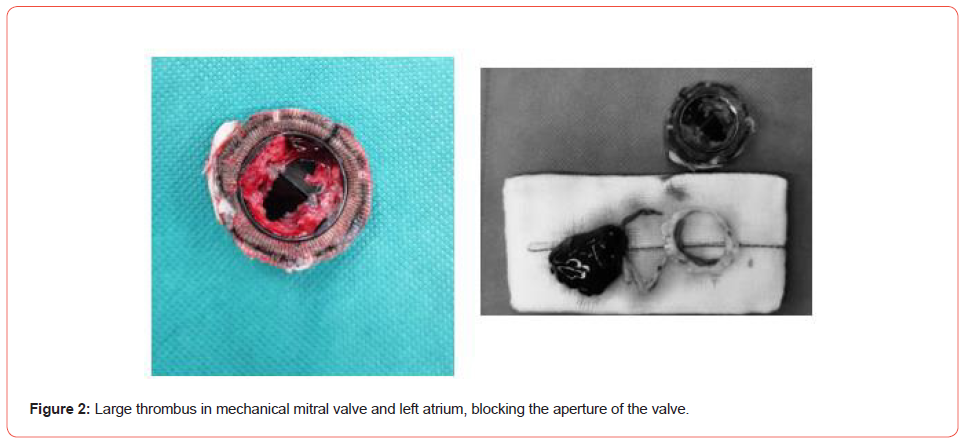
During the operation, she was found to have large blood clot blocking the mitral valve aperture and left atrium. (Figure 2) The mechanical mitral valve was replaced by another mechanical valve. Her right ventricular contractility was mildly impaired and left ventricular contractility was noted to be satisfactory upon valvular replacement. VA-ECMO support was continued during the perioperative period and was withdrawn on the second day in AICU. She made good recovery and discharged from the AICU 4 days afterwards. She continued taking oral warfarin.
Discussion
Valvular replacement is indicated in selected groups of patients with valvular heart disease. Choice of valve depends on patient factors including age, drug compliance, risk of thrombosis, risk of bleeding for vitamin K antagonist treatment, co-morbidity, risk of re-intervention when replaced valve dysfunctions. Informed choice has to be made by patient with the necessary information being provided.
Mechanical valve replacement is advocated for patient <50 years old, as risk of structural deterioration of bioprosthetic valve is higher in young patient [1]. Despite the increasing use of nonoral anti-coagulant, vitamin K antagonist is necessary to prevent thromboembolism in patients with mechanical heart valve [1]. Target of anticoagulation has to be achieved by close monitoring and dosage adjustment. The recommended INR is 2.5-4 for VKA anticoagulation for mechanical mitral valve, which carries a higher thromboembolism risk as compared to other valves.
Nonvitamin K-dependent oral anticoagulants (NOAC) are increasing used in clinical practice, which include direct thrombin inhibitor and oral direct factor Xa inhibitor. There are several researches studying the role of NOAC in thromboembolism prevention for patients with valvular replacement. Oral direct factor Xa inhibitors, Rivaroxaban and Apixaban, have been studied as antithrombotic regimen following transcatheter aortic valve replacement in the GALILEO and ATLANTIS trials respectively [1,2]. GALILEO study was terminated prematurely as Rivaroxaban strategy associated with higher risk of death or thromboembolism than the antiplatelet-based strategy. The ATLANTIS trial was designed to compare oral Apixaban 2.5mg is superior to VKA and aspirin in reducing post-TAVR thromboembolic and bleeding complications [3]. At the current moment, there is lack of evidence to support the use of oral direct factor Xa inhibitor to prevent thromboembolism in mechanical mitral valve replacement. There are several studies ongoing, and more evidence is necessary before oral factor Xa inhibitor can be used in this clinical setting.
Use of direct thrombin inhibitor has a longer history than oral factor Xa inhibitor. The RE-ALIGN investigators published the result of the study comparing dabigatran versus warfarin in patients with mechanical heart valves in year 2013. The trial was terminated prematurely because of excess of thromboembolic and bleeding events in the dabigatran group [4]. It is believed that the highly thrombogenic surface of the mechanical valve requires a very high dose of NOAC to be effective in preventing thromboembolism [5]. There may be new insights into the use of NOAC in thromboembolism prevention in mechanical valve since new valve material has been developed [6].
For recent 20 years, with advances in technology and consumables, VA-ECMO has been increasingly initiated outside the operation theatre [7]. Peripheral VA-ECMO has been used in selected patients with refractory cardiogenic shock or cardiac arrest, although survival rates are <40%. There are no hard and fast rules on selection of patients and timing of initiation due to the fact that peripheral VA-ECMO is usually performed in emergency setting [8]. Examples of inclusion criteria for extracorporeal cardiopulmonary resuscitation (ECPR) are witnessed arrest, arrest to first CPR <5 minutes with bystander CPR, arrest to ECMO flow <60 minutes, and others. It requires a competent ECMO team to establish ECMO support in a timely manner. Cannulation has to be commenced after 10-20 minutes of failed resuscitation efforts [8].
The goal of peripheral VA-ECMO support is to bridge to decision and bridge to therapy. It is a temporizing hemodynamic support [7]. With the availability of more compact and efficient mechanical cardiac support devices, some patients may require prolonged support and switch to other devices.
Conclusion
Prevention of thromboembolism is very important after mechanical heart valve replacement. VKA is still the only anticoagulant of choice for mechanical mitral valve and clotting profile should be monitored. Clinician should practice evidencebased care. Peripheral VA-ECMO is a life sustaining support for bridging to treatment in selected patient.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Ethics Committee approval
Approval number: hkuszh2021191.
Funding statement
The authors received no funding support.
References
- Otto CM, Nishimura RA, Bonow RO, Carabello BA, John P Erwin 3rd, et al. (2021) 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 143(5): e35-e71.
- Windecker S, Tijssen J, Giustino G, Guimarães AHC, RoxanaMehran MD, et al. (2017) Trial design: Rivaroxaban for the prevention of major cardiovascular events after transcatheter aortic valve replacement: Rationale and design of the GALILEO study. Am Heart J 184: 81-87.
- Collet JP, Berti S, Cequier A, Belle EV, Thierry Lefevre, et al. (2018) Oral anti-Xa anticoagulation after trans-aortic valve implantation for aortic stenosis: The randomized ATLANTIS trial. Am Heart J 200: 44-50.
- Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Arie P Kappetein, et al. (2013) Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 369(13): 1206-1214.
- Aimo A, Giugliano RP, De Caterina R (2018) Non-Vitamin K Antagonist Oral Anticoagulants for Mechanical Heart Valves: Is the Door Still Open? Circulation 138(13): 1356-1365.
- Chaudhary R, Garg J, Krishnamoorthy P, Shah N, Matthew W Martinez, et al. (2017) On-X Valve: The Next Generation Aortic Valve. Cardiol Rev 25(2): 77-83.
- Rao P, Khalpey Z, Smith R, Burkhoff D, aniel Burkhoff, Robb D Kociol, et al. (2018) Venoarterial Extracorporeal Membrane Oxygenation for Cardiogenic Shock and Cardiac Arrest. Circ Heart Fail 11: e004905.
- Alexander (Sacha) C, Richardson, Tonna JE, Nanjayya V, Paul Nixon, et al. (2021) Extracorporeal Cardiopulmonary Resuscitation in Adults. Interim Guideline Consensus Statement from the Extracorporeal Life Support Organization. ASAIO Journal 67(3): 221-228.
-
Qian Du, Jun Jin, Si-hao Zheng, Xu Li, Lin Huang, Chak-Kwan Tong. Use of VA-ECMO in Mechanical Mitral Valve thrombosis. Anaest & Sur Open Access J. 3(3): 2022. ASOAJ.MS.ID.000562.
-
Thrombosis, Anticoagulation, VA-ECMO, Chronic rheumatic heart disease, Return of spontaneous circulation (ROSC)
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






