Case Report
Case Report
Systemic Mastocytosis: Case Report and Narrative Review with Practical Recommendations to The Anesthesiologists
Mathias Gutzwiller1*, Christian Grob1, Thierry Girard1, Karin Hartmann2,3 and Salome Dell-Kuster1,4
1Clinic for Anaesthesia, Intermediate Care, Preclinical Emergency Medicine and Pain Therapy, University Hospital Basel, Switzerland
2Department of Dermatology, Division of Allergy, University Hospital Basel and University of Basel, Switzerland
3Department of Biomedicine, University Hospital Basel and University of Basel, Switzerland
4Department of Clinical Research, University of Basel,Switzerland
Mathias Gutzwiller, Clinic for Anaesthesia, Intermediate Care, Preclinical Emergency Medicine and Pain Therapy, University Hospital Basel, Switzerland.
Received Date: August 03, 2022; Published Date:August 18, 2022
Abstract
Mastocytosis is a rare disease with increased clonal mast cells prone to activation and degranulation that has a substantial impact on choice of anesthetic agents and perioperative management. Patients with mastocytosis have up to a six-fold increased risk for anaphylaxis and an increased risk for severe perioperative immediate hypersensitivity reactions (IHR). The prevalence for allergic (immunoglobulin E- mediated) IHR is similar to the general population even if concurrent allergic diseases, which are generally frequent, are also possible. Non-immunological mechanisms involving mast cell receptors are mainly responsible for mast cell degranulation. Risk factors for perioperative IHR in adult patients with mastocytosis are previous anaphylaxis, major surgery, general anesthesia, and lack of adequate premedication. While unspecific triggers (physical, thermal, psychological) are well-accepted cofactors in perioperative IHR, a definitive classification of safe or unsafe drugs remains controversial. Management of patients with mastocytosis requires multidisciplinary and specific preparation for perioperative management, adequate premedication, avoidance of triggering factors, and careful choice of anesthetic drugs.
Keywords:Systemic mastocytosis; General anesthesia; Perioperative management; Neuromuscular blocking drugs; Case report; Narrative review
Abbreviations:ASA: American Society of Anesthesiology; aVWS: acquired von Willebrand syndrome; HES: hydroxyethyl starch; IgE: immunoglobulin E; IHR: immediate hypersensitivity reaction; LA: local anesthetic; MPCM: maculopapular cutaneous mastocytosis; MRGPRX2: Masrelated G-protein coupled receptor member X2; NMBA: neuromuscular blocking agents; NSAIDs: non-steroidal anti-inflammatory drugs; VWF: von Willebrand factor
Introduction
Mastocytosis is a rare disease characterized by abnormal accumulation of clonal mast cells prone to activation and degranulation triggered by different stimuli [1]. Despite its rarity, mastocytosis is a challenge for the anesthetist, as it may be associated with perioperative immediate hypersensitivity reactions (IHR) [2]. Depending on the mechanism of action, IHRs may be allergic (i.e., elicited by an immune reaction, most commonly immunoglobulin E (IgE)-mediated) or non-allergic (i.e., elicited by a non-immune pathway) [3]. Mastocytosis is mainly associated with non-allergic immediate hypersensitivity reaction. Even if mastocytosis is not an allergic disorder, concomitant allergic diseases – which are generally frequent – are also possible in patients with mastocytosis. Some drugs typically used during the perioperative period, especially neuromuscular blocking agents (NMBA), but also hypnotics, opioids, non-steroidal antiinflammatory drugs (NSAIDs), and radiocontrast media may trigger potentially severe IHRs [4-7]. However, the clinical relevance of mast cell degranulation elicited by these drugs may be overestimated in the literature and many uneventful procedures in mastocytosis patients probably go unreported. Of clinical significance for the anesthetist, further anesthesia-related triggers of perioperative IHRs include emotional stress, temperatures changes, pressure, and friction [2, 6]. Controversial recommendations exist in the current literature by different authors. The aim of this narrative review is to provide a thorough update of literature published after the latest review article [2] about the management of patients with mastocytosis in the perioperative setting.
Case presentation
Written informed consent to publish this case report was obtained from the patient. The patient, a 64- year-old Caucasian ASA III male, suffered from an unstable pathological vertebral fracture. Diagnostic workup revealed the diagnosis of systemic mastocytosis and a plasma cell myeloma. The patient was scheduled for percutaneously navigated spondylodesis to stabilize the fracture. His past medical history was uneventful, in particular he did not report on allergies. He had undergone total intravenous general anesthesia 13 years ago, which was uneventful except for postoperative nausea and vomiting. Following the advice of the allergist, premedication with an H1-receptor antagonist (clemastine 1 mg orally) on the evening before and a leukotriene receptor antagonist (montelukast 10 mg orally) on the day of surgery was administered. Anxiolytic medication was deliberately omitted as the patient felt relaxed. Prewarming was initiated with a forced-air warming system at arrival in the anesthetic room. In addition to ASA monitoring standards, invasive blood pressure measurement, continuous esophageal body temperature monitoring, and bispectral index monitoring were installed. Additional intravenous prophylaxes with H1-receptor antagonist (clemastine 2 mg), H2-receptor antagonist (ranitidine 50 mg), and corticosteroids (dexamethasone 4 mg) were administered 20 minutes before induction of anesthesia. Target-controlled infusion of propofol and remifentanil were used together with cisatracurium for neuromuscular blockade and daptomycin as antibiotic prophylaxis. The procedure was performed in prone position with special attention to limit friction during the positioning of the patient. Multimodal analgesia was ensured with fentanyl, remifentanil, and low-dose ketamine. Neuromuscular blockade was maintained with subsequent doses of cisatracurium according to accelerometry following ulnar nerve stimulation (IntelliVue NMT-Module, Philips Amsterdam, the Netherlands). A decrease in mean arterial pressure below 65 mmHg was registered 40 minutes after induction. In absence of other clinical signs of mast cell degranulation, the hypotension was treated with 5-10 μg intravenous boluses of adrenaline up to a total dose of 55 μg. The remaining three hours of anesthesia were uneventful; normothermia could be maintained. After complete spontaneous regression of the neuromuscular blockade to a train-of-four ratio > 0.9, the patient’s trachea was extubated and, the patient was transferred to the intermediate care unit. Analgesia was sustained with paracetamol and intravenous fentanyl. The H1- and H2-receptor antagonists as well as the leukotriene receptor antagonist were continued postoperatively for five days. The postoperative course was only complicated by urinary retention necessitating the placement of a urinary catheter for three days. However, the patient could be transferred to the ward on postoperative day one.
Literature Search
We performed a non-systematic literature search, focusing on any type of articles related to perioperative management of patients with mastocytosis published after 2014 up until March 2021, 2014, being the year of publication of a well referred narrative review on this topic [2]. We searched the PubMed database with the following MeSH terms: “mastocytosis”, “anesthesia”, “surgery”, “case management”, “anaphylaxis”, “Histamine release”, “anesthesia, obstetrical”. Publications were selected in English, German, and French. References were searched for further relevant literature.
Mastocytosis
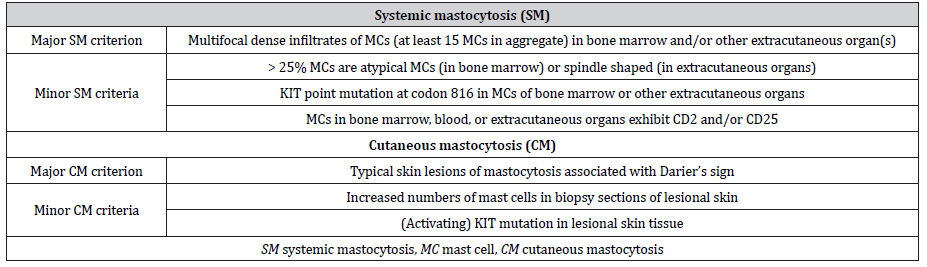
Mastocytosis occurs with an estimated prevalence of 10- 13:100,000 [8, 9] and is frequently related to a gain-of-function variant at amino acid position 816 of the gene KIT (OMIM: https://www.omim.org/entry/154800), which encodes the tyrosine kinase receptor KIT. The variant D816V is associated with autophosphorylation of KIT, resulting in increased mast cell proliferation and activation [5, 10]. Up to 90% of adults with the systemic form and also most patients with the cutaneous form have been found to express the variant KIT D816V. Mastocytosis is characterized by abnormal accumulation of clonal mast cells in one or more organs such as skin, bone marrow, gastrointestinal tract, liver, and spleen [1, 11, 12]. The severity of mastocytosis depends on the degree of mast cell accumulation and releasability, the organs involved, and the presence or absence of specific organ dysfunctions [1]. Clinical manifestation of mast cell activation depends on the effect of preformed mediators (histamine, serotonin, proteases (e.g., tryptase) and heparin), newly formed lipid mediators (e.g., thromboxane, prostaglandin D2, leukotriene C4) and various cytokines (e.g., tumor necrosis factor alpha, interleukin-4) [1, 13]. Symptoms range from benign, such as pruritus, flush, headache, abdominal cramping, and diarrhea to potentially fatal anaphylaxis and cardiac arrest [1]. Clinical signs of end-organ failure are often related to advanced forms of systemic mastocytosis [14]. In 2016, the World Health Organization classified mastocytosis, formerly a myeloproliferative neoplasia, as an independent disease, distinguishing between cutaneous mastocytosis, systemic mastocytosis, and localized mast cell tumors (Table 1) [11].
Cutaneous mastocytosis
Cutaneous mastocytosis mainly occurs during childhood, typically manifesting as maculopapular or nodular hyperpigmented lesions of the skin and has a good prognosis. It is classified into several sub- types (Table 1) [1, 11, 15].
Systemic mastocytosis
Systemic mastocytosis mainly occurs in adults. In addition to possible cutaneous involvement, mast cell accumulation is usually localized in the bone marrow and variably also in other extracutaneous organs (e.g., liver, spleen, gastrointestinal tract, or lymph nodes; systemic mastocytosis) or results in mast cell tumors (mast cell sarcoma) [1, 11]. It can be subdivided into indolent, smoldering, or aggressive forms depending on the presence of organ affection by mast cell infiltration and the presence of organ dysfunction (Table 1) [11, 15]. The diagnosis of systemic mastocytosis is defined by the presence of either at least 1 major plus 1 minor criterion or 3 minor criteria (Table 2) [11, 15]. In addition, patients with anaphylaxis and monoclonal mast cell disorder presenting with KIT D816V, who do not fulfill the abovementioned criteria for the diagnosis of mastocytosis, are referred to as having monoclonal mast cell activation syndrome [7].
Table 1:Updated World Health Organization classification of mastocytosis [11, 15].
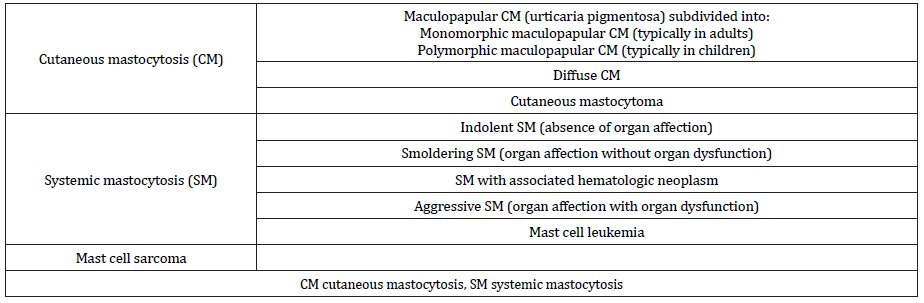
Table 2:Diagnostic criteria for systemic mastocytosis [11] and refined criteria for cutaneous involvement in patients with mastocytosis [11, 15].
Mastocytosis and anesthesia
Latest evidence about mastocytosis and anesthesia
A few case reports and small case series have been published on adult mastocytosis patients undergoing surgery, but we could not find any guidelines on safe perioperative management published by anesthesia societies. We identified only one retrospective cohort study investigating IHRs during 726 anesthetic procedures in mastocytosis patients [16]. The coauthors of this study report a perioperative frequency of mast cell mediator-related
symptoms and anaphylaxis of 2% and 0.4%, respectively, in the adult population and of 4% and 2%, respectively, in the pediatric population. In the subgroup of patients undergoing general anesthesia, the incidence of anaphylaxis was 3% in the adult and 4% in the pediatric population. Triggers of anaphylaxis were postulated to be gastrointestinal manipulation, anxiety and pain in two out of four cases. Hypersensitivity or allergy to drugs was systematically ruled out. These data show an increased frequency of perioperative anaphylaxis in patients with mastocytosis compared to perioperative anaphylaxis in the general Spanish population (0.01% to 0.009%) [17]. All English-language case reports published between 1998 and 2018 on the perioperative management of patients with mastocytosis have recently been summarized in the context of pre-existing allergic preconditions [18]. In these 21 case reports, 52% of the procedures were uneventful [18]. Most of the patients received prophylactic anti-mediator therapy. Concurrent suspected or proven IgE-mediated allergic reactions were responsible for the reported event in four cases (atracurium twice, rocuronium and gelatin, once each) and non-allergic IHR were responsible for the event in five cases (atracurium, vancomycin, linezolid, tourniquet, and colon traction) [18]. Since 2018, another three case reports describing the perioperative course of mastocytosis patients have been published [19-21]. Ripoll et al. report on a patient newly diagnosed with systemic mastocytosis during a recurrent episode of distributive shock during and after cardiac surgery, for which no specific trigger but rather a combination of surgery, general anesthesia, and protamine has been suspected as the elicitor of the mast cell degranulation [19]. Requena Lopez et al. report on postoperative anaphylaxis in a pediatric patient with an undiagnosed solitary mastocytoma [20]. The specific trigger of the reaction remains unknown, as an allergic reaction to any of the drugs administered during the perioperative period was ruled out by subsequent testing. Becerra-Bolanos et al. report on an uneventful general anesthesia in a patient suffering from systemic mastocytosis administered prophylactic premedication and provide a systematic review about the safety of using sugammadex [21]. The paucity of scientific literature on this subject, the severity of the cases presented in earlier case reports, and the fact that most uneventful procedures likely go unreported might have led to an overestimation of the perioperative risk for mastocytosis patients [22]. Increased attention is being paid to the perioperative environment in general in terms of temperature, mechanical pressure, and anxiolytic premedication [22].
Mechanism of IHR in mastocytosis patients and clinical workup
In patients with mastocytosis, mast cell activation and degranulation resulting in a non-allergic IHR can be triggered by non-specific factors (drugs, physical stimuli, temperature changes, or emotional stress) [2, 23]. Patients with mastocytosis might also present with a concurrent drug-induced IgE-mediated reaction (i.e., an allergic IHR) [2, 22]. Beside activation by IgE, other mechanisms play a role in mast cell activation through different types of pathways, including stem cell factor receptor (KIT, CD117), protease-activated receptors, opioid receptors, complement receptors and Mas-related G-protein-coupled receptor member X2 (MRGPRX2) [13, 24, 25]. Nevertheless, the specific role in the perioperative setting and in humans remains unproven for most of these pathways [13, 25].
Clinical presentation of perioperative IHRs
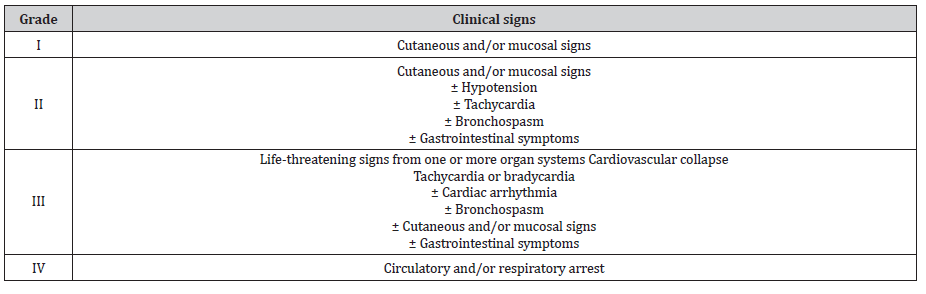
Clinical severity of perioperative IHRs is classified into four grades (modified Ring and Messmer grading; Table 3) [2, 3, 26, 27]. Grades I and II are mild to moderate in presentation and are often caused by non- allergic triggers, whereas grades III and IV are severe and sometimes life-threatening and usually are caused by allergic triggers (IgE-mediated) [3, 27]. Perioperative clinical manifestation of mast cell degranulation is more likely in patients suffering from systemic mastocytosis but can also occur in patients with cutaneous mastocytosis [2, 16, 20, 28]. Perioperative IHR often develops during the induction phase of anesthesia. Drug-induced IHR, by definition, occurs within 1-6 hours after exposure but usually occurs within minutes after exposure in the perioperative setting [22, 29]. Clinical symptoms can range from mild cutaneous symptoms (generalized erythema, flushing, rash) to cardiovascular collapse (hypotension with or without tachycardia) and lifethreatening anaphylaxis [2, 3]. Angioedema, gastrointestinal symptoms, and bronchospasm in absence of underlying airway hyperreactivity are seldom observed in the perioperative setting [2, 6, 30]. Increased number and releasability of mast cells have been reported to lead to an increased frequency of mostly nonallergic drug-induced IHR and an increased clinical severity of all drug-induced IHR [22]. Nevertheless, this has not been reported in clinical practice.
Table 3:Modified Ring and Messmer grading system for perioperative immediate hypersensitivity reactions [2, 3, 26, 27].
Management of anaphylactic reactions
Regarding management of perioperative allergic reactions, we refer the reader to a recently published review by Garvey et al. [26]. The management of perioperative anaphylactic reactions aims to restore vital functions by first removing the suspected trigger. Further therapeutic measures include rapid infusion of crystalloids and increasing oxygen delivery [2, 26, 27, 31]. Intravenous adrenaline is the first-line drug to restore coronary and cerebral perfusion and to maintain cardiac output [27, 31]. Recent guidelines recommend dosing adrenaline and fluid administration according to the severity of the reaction [26].
Workup of perioperative IHRs
Perioperative IHRs require a thorough workup after an event. The main goals are to identify the eliciting factor and to determine a safe alternative for future anesthetic procedures even if the eliciting factor cannot be identified [26]. Systematic multidisciplinary investigation should include complete perioperative documentation, a thorough clinical history, and in vitro and in vivo tests within a recommended period of 1 - 4 months post event [22, 26]. Testing includes skin tests to drugs administered just prior to the IHR, measurement of specific IgE if available for suspected drugs, and possibly a basophil activation test [3]. In some cases, a challenge test can also be performed. Current recommendations to measure serum tryptase level are to collect two blood samples, a first sample at one hour (peak of serum tryptase at 30-90 minutes after symptom onset) and a second at 2 - 4 hours after the reaction for comparison with the baseline value [4, 26]. The definition of increased tryptase level at the time of reaction has recently been changed from > 11.4 ng/mL to a change in tryptase of (1.2x baseline) + 2 ng/mL [32]. In absence of a preoperative tryptase measurement, a serum baseline tryptase level can be measured later, at least 24 hours after the event [26]. Life-threatening reactions associated with an increased tryptase level and positive skin tests to the culprit agent are indicative of an IgE-mediated allergy, whereas mild to moderate reactions, independent of tryptase level, with negative skin tests suggest a non-allergic reaction [18, 26, 33].
Epidemiology of hypersensitivity in the general population and in mastocytosis patients
A reported substantially elevated risk for anaphylaxis in mastocytosis patients is based on investigations on the cumulative incidence of anaphylaxis in patients followed in referral centers [34, 35]. A more realistic estimate has been recently published in a Danish population-based study [36], reporting an incidence rate for anaphylaxis of 6.5 per 1000 person-years in systemic mastocytosis patients as opposed to 0.9 per 1000 person-years in the general population [7, 36]. However, the reported prevalence of IgE- mediated IHR in patients with mastocytosis is similar to the general population [18, 22, 34, 37]. Nevertheless, the prevalence of atopic predisposition was higher in patients with systemic mastocytosis presenting with anaphylaxis than in those presenting without anaphylaxis [38]. The following risk factors for anaphylaxis in patients with systemic mastocytosis have been identified: male sex, absence of mastocytosis in the skin, presence of atopy, total IgE level ≥ 15 kU/L, and baseline tryptase level < 40 ng/mL [34, 36, 38]. Interestingly, the risk of anaphylaxis in systemic mastocytosis patients was not increased at baseline tryptase level > 40 ng/mL [35, 38]. The main triggers of anaphylaxis in adult mastocytosis patients are Hymenoptera sting (27%), food (24%), and medication (18%); in 20% the trigger is idiopathic [35]. NSAIDs, antibiotics (beta-lactam, aminoglycosides), contrast media, phenylephrine, codeine, local anesthetic (LA) and general anesthesia have also been reported as triggers [34, 35], however, a report of formal confirmation by positive testing in these studies is still lacking [37]. While there is a high incidence of patients with clonal mast cell disorders in those presenting with systemic reactions to Hymenoptera venom and elevated serum baseline tryptase level (> 11.4 ng/mL) [39], a similar association between the presence of IHR to drugs and increased serum baseline tryptase level could not be demonstrated [40]. In the general population, data on perioperative IHRs highlight some regional differences in both the frequencies and triggers of such reactions [41]. The 6th National Audit Project in the UK indicated an incidence of severe life-threatening perioperative IHRs (Grade III and IV) of about 1:10,000 anesthetic procedures, with a probable underestimation of about 70% due to methodological limitations [42]. Data from the USA reports a higher incidence of perioperative IHR between 1:677 [43] and 1:6,531 [44]. In about 60-70% of cases, perioperative IHRs are IgE-mediated [13, 33]. NMBA and antibiotics are the most common triggers of perioperative IHRs [33, 41, 42], followed by chlorhexidine (9%) and dyes (patent blue dye 4.5%). Other drugs like hypnotics, opioids, and LA remain rare triggers of IHRs in the general population [41, 42].
Perioperative Management
The main goal of perioperative management in mastocytosis is to reduce the probability of mast cell degranulation and anaphylaxis [2]. The following considerations are important to reach this goal: 1) knowledge and awareness of the disease and its implication for anesthesia, 2) multidisciplinary management, 3) thorough preoperative patient workup, 4) reduction of potential triggers when selecting the anesthesia management, and 5) preparation for a potential anaphylactic reaction.
Preoperative considerations
Patients’ history and workup
The patient’s history should focus on past symptoms specific for mastocytosis, known triggers, prior perioperative reactions, and known drug allergies [4]. However, an uneventful general anesthesia in the past does not guarantee an uneventful course in the future. As highlighted in a population-based study, mastocytosis patients have a mildly increased risk to suffer from anesthesiarelevant comorbidities such as venous thromboembolism, stroke, anaphylaxis, and possibly myocardial infarction [36]. Due to the elevated risk of anaphylaxis associated with increased tryptase values, particularly in the range of 12 to 40 ng/mL, the anesthesia team must acknowledge above normal baseline values [28, 38, 45]. In case of prior perioperative IHR, which has not been further investigated, allergological workup should be preoperatively organized [2, 16, 22]. However, skin tests are not routinely recommended in the absence of previous perioperative IHR [2, 16, 46]. Risk factors for perioperative mast cell mediator-related symptoms in the adult mastocytosis population include previous uninvestigated perioperative IHR, major surgery (particularly gastrointestinal or cardiac), general anesthesia, and lack of prophylactic anti-mediator therapy consisting of at least H1/ H2 antihistamines and benzodiazepine given at least one hour preoperatively [6, 16]. Individual patient risk should be assessed in a multidisciplinary team (anesthesiology, surgery, internal medicine, and/or allergology) resulting in a plan including all aspects of the perioperative management, especially back-up plans [22, 28, 47].
Premedication
Current literature lacks evidence and consensus on the utility of prophylactic antimediator administration. While some authors only focus on maintaining the established antimediator treatment [2], others strongly advise prophylactic anti-mediator administration before undergoing anesthetic procedures. Administration of prophylactic antimediator therapy was shown to reduce the frequency of perioperative IHR in one retrospective cohort study without adverse effects [16]. As a premedication, several authors recommend the administration of antihistamines (mainly H1- receptor antagonists, possibly also combined with H2-receptor antagonists) and sometimes also corticosteroids to block the release of mast cell mediators [2, 4, 6, 22, 24, 30, 48]. Leukotriene receptor antagonists are less widely recommended, but individual reports have found them to be highly effective [6, 16]. Preoperative administration of an anxiolytic drug to reduce emotional stress as an unspecific potential trigger of mast cell degranulation is also widely recommended [2, 4, 6, 16, 22, 30].
Intraoperative considerations
A physical stimulus is rarely the only cause for anaphylaxis but can play a role as a cofactor [6]. Nevertheless, unnecessary friction or pressure should be avoided when manipulating the patient or while operating on organs containing large amounts of mast cells, especially during gastrointestinal tract surgery [2, 6, 28, 49]. Interestingly, manipulation of the airway does not elicit the same reaction, as airway mast cells seem to be less reactive [6]. The patient’s temperature should be monitored and maintained by increasing the ambient air temperature and warming the patient with forced-air warming system and warm infusion. There is a lack of randomized-controlled trials concerning the safe use of anesthetics during the perioperative period, and information on the tolerance of and the reaction to single drugs and drug groups is conflicting [7, 22]. All previous studies recommend avoiding drugs known to be associated with histamine liberation in patients with mastocytosis. Some authors further recommend the avoidance of drugs associated with a higher risk of immediate perioperative hypersensitivity reaction (IgE-mediated or not) [4, 6, 16, 30, 48]. In Table 4, we summarize drugs with a low risk for a perioperative IHR in patients with mastocytosis, and those drugs that should be avoided [2, 4, 6, 18, 28, 30, 37, 47, 48, 50]. We have added a supplementary category of drugs for which data are controversial, especially those known to often elicit perioperative IHR in the general population. While their use is not contraindicated, slow administration is recommended when there is no alternative of an equally effective and safe drug [4, 48].
Table 4:Safety of perioperative drugs for patients with mastocytosis [2, 4, 6, 14, 18, 28, 30, 37, 47, 48].
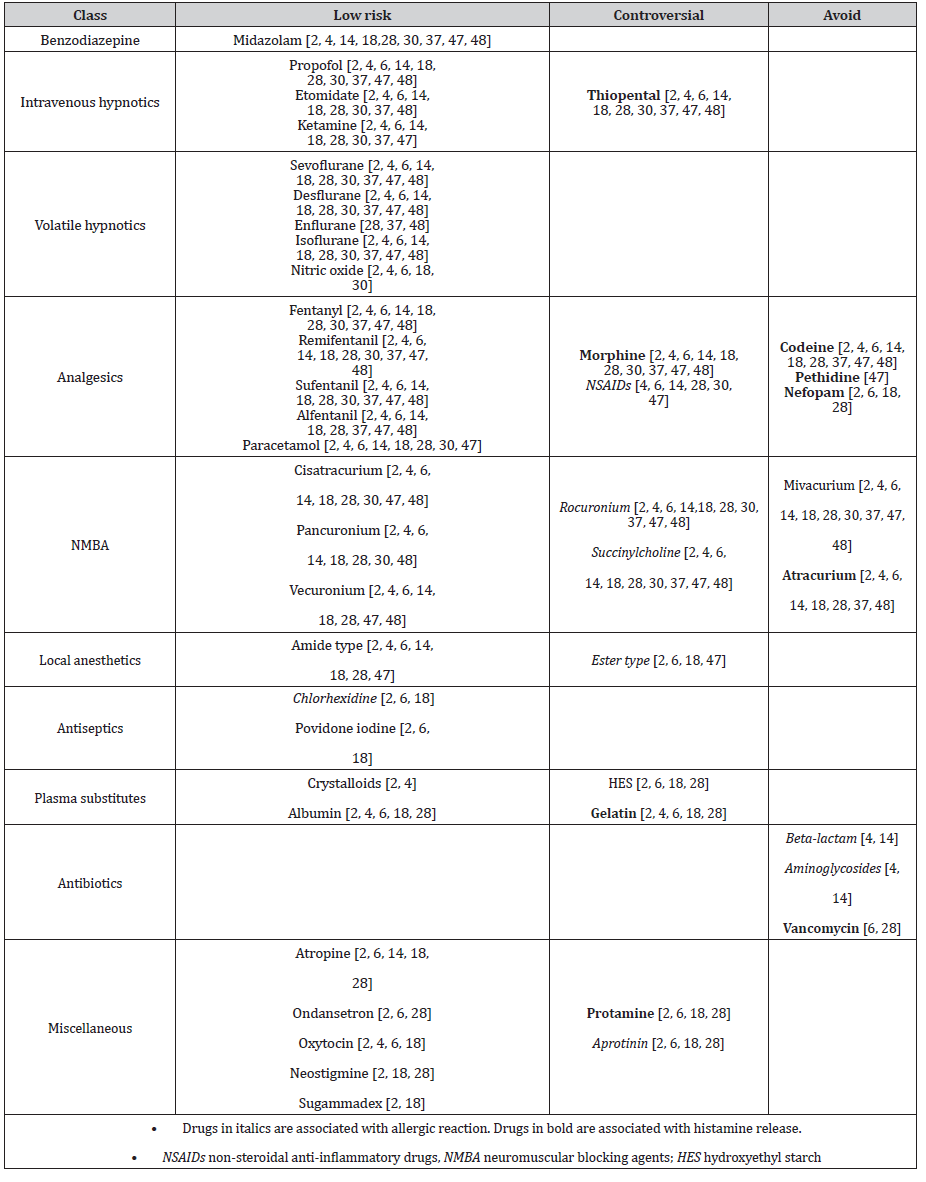
Neuromuscular blocking agents
Data provided by different authors diverge on the safety of different NMBA. Unanimity exists concerning the low-risk use of cisatracurium, pancuronium, and vecuronium, and the avoidance of mivacurium and atracurium as the most potent mast cell activators [2, 4, 6, 28, 30, 37, 47, 48, 50-52]. There are controversial recommendations on the use of rocuronium and succinylcholine [2, 6, 48]. Most publications advise against the use of rocuronium due to its association with allergic reactions [4, 6, 30, 37, 41, 53]. One publication considers succinylcholine as a drug of first choice in patients with mastocytosis, probably based on its low potency for histamine release by mast cells [6, 51, 52], but succinylcholine has also been associated with a high risk of anaphylaxis [41, 42, 53]. The reported incidence of anaphylaxis after the administration of sugammadex, a NMBA reversal drug, is 4:10,000 based on a retrospective, single- center Japanese study [54]. Although, there is a lack of data on the safety of sugammadex in mastocytosis patients, three case reports describe an uneventful administration of sugammadex in patients with this condition [21, 48, 55].
Volatile and intravenous anesthetics
Volatile hypnotics are considered safe and have not been reported to cause perioperative anaphylactic reactions [2, 56]. Recent epidemiologic data show few cases of perioperative anaphylactic reactions to intravenous hypnotic drugs in the general population [33, 42]. There are no reports of perioperative reactions after administration of propofol, thiopental, and ketamine despite a marked heterogeneity of histamine release from human mast cells in response to these often-used hypnotic drugs in an in vitro study [57]. Hence, most authors agree on the low risk of ketamine, propofol, and etomidate in mastocytosis patients [2, 4, 6, 37]. Avoidance of thiopental is advocated by some authors due to the high number of reports of perioperative IHRs [4, 37]. There is no report of perioperative IHRs after administration of midazolam, and it is widely accepted as a low-risk drug in mastocytosis [2, 4, 37, 48], despite some in vitro results of histamine liberation from the lung and skin triggered by this drug [57].
Opioids
While IgE-mediated IHRs to opioids are extremely rare, many opioids are known for direct histamine release possibly due to an off-target occupation of the MRGPRX2 receptor, which has been demonstrated to be involved in histamine release by morphine in an in vitro study [26, 41, 58]. According to an in-vivo study using intradermal microdialysis, fentanyl, alfentanil, sufentanil, remifentanil, buprenorphine, and the opioid antagonist naloxone did not induce the release of histamine and tryptase from mast cells, whereas codeine and meperidine did [59]. Unanimity exists about the low risk of administration of fentanyl, remifentanil, sufentanil, and alfentanil [2, 4, 6, 30, 37, 47, 48], and the avoidance of pethidine and codeine [4, 6, 37, 47, 48]. Hence, morphine remains the most controversial opioid. While it is contraindicated by many authors due to its histamine-releasing effect [4, 30, 37, 47, 48, 57, 60], others support its cautious use with titrated administration in the absence of an alternative [2, 6].
Local anesthetics
The overall incidence of an allergic reaction to LA has been reported to be less than 1% in the general population [61], and recent epidemiologic data have shown no proven allergic reaction to LA in the perioperative setting [33, 41, 42]. Anaphylaxis following LA administration in patients with mastocytosis is rare and has only been reported in a few case reports and in a few studies [22, 35]. The uneventful administration of LA for bone marrow examination further supports its safe use in mastocytosis patients [22]. An amide-type LA is considered safer than ester-type LA in mastocytosis patients [4, 6, 48], as ester- type LA is generally considered to be more antigenic of the two [41].
Plasma substitutes
Most authors agree on the low risk of administering crystalloids and albumin, but controversy exists about hydroxyethyl starch (HES) and gelatin administration in patients with mastocytosis [2, 4, 6]. Recent French [33] and British [42] data show few IHRs to gelatins in the general population, but data concerning HES is lacking due to restricted use in these countries [41].
Antibiotics
Antibiotics, particularly beta-lactams such as amoxicillin/ clavulanic acid, cefazolin, and cefuroxime, are among the leading causes of perioperative IHRs among the general population [33, 41, 42]. Beta-lactam and aminoglycoside antibiotics have also been identified as triggers of anaphylaxis in studies involving mastocytosis patients [34, 35], although to our knowledge it has not been demonstrated that IHRs to these antibiotics were more frequent than in the general population. Only vancomycin has been shown in vitro to elicit histamine liberation from mast cells through the MRGPRX2-mediated mechanism and should thus be avoided in mastocytosis patients if possible [6, 28, 58].
Antiseptics
The antiseptic agent chlorhexidine is widely used in medical and non-medical products, accounting for 9% (number three cause) of perioperative anaphylaxis in the general population [42]. On the other hand, povidone-iodine has only been reported as the culprit agent of perioperative IHR in a few cases [41]. As there are no reports of perioperative IHR to antiseptic agents in mastocytosis patients in the literature, chlorhexidine and povidone iodine are considered low risk [2, 6].
Postoperative considerations
During the postoperative period, pharmacological, psychological, mechanical, and thermal factors pose a risk for triggering mast cell degranulation. As the risk of mast cell degranulation is increased up to 6 hours after the administration of drugs, prolonged postoperative surveillance should be considered [30], and patients with mastocytosis may not qualify for ambulatory surgery [62]. Biphasic perioperative anaphylaxis with an interval of 14 hours after resolution of the first event has also been reported [37]. Special attention should be paid to achieve adequate postoperative analgesia while avoiding potential triggers. Although NSAIDs rarely cause perioperative IHRs in the general population [33, 41, 42], the risk for an IHR to NSAIDs seems to be higher in mastocytosis with an incidence up to 14% [34, 35, 63]. Unable to show an association between IHR to NSAIDs and elevated tryptase level, Seitz and colleagues have concluded that NSAIDs might be administered safely [64]. In contrast, a more recent prospective placebo-controlled challenge study with a cyclooxygenase-1 inhibitor in 50 mastocytosis patients combined with a retrospective chart review has found an elevated risk of IHRs to acetylsalicylic acid and NSAIDs compared to the general population [65]. In addition, 87.5% of patients with an IHR to NSAIDs in the retrospective study have also reported mast cell mediator-related symptoms after physical stimuli and an IHR to other drugs or alcohol. Further, NSAIDs may not only play a role as an elicitor of IHRs, but also as a co-factor in anaphylaxis [7, 22]. These data suggest that treatment with NSAIDs is not contraindicated in patients with mastocytosis, however, careful case-by-case evaluation should be undertaken considering a history of previous IHRs to NSAIDs or other drugs and any risk factors for IHRs to NSAIDs [7, 64, 65]. The risk of adverse reactions after administration of aspirin seems higher than after the administration of other NSAIDs, but for more detail we refer the reader to a recently published review [7]. There are no reports of IHRs to paracetamol, and it is widely accepted as a safe drug [2, 4, 6, 30, 63]. Conclusive literature concerning the use of regional anesthesia in mastocytosis is lacking. However, regional and neuraxial anesthesia using amide-type LA may be a good option for patients at increased risk for perioperative pain, particularly because pain is known as an important trigger for mast cell degranulation and the options for low-risk analgesics are limited (Table 4) [22, 66, 67]. Concerning neuraxial anesthesia, data show good tolerance of epidural and spinal anesthesia with amide-type LA [66, 67]. An undulating effect has been described. Uncertainty exists whether this effect is due to tachyphylaxis [68] or to a local effect of mast cell degranulation [69].
Special considerations
Pediatric anesthesia
Klein and Misseldine have written an extensive review on anesthetic considerations in children with mastocytosis [46]. We refer to this review for pediatric anesthesia management. Based on more recent literature, there are a few updates worth mentioning: The recent recommendation for the subdivision of maculopapular cutaneous mastocytosis (MPCM) into a monomorphic and a polymorphic form [15] has its rationale in the prognostic impact of these two forms in children. While the polymorphic form tends to display normal serum tryptase levels and to spontaneously resolve in adolescence, the monomorphic form frequently has elevated serum tryptase levels and persists into adulthood, usually progressing to a systemic disease. Similar to adults, symptoms in children typically consist of cutaneous manifestations including pruritus, bullous lesions, and flushing related to release of histamine, prostaglandine-D2 and leukotriene-C4, sometimes also abdominal pain, and only rarely IgE-mediated bronchospasm [34, 70, 71]. In contrast to adults, cardiovascular collapse is rarely seen in the pediatric mastocytosis population [72, 73]. Similar to adults, elevated tryptase and/or the presence of flushing are associated with a higher risk of anaphylaxis [35, 74-77]. According to a recent prospective study in children who mostly suffer from MPCM, investigations have shown no association between KIT mutation with cutaneous features of pediatric mastocytosis and evolution of the disease [78, 79]. The authors could also demonstrate that mastocytosis-related symptoms can occur at any age [78]. Careful perioperative management for patients with a history of pediatric mastocytosis is, therefore, recommended even in the absence of cutaneous lesions [78]. However, a review of retrospective records [46] and one prospective study in patients with urticaria pigmentosa [70] indicate a greater risk for major perioperative adverse reactions is unlikely in children with mastocytosis limited to the skin, with or without premedication.
During pregnancy, between 20% to 30% of women with mastocytosis complain about increased disease- related symptoms. However, up to one third describe reduced symptoms or complete relief [66, 67]. Pregnancy outcome is generally good. We refer to a summary of reports about parturients with any form of mastocytosis published between 1995 and 2013 [2]. Since then, an additional prospective study has been published in 2016, which showed no increased probability of severe maternal or infant complications but a slightly increased risk of spontaneous miscarriage during the first trimester [80, 81]. Although cases of preterm delivery and low birth weight are reported, their frequency does not seem to be relevantly different from the general population [66, 67, 81]. Special attention should be paid to severe or insufficiently treated mastocytosis. As many triggers such as pain and stress are present in the peripartum period, administration of early epidural analgesia might be an effective intervention to prevent mast cell degranulation [2]. In addition, most authors recommend periportal premedication with antihistamines with or without corticosteroids according to a recent review [66, 67, 80, 81]. Second generation H1-antagonists like loratadine and cetirizine are recommended [66]. Clemastine may be given if an additional sedating effect is desired. Most other first-generation H1-antagonists should be avoided due to an increased risk of birth defects and fetal abnormalities [81]. Ranitidine is the H2- antagonist of choice [66]. Regarding corticosteroids, most literature considers its short-term use to be generally safe throughout labour and delivery. Non-fluorinated corticosteroids like prednisone are preferable to fluorinated ones like dexamethasone because of reduced placental transfer. Furthermore, oxytocin can be given safely to induce and intensify contractions [66, 67, 80].
Coagulation disorders
A small subgroup of patients with mastocytosis shows an increased risk for bleeding and coagulation abnormalities [19, 69, 82-84]. This might be caused by mast cell release of heparin, heparin-like anticoagulants, and antithrombin III as well as hyperfibrinolysis-inducing mediators like tryptase and tissue plasminogen activator [19, 82-85]. Further, mastocytosisassociated acquired von Willebrand syndrome (aVWS) has been described in two recent retrospective studies in up to 38% of the cases [83, 86]. Diagnostic laboratory testing including coagulation status with prothrombin time, activated partial thromboplastin time, activated clotting time, anti-Xa assay, thrombin time, von Willebrand factor (VWF), and thromboelastography or rotational thromboelastometry should be considered in case of increased bleeding. The most important treatment in these patients is the administration of a mast cell stabilizing therapy including corticosteroids, because standard hemostatic management might be insufficient [83, 86]. Another therapeutic option is tranexamic acid to treat hyperfibrinolysis [69]. In case of proven aVWS, VWFcontaining factor VIII concentrates and desmopressin may be administered [87]. Protamine should be given carefully and only if hyperheparinemia has been shown to be causative for bleeding. Protamine itself has a mast cell degranulating effect and may also cause bleeding when overdosed [19, 69, 82]. In addition, an insufficient neutralizing effect of protamine has been described due to a different structure of released heparin-like anticoagulants [82]. Apart from bleeding complications, the risk of venous thromboembolism seems to be slightly increased in patients with mastocytosis [36]. Therefore and because of a possible prothrombotic mast cell mediator release, some literature advise mandatory postoperative thrombosis prophylaxis [69].
Conclusions
Patients with mastocytosis have a higher frequency and severity of perioperative immediate hypersensitivity reactions, including anaphylaxis, than the general population. However, the incidence of allergic diseases in general is not increased. Because it is unclear whether to avoid only drugs releasing histamine or also drugs known to elicit an allergic reaction, the classification of drugs as safe or unsafe remains controversial. Factors triggering mast cell release such as physical, thermal, and psychological factors are generally accepted as co-factors in perioperative IHRs. Thorough preparation including gathering information about the patient’s history, administering adequate premedication, careful choice of perioperative anesthesia management that considers the expertise of specialists, avoids factors inducing mast cell degranulation, and includes postoperative surveillance are key to an uneventful perioperative course.
Acknowledgments
We would like to thank our patient for the permission to publish this case report. Allison Dwileski’s editorial assistance for this manuscript is gratefully acknowledged.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
None.
References
- Valent P, Akin C, Hartmann K, Alvarez Twose I, Brockow K, et al. (2021) Updated diagnostic criteria and classification of mast cell disorders: A consensus proposal. Hemasphere 5(11): e646.
- Dewachter P, Castells MC, Hepner DL, Mouton Faivre C (2014) Perioperative management of patients with mastocytosis. Anesthesiology 120(3): 753-759.
- Garvey LH, Ebo DG, Mertes PM, Dewachter P, Garcez T, et al. (2019) An eaaci position paper on the investigation of perioperative immediate hypersensitivity reactions. Allergy 74(10): 1872-1884.
- Bonadonna P, Lombardo C (2014) Drug allergy in mastocytosis. Immunol Allergy Clin North Am. 34(2): 397-405.
- Theoharides TC, Valent P, Akin C (2015) Mast cells, mastocytosis, and related disorders. N Engl J Med 373(2): 163-172.
- Hermans MAW, Arends NJT, Gerth van Wijk R, van Hagen PM, Kluin Nelemans HC et al. (2017) Management around invasive procedures in mastocytosis: An update. Ann Allergy Asthma Immunol 119(4): 304-309.
- Carter MC, Metcalfe DD, Matito A, Escribano L, Butterfield JH, et al. (2019) Adverse reactions to drugs and biologics in patients with clonal mast cell disorders: A work group report of the mast cells disorder committee, american academy of allergy, asthma & immunology. J Allergy Clin Immunol 143(3): 880-893.
- Cohen SS, Skovbo S, Vestergaard H, Kristensen T, Moller M, et al. (2014) Epidemiology of systemic mastocytosis in denmark. Br J Haematol 166(4): 521-528.
- van Doormaal JJ, Arends S, Brunekreeft KL, van der Wal VB, Sietsma J, et al. (2013) Prevalence of indolent systemic mastocytosis in a dutch region. J Allergy Clin Immunol 131(5): 1429-1431e1.
- Valent P, Akin C, Escribano L, Fodinger M, Hartmann K, et al. (2007) Standards and standardization in mastocytosis: Consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest 37(6): 435-453.
- Valent P, Akin C, Metcalfe DD (2017) Mastocytosis: 2016 updated who classification and novel emerging treatment concepts. Blood 129(11): 1420-1427.
- Schuch A, Brockow K (2017) Mastocytosis and anaphylaxis. Immunol Allergy Clin North Am 37(1): 153-164.
- Spoerl D, Nigolian H, Czarnetzki C, Harr T (2017) Reclassifying anaphylaxis to neuromuscular blocking agents based on the presumed patho-mechanism: Ige-mediated, pharmacological adverse reaction or "innate hypersensitivity"? Int J Mol Sci 18(6): 1223.
- Bonadonna P, Lombardo C, Zanotti R (2014) Mastocytosis and allergic diseases. J Investig Allergol Clin Immunol 24(5): 288-297.
- Hartmann K, Escribano L, Grattan C, Brockow K, Carter MC, et al. (2016) Cutaneous manifestations in patients with mastocytosis: Consensus report of the european competence network on mastocytosis; the american academy of allergy, asthma & immunology; and the european academy of allergology and clinical immunology. J Allergy Clin Immunol 137(1): 35-45.
- Matito A, Morgado JM, Sanchez-Lopez P, Alvarez-Twose I, Sanchez Munoz L, et al. (2015) Management of anesthesia in adult and pediatric mastocytosis: A study of the Spanish network on mastocytosis (rema) based on anesthetic procedures. Int Arch Allergy Immunol 167(1): 47-56.
- Escolano F, Valero A, Huguet J, Baxarias P, de Molina M, et al. (2002) [prospective epidemiologic study of perioperative anaphylactoid reactions occurring in catalonia (1996-7) Rev Esp Anestesiol Reanim 49(6): 286-293.
- Dewachter P, Kopac P, Laguna JJ, Mertes PM, Sabato V et al. (2019) Anaesthetic management of patients with pre-existing allergic conditions: A narrative review. Br J Anaesth 123(1): e65-e81.
- Ripoll JG, Ritter MJ, Comfere TB, Smith MM, Schaff HV, et al. (2019) Undiagnosed systemic mastocytosis presenting as postoperative distributive shock: A case report. AA Pract 13(10): 392-395.
- Requena Lopez S, Matito A, Alvarez Twose I, Torrelo A (2019) Perioperative anaphylaxis in a patient with a solitary mastocytoma. Pediatr Dermatol 36(3): 352-354.
- Becerra Bolanos A, Muino Palomar V, Cabrera Doreste S, Rodriguez Perez A (2021) Sugammadex in systemic mastocytosis: Case report and a systematic review of literature. Anaesthesist 9.
- Bonadonna P, Pagani M, Aberer W, Bilo MB, Brockow K et al, (2015) Drug hypersensitivity in clonal mast cell disorders: Enda/eaaci position paper. Allergy. 70(7): 755-763.
- Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ et al, (2004) Revised nomenclature for allergy for global use: Report of the nomenclature review committee of the world allergy organization, october 2003. J Allergy Clin Immunol. 113(5): 832-836.
- Romantowski J, Gorska A, Niedoszytko M, Gulen T, Gruchala Niedoszytko M et al, (2021) A challenge for allergologist: Application of allergy diagnostic methods in mast cell disorders. Int J Mol Sci. 1; 22(3):1454.
- Falduto GH, Pfeiffer A, Luker A, Metcalfe DD, Olivera A (2021) Emerging mechanisms contributing to mast cell-mediated pathophysiology with therapeutic implications. Pharmacol Ther. 220107718.
- Garvey LH, Dewachter P, Hepner DL, Mertes PM, Voltolini S et al, (2019) Management of suspected immediate perioperative allergic reactions: An international overview and consensus recommendations. Br J Anaesth. 123(1): e50-e64.
- Dewachter P, Savic L (2019) Perioperative anaphylaxis: Pathophysiology, clinical presentation and management. BJA Education. 19(10): 313-320.
- Dewachter P, Mouton-Faivre C, Cazalaa JB, Carli P, Lortholary O et al, (2009) [mastocytosis and anaesthesia]. Ann Fr Anesth Reanim. 28(1): 61-73.
- Demoly P, Adkinson NF, Brockow K, Castells M, Chiriac AM et al, (2014) International consensus on drug allergy. Allergy. 69(4): 420-437.
- Konrad FM, Unertl KE, Schroeder TH (2009) Mastocytosis A challenge in anaesthesiology. Anaesthesist. 58(12): 1239-1243.
- Muraro A, Roberts G, Worm M, Bilo MB, Brockow K et al, (2014) Anaphylaxis: Guidelines from the european academy of allergy and clinical immunology. Allergy. 69(8):1026-1045.
- Hopkins PM, Cooke PJ, Clarke RC, Guttormsen AB, Platt PR et al, (2019) Consensus clinical scoring for suspected perioperative immediate hypersensitivity reactions. Br J Anaesth. 123(1): e29-e37.
- Tacquard C, Collange O, Gomis P, Malinovsky JM, Petitpain N et al, (2017) Anaesthetic 602 hypersensitivity reactions in france between 2011 and 2012: The 10th gerap epidemiologic 603 survey. Acta Anaesthesiol Scand. 61(3): 290-299.
- Gonzalez de Olano D, de la Hoz Caballer B, Nunez Lopez R, Sanchez Munoz L, Cuevas Agustin M et al, (2007) Prevalence of allergy and anaphylactic symptoms in 210 adult and pediatric 606 patients with mastocytosis in spain: A study of the spanish network on mastocytosis (rema). 607. Clin Exp Allergy. 37(10):1547-1555.
- Brockow K, Jofer C, Behrendt H, Ring J (2008) Anaphylaxis in patients with mastocytosis: study on history, clinical features and risk factors in 120 patients. Allergy. 63(2): 226-232.
- Broesby Olsen S, Farkas DK, Vestergaard H, Hermann AP, Moller MB et al, (2016) Risk of solid cancer, cardiovascular disease, anaphylaxis, osteoporosis and fractures in patients 612 with systemic mastocytosis: A nationwide population-based study. Am J 91(11): 1069-1075.
- Brockow K, Bonadonna P (2012) Drug allergy in mast cell disease. Curr Opin Allergy Clin Immunol. 12(4): 354-360.
- Gulen T, Ljung C, Nilsson G, Akin C (2017) Risk factor analysis of anaphylactic reactions in patients with systemic mastocytosis. J Allergy Clin Immunol Pract. 5(5): 1248-1255.
- Bonadonna P, Perbellini O, Passalacqua G, Caruso B, Colarossi S et al, (2009) Clonal mast cell disorders in patients with systemic reactions to hymenoptera stings and increased serum 620 tryptase levels. J Allergy Clin Immunol. 123(3): 680-686.
- Bonadonna P, Zanotti R, Pagani M, Caruso B, Perbellini O et al, (2009) How much specific is the association between hymenoptera venom allergy and mastocytosis? Allergy. 64(9):1379-1382.
- Mertes PM, Ebo DG, Garcez T, Rose M, Sabato V, et al. (2019) Comparative epidemiology of suspected perioperative hypersensitivity reactions. Br J Anaesth 123(1): e16-e28.
- Harper NJN, Cook TM, Garcez T, Farmer L, Floss K, et al. (2018) Anaesthesia, surgery, and life-threatening allergic reactions: Epidemiology and clinical features of perioperative anaphylaxis in the 6th national audit project (nap6). Br J Anaesth 121(1): 159-171.
- Saager L, Turan A, Egan C, Mascha EJ, Kurz A, et al. (2015) Incidence of intraoperative hypersensitivity reactions: A registry analysis: A registry analysis. Anesthesiology 122(3): 551-559.
- Gonzalez-Estrada A, Carrillo-Martin I, Renew JR, Rank MA, Campbell RL, et al. (2021) Incidence of and risk factors for perioperative or periprocedural anaphylaxis in the United States from 2005 to 2014. Ann Allergy Asthma Immunol 126(2): 180-186e3.
- van Anrooij B, van der Veer E, de Monchy JG, van der Heide S, Kluin-Nelemans JC, et al. (2013) Higher mast cell load decreases the risk of hymenoptera venom-induced anaphylaxis in patients with mastocytosis. J Allergy Clin Immunol 132(1): 125-130.
- Klein NJ, Misseldine S (2013) Anesthetic considerations in pediatric mastocytosis: A review. J Anesth 27(4): 588-598.
- Ulbrich F, Engelstadter H, Wittau N, Steinmann D (2013) Anaesthetic management of emergency caesarean section in a parturient with systemic mastocytosis. Int J Obstet Anesth 22(3): 243-246.
- Unterbuchner C, Hierl M, Seyfried T, Metterlein T (2017) Anaesthesia and orphan disease: Rapid sequence induction in systemic mastocytosis. Eur J Anaesthesiol 34(3): 176-178.
- Ishii K, Miyoshi H, Akiyama D, Makita T, Sumikawa K, et al. (2018) Anesthetic management of patients with systemic mastocytosis. J Clin Anesth 50: 61.
- Bonadonna P, Lombardo C, Zanotti R (2014) Mastocytosis and allergic diseases. J Investig Allergol Clin Immunol 24(5): 288-297.
- Stellato C, de Paulis A, Cirillo R, Mastronardi P, Mazzarella B, et al. (1991) Heterogeneity of human mast cells and basophils in response to muscle relaxants. Anesthesiology 74(6): 1078-1086.
- Koppert W, Blunk JA, Petersen LJ, Skov P, Rentsch K, et al. (2001) Different patterns of mast cell activation by muscle relaxants in human skin. Anesthesiology 95(3): 659-667.
- Petitpain N, Argoullon L, Masmoudi K, Fedrizzi S, Cottin J, et al. (2018) Neuromuscular 655 blocking agents induced anaphylaxis: Results and trends of a french pharmacovigilance survey from 2000 to 2012. Allergy 73(11): 2224-2233.
- Miyazaki Y, Sunaga H, Kida K, Hobo S, Inoue N, et al. (2018) Incidence of anaphylaxis associated with sugammadex. Anesth Analg 126(5): 1505-1508.
- Dewachter P, Mouton-Faivre C (2018) Concurrent immunoglobulin e-mediated neuromuscular blocking agent allergy in systemic mastocytosis. Ann Allergy Asthma Immunol 120(6): 663-664.
- Mertes PM, Malinovsky JM, Jouffroy L, Working Group of the S, Sfa, et al. (2011) Reducing the risk of anaphylaxis during anesthesia: 2011 updated guidelines for clinical practice. J Investig Allergol Clin Immunol 21(6): 442-453.
- Marone G, Stellato C, Mastronardi P, Mazzarella B (1993) Mechanisms of activation of 666 human mast cells and basophils by general anesthetic drugs. Ann Fr Anesth Reanim 12(2): 116-125.
- Navines-Ferrer A, Serrano-Candelas E, Lafuente A, Munoz-Cano R, Martin M et al. (2018) Mrgprx2-mediated mast cell response to drugs used in perioperative procedures and anaesthesia. Sci Rep 8(1): 11628.
- Blunk JA, Schmelz M, Zeck S, Skov P, Likar R, et al. (2004) Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: An in vivo microdialysis study in human skin. Anesth Analg 98(2): 364-370.
- Stellato C, Cirillo R, de Paulis A, Casolaro V, Patella V, et al. (1992) Human basophil/mast cell releasability. Ix. Heterogeneity of the effects of opioids on mediator release. Anesthesiology 77(5): 932-940.
- Bhole MV, Manson AL, Seneviratne SL, Misbah SA (2012) Ige-mediated allergy to local 678anaesthetics: Separating fact from perception: A uk perspective. Br J Anaesth 108(6): 903-911.
- Konrad FM, Schroeder TH (2009) Anaesthesia in patients with mastocytosis. Acta Anaesthesiol Scand 53(2): 270-271.
- Sanchez-Matas I, Matito-Bernechea A, Gonzalez de Olano D, Alvarez-Twose I, Sanchez Munoz L (2009) Prevalence of hypersensitivity reactions to nonsteroidal anti-inflamatory 684 drugs in 212 patients with mastocytosis in spain. Allergy 64551-64598.
- Seitz CS, Brockow K, Hain J, Trautmann A (2014) Non-steroidal anti-inflammatory drug hypersensitivity: Association with elevated basal serum tryptase?. Allergy Asthma Clin Immunol 10(1): 19.
- Hermans MAW, van der Vet SQA, van Hagen PM, van Wijk RG, van Daele PLA (2018) Low frequency of acetyl salicylic acid hypersensitivity in mastocytosis: The results of a double- blind, placebo-controlled challenge study. Allergy 73(10): 2055-2062.
- Matito A, Alvarez-Twose I, Morgado JM, Sanchez-Munoz L, Orfao A, et al. (2011) Clinical impact of pregnancy in mastocytosis: A study of the spanish network on mastocytosis (rema) in 45 cases. Int Arch Allergy Immunol 156(1): 104-111.
- Worobec AS, Akin C, Scott LM, Metcalfe DD (2000) Mastocytosis complicating pregnancy. Obstet Gynecol 95(3): 391-395.
- Villeneuve V, Kaufman I, Weeks S, Deschamps A (2006) Anesthetic management of a labouring parturient with urticaria pigmentosa. Can J Anaesth 53(4): 380-384.
- B Sido, J Homann, H-J Hertfelder, T Zienkiewicz, K-P Christians, et al. (2019) Surgical interventions in patients with systemic mast cell activation disease: Recommendations for perioperative management. Chirurg 90(7): 548-556.
- Heinze A, Kuemmet TJ, Chiu YE, Galbraith SS (2017) Longitudinal study of pediatric urticaria pigmentosa. Pediatr Dermatol 34(2): 144-149.
- Ben-Amitai D, Metzker A, Cohen HA (2005) Pediatric cutaneous mastocytosis: A review of patients. Isr Med Assoc J 7(5): 320-322.
- Tirel O, Chaumont A, Ecoffey C (2001) [circulatory arrest in the course of anesthesia for a child with mastocytosis]. Ann Fr Anesth Reanim 20(10): 874-875.
- Castells M, Metcalfe DD, Escribano L (2011) Diagnosis and treatment of cutaneous mastocytosis in children: Practical recommendations. Am J Clin Dermatol 12(4): 259-270.
- Brockow K, Ring J, Alvarez-Twose I, Orfao A, Escribano L (2012) Extensive blistering is a predictor for severe complications in children with mastocytosis. Allergy 67(10): 1323-1324.
- Brockow K, Akin C, Huber M, Metcalfe DD (2003) Assessment of the extent of cutaneous involvement in children and adults with mastocytosis: Relationship to symptomatology, tryptase levels, and bone marrow pathology. J Am Acad Dermatol 48(4): 508-516.
- Alvarez-Twose I, Vano-Galvan S, Sanchez-Munoz L, Morgado JM, Matito A, et al. (2012) Increased serum baseline tryptase levels and extensive skin involvement are predictors for the severity of mast cell activation episodes in children with mastocytosis. Allergy 67(6): 813-821.
- Lange M, Niedoszytko M, Renke J, Glen J, Nedoszytko B (2013) Clinical aspects of paediatric mastocytosis: A review of 101 cases. J Eur Acad Dermatol Venereol 27(1): 97-102.
- Meni C, Georgin-Lavialle S, Le Sache de Peufeilhoux L, Jais JP, et al. (2018) Paediatric mastocytosis: Long-term follow-up of 53 patients with whole sequencing of kit. A 722 prospective study. Br J Dermatol 179(4): 925-932.
- Wiechers T, Rabenhorst A, Schick T, Preussner LM, Forster A, et al. (2015) Large maculopapular cutaneous lesions are associated with favorable outcome in childhood-onset mastocytosis. J Allergy Clin Immunol 136(6): 1581-1590e3.
- Ciach K, Niedoszytko M, Abacjew-Chmylko A, Pabin I, Adamski P et al. (2016) Pregnancy and delivery in patients with mastocytosis treated at the polish center of the european competence network on mastocytosis (ecnm). PLoS One 11(1): e0146924.
- Lei D, Akin C, Kovalszki A (2017) Management of mastocytosis in pregnancy: A review. J Allergy Clin Immunol Pract 5(5): 1217-1223.
- Sucker C, Mansmann G, Steiner S, Gattermann N, Schmitt-Graeff A, et al. (2008) Fatal bleeding due to a heparin-like anticoagulant in a 37-year-old woman suffering from systemic mastocytosis. Clin Appl Thromb Hemost 14(3): 360-364.
- Carvalhosa AB, Aouba A, Damaj G, Canioni D, Brouzes C, et al. (2015) A french national survey on clotting disorders in mastocytosis. Medicine (Baltimore) 94(40): e1414.
- Moreno-Ancillo A, Gil-Adrados AC, Dominguez-Noche C, Jurado-Palomo J (2013) Bleeding and shock in a 44-year-old woman with systemic mastocytosis. J Investig Allergol Clin Immunol 23(7): 517-518.
- Thomas VA, Wheeless CJ, Stack MS, Johnson DA (1998) Human mast cell tryptase fibrinogenolysis: Kinetics, anticoagulation mechanism, and cell adhesion disruption. Biochemistry 37(8): 2291-2298.
- Mital A, Prejzner W, Hellmann A (2018) Acquired von willebrand syndrome during systemic mastocytosis: An analysis of 21 cases. Pol Arch Intern Med 128(7-8): 491-493.
- Majeranowski A, Mital A, Zawilski J, Czarnogórski M, Janowiak-Majeranowska A (2020) Acquired von willebrand syndrome associated with indolent systemic mastocytosis. Journal of Transfusion Medicine 13(2): 151-152.
-
Mathias Gutzwiller*, Christian Grob, Thierry Girard, Karin Hartmann and Salome Dell-Kuster. Systemic Mastocytosis: Case Report and Narrative Review with Practical Recommendations to The Anesthesiologists. Anaest & Sur Open Access J. 3(4): 2022. ASOAJ.MS.ID.000567.
-
Immediate hypersensitivity reactions (IHR), Mast cell tumors, Surgery, Anxiety, Pain, Local anesthetic, Tryptase, Gastrointestinal tract surgery, NSAIDs.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.