 Case Report
Case Report
Pannus-Induced Delayed Coronary Obstruction After TAVR
Steven J Chen1*, Daniel Mattimore2, Jonathan Price3 and Jeremy Poppers2
1Renaissance School of Medicine, Stony Brook University, Stony Brook, NY, 11794, United States
2Department of Anesthesiology, Stony Brook University Hospital, Stony Brook, NY, 11794, United States
3Division of Cardiothoracic Surgery, Department of Surgery, Stony Brook University Hospital, Stony Brook, NY, 11794, United States
Steven Chen, Renaissance School of Medicine, Stony Brook University, Stony Brook, NY, 11794, United States
Received Date: July 22, 2024; Published Date: August 01, 2024
Abstract
Transcatheter aortic valve replacement (TAVR) is now a well-established and widely employed minimally invasive alternative to open surgery for the definitive treatment of aortic valve stenosis. While TAVR avoids many risks inherent to open valve surgery, there are other complications unique to the TAVR procedure. One such risk is coronary obstruction, which can be attributed to several mechanisms. Examples include valve displacement and disruption of atherosclerotic plaques, which most commonly occur within seconds to minutes after initial valve deployment, and less commonly, delayed coronary obstruction (DCO), which can occur months or even years after valve replacement surgery as a result of fibrosis and pannus formation. Herein we report a case of left main coronary artery obstruction presenting as an ST-elevation myocardial infarction (STEMI), which occurred nearly five years after the initial TAVR implantation. The patient underwent an urgent surgical aortic valve replacement and coronary bypass grafting as both the preoperative imaging and intraoperative findings revealed organized pannus formation with near total occlusion of the left sinus of Valsalva. This case illustrates a rare case of DCO, the etiology of which may be influenced by several modifiable factors, including lifestyle and preemptive pharmacologic therapy, and anesthetic considerations when DCO is suspected. Pannus-Induced Delayed Coronary Obstruction After TAVR.
Keywords: TAVR; Pannus formation; Delayed coronary obstruction; Sinus of Valsalva; Granulomatous inflammation; Stent thrombosis
Introduction
TAVR is a minimally invasive alternative for open surgical replacement for calcific aortic stenosis, with numerous landmarks randomized controlled trials supporting its use in patients with intermediate and high surgical risk [1-6]. With the indications for TAVR expanding to the treatment of patients with low surgical risk, and as life expectancy in developed countries continues to grow, TAVR use has become more ubiquitous [1]. However, several life-threatening complications of TAVR have now been documented, one of which is delayed obstruction of the coronary ostia after valve placement [7-9].
Delayed coronary artery obstruction (DCO) after TAVR implantation is a rare complication occurring in fewer than 1% of implanted TAVRs and carries a 30-day mortality rate approaching 50% [10- 12]. DCO has been previously classified into two broad categories based on its time of presentation [11,13]. Early coronary obstruction occurs within seconds and up to seven days after TAVR due to obstruction from the calcified native valve leaflet, [10] whereas DCO presents any time after seven days of valve implantation [11]. Risk factors for early coronary obstruction include anatomical variations that predispose to occlusion after valve deployment, such as a narrow sinus of Valsalva, low coronary height, and/or excessive prior calcification of the stenotic aortic valve [5]. In contrast to early obstruction, the most common etiologies for DCO involve inflammatory-mediated thrombosis, endothelialization of the valve implant, and valve-in-valve procedures [11].
While multiple reports have been published regarding DCO in the span of months to years after implantation, information on DCO presenting multiple years after the initial operation is sparse [14,15]. Here, we present a case of a patient who experienced unstable angina for several months, which was ultimately attributed to DCO secondary to pannus formation within the stent of the valve prosthesis that had been implanted five years prior to presentation.
Case Report
A 62 year-old female with a BMI of 31.2 kg/m2 presented to the emergency department with chest pain and evidence of an ST-elevation myocardial infarction. She had undergone an implantation of a 20 mm Edwards Sapien 3 TAVR five years prior to admission for severe symptomatic aortic stenosis. Other relevant medical history included hypertension, hyperlipidemia, right bundle branch block (RBBB), and chronic obstructive pulmonary disease (COPD), and an active 80-pack-year smoking history. Aortic valve replacement was recommended and offered to the patient, but she declined to pursue an evaluation for TAVR.
Initially, the patient presented with shortness of breath after climbing one flight of stairs. Her pulmonary function testing (PFT) baseline was an FEV1 of 1.39 L, FVC of 2.05 L, and an FEV1/FVC ratio of 67.8%. On ECG, ST elevations were noted in a VR with diffuse ST depressions in the anterior and inferior leads. Troponins the next day were elevated to 4.15 ng/mL (normal range 0-0.04 ng/ mL). Subsequent cardiac catheterization revealed 80% stenosis of the first diagonal branch for which a drug-eluting stent (DES) was deployed. Residual stenosis was 0%. The patient was discharged 2 days later on aspirin 81 mg daily and ticagrelor 90 mg twice daily for at least one year.
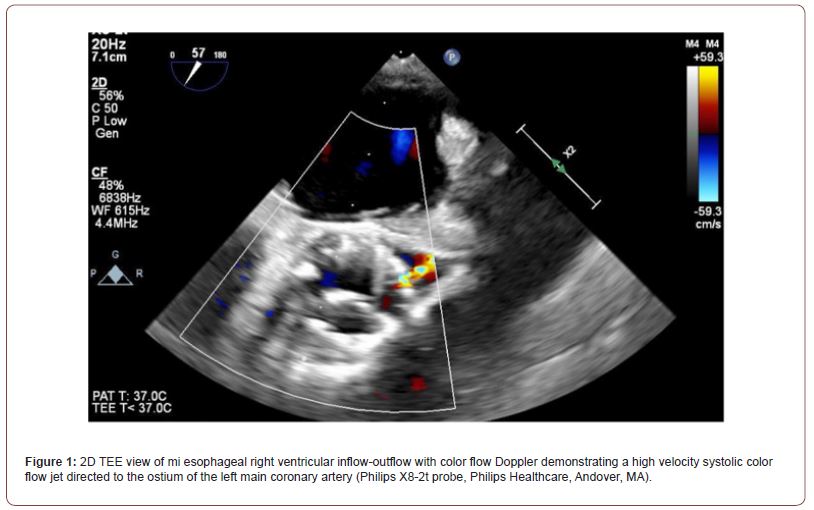
Eight months after her coronary stent placement, the patient presented with complaints of daily anginal symptoms. During cardiac catheterization, initial attempts to cannulate the left main orifice were unsuccessful because the catheter would not traverse the cells of the stent of the TAVR prosthesis. Once the catheter was within the valve stent, the patient experienced transient episodes of diffuse ST elevation that resolved upon withdrawal of the catheter, but no evidence of significant coronary obstruction was noted. Further evaluation included a transeophageal echocardiogram (TEE), which revealed a high velocity color flow Doppler jet at the left main coronary ostium, supporting the diagnosis that the TAVR leaflet may be responsible for occlusion of the coronary artery. Since the patient had been taking ticagrelor, surgery was deferred by one week to minimize bleeding risks.
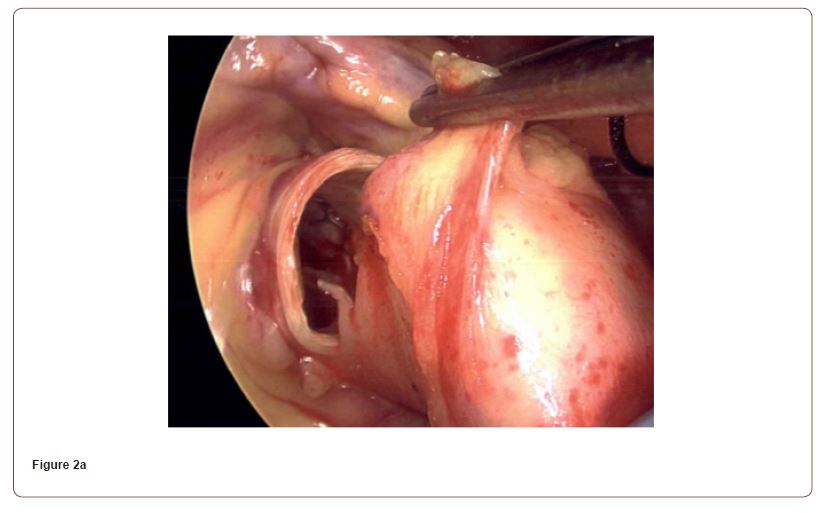
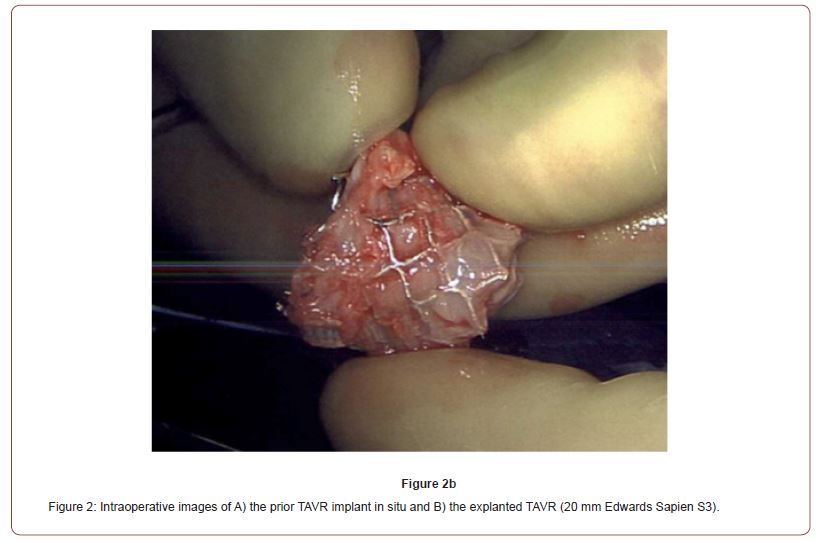
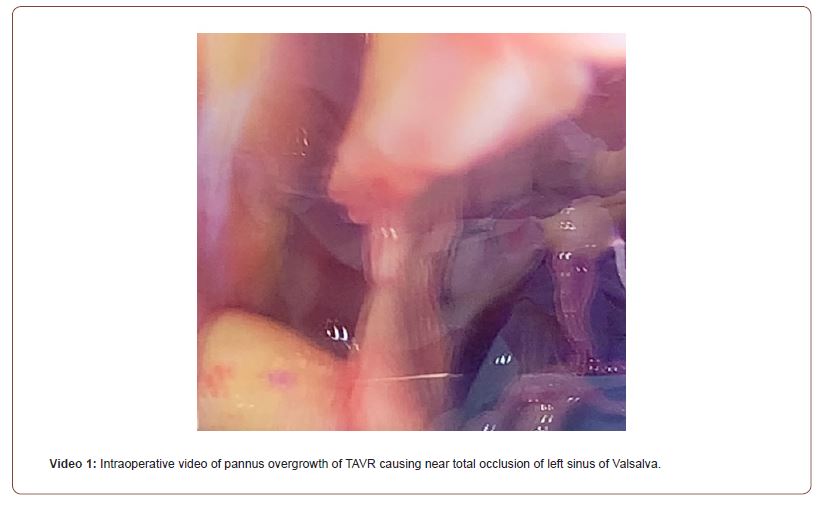
One week after cardiac catheterization, the patient was brought to the operating room. General anesthesia was induced with judicious use of midazolam, fentanyl, propofol, rocuronium, and phenylephrine. Intraoperative TEE confirmed the presence of a high velocity color flow Doppler jet directed towards the left main coronary ostium (Figure 1). After initiation of cardiopulmonary bypass, an intraoperative assessment demonstrated chronic organized pannus with near total occlusion of the metal TAVR scaffold overlying the left sinus of Valsalva (Video 1). A slit-like opening was noted in which blood could flow through the stent into the left coronary sinus, but the sinus was otherwise completely obstructed. The TAVR had also been heavily incorporated into the right sinus of Valsalva (Figure 2). The sinotubular junction down to the aortic annulus was noted to be of narrow caliber and able to accommodate a 19 mm valve. An Edwards Inspiris Resilia 19 mm aortic valve bioprosthesis was implanted. At the time of valve replacement also underwent a single vessel coronary artery bypass graft of the left anterior descending artery utilizing the left internal mammary artery. Surgical pathology of the explanted bioprosthesis revealed three pale, yellow, partially calcified valve leaflets in the lumen.
The patient had a benign postoperative course, as she was extubated on post-operative (POD) 0 and subsequently discharged on POD 7. At her three-month post-operative visit, she was recovering well and ambulating around the home without difficulty. She continues to smoke 0.5 packs per day, decreased from her previous smoking history of 2-2.5 packs per day.
Discussion
Minimally invasive structural heart techniques to address aortic valve diseases have grown significantly in the past decade [1,2,5,16,17]. The indications have now expanded to moderate and low-risk cases of aortic stenosis.16 Coronary obstruction is a critical complication that requires greater attention given the increasing frequency of TAVRs that are performed today [10]. The main causes include immediate coronary ostia obstruction due to valve deployment and native valve leaflet displacement, with longer-term complications resulting from thrombus and fibrous plaque development [11,12,17,18]. This case illustrates how pannus formation can be a significantly delayed cause of coronary ostia obstruction several years after the initial TAVR deployment.
Pannus formation is described as a proliferation of fibroelastic tissue usually organized at the valve annulus [19]. Chronic inflammatory processes to the prosthetic valve result in proliferation of myofibroblasts with upregulated TGF-β-R1 and other growth factor receptors, leading to deposition of collagen and elastic fibrous tissue [19].
Strategies to reduce the likelihood of late DCO may be directed towards post-operative anticoagulation regimens and TAVR design. The optimal post-operative anticoagulation regimen after TAVR implantation remains unknown and guidelines are conflicting [20,21]. Indeed, it is critically important to balance the risk of stroke against increased risk of bleeding when anticoagulating patients after TAVR. Overall, the most effective therapy focuses on mitigating patient-specific risk factors with newer evidence favoring single antiplatelet therapy over dual antiplatelet therapy as a safer and noninferior approach [22].
Another possible approach to reduce the occurrence DCO would focus on the metal scaffold employed in TAVR design. Currently, antiproliferative medication is used in DES design but is not utilized in TAVR design. Antiproliferative medication such as sirolimus and everolimus are widely used to coat coronary stents and serve to inhibit endothelial fibrosis and rethrombosis [23-28]. It is possible that the use of these medications in TAVR metallic scaffolding could mitigate the late pannus formation seen in this case report.
This case highlights a rare and potentially devastating mechanism for developing DCO several years after TAVR implantation. Optimizing post-implantation anticoagulation and exploring the use of anti-thrombotic materials in TAVR design may help mitigate and minimize such complications. Lastly, although cases are fortunately rare, anesthesiologists should be aware of DCO. DCO should be considered when patients with prior TAVR implantation present with symptoms of unstable angina and are found to have non-obstructive coronary disease. Not surprisingly, the hemodynamic parameters for DCO mirror that of flow-limiting coronary obstruction by minimizing the oxygen demands of the heart. Specifically, the anesthesiologist should aim to reduce preload, heart rate, and contractility, while increasing afterload and maintaining sinus rhythm.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author Contributions
Steven Chen: Investigation, Writing–Original draft preparation. Daniel Mattimore: Investigation, Writing–Original draft preparation. Jonathan Price: Investigation, Validation. Jeremy Poppers: Conceptualization, Investigation, Writing–Review and Editing.
Patient Consent
Consent for publication was obtained from the patient before submission. This manuscript adheres to the applicable EQUATOR guidelines. Pannus-Induced Delayed Coronary Obstruction After TAVR.
References
- Leon MB, Smith CR, Mack MJ, Raj R Makkar, Lars SV, et al. (2016) Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 374(17): 1609-1620.
- Reardon MJ, Van Mieghem NM, Popma JJ, Kleiman NS, Sondergaard L, et al. (2017) Surgical or Transcatheter Aortic-Valve Replacement in Intermediate-Risk Patients. New England Journal of Medicine 376(14): 1321-1331.
- Thyregod HGH, Ihlemann N, Jørgensen TH, Nissen H, Kjeldsen BJ, et al. (2019) Five-Year Clinical and Echocardiographic Outcomes from the NOTION Randomized Clinical Trial in Patients at Lower Surgical Risk. Circulation 139(24): 2714-2723.
- Schneeberger Y, Seiffert M, Schaefer A, et al. (2022) TAVI for Pure Non-calcified Aortic Regurgitation Using a Self-Expandable Transcatheter Heart Valve. Front Cardiovasc Med 8: 743579.
- Makkar RR, Thourani VH, Mack MJ, Kodali SK, Kapadia S, et al. (2020) Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. New England Journal of Medicine 382(9): 799-809.
- Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, et al. (2011) Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N Engl J Med 364(23): 2187-2198.
- Valvo R, Costa G, Tamburino C, Barbanti M (2022) Usefulness of intravascular ultrasound to assess coronary occlusion after transcatheter aortic valve replacement. Catheterization and Cardiovascular Interventions 99(5): 1687-1690.
- Naar J, Vondrakova D, Kruger A, Janotka M, Zemanova I, et al. (2023) Cardiac Arrest as an Uncommon Manifestation of Late Type A Aortic Dissection Associated with Transcatheter Aortic Valve Replacement. J Clin Med 12(16): 5318.
- Ribeiro HB, Webb JG, Makkar RR, Cohen MG, Kapadia SR, et al. (2013) Predictive Factors, Management, and Clinical Outcomes of Coronary Obstruction Following Transcatheter Aortic Valve Implantation. Journal of the American College of Cardiology 62(17):1552-1562.
- Limani SM, Roberts JD, Desai NK, Yamini S (2022) A Rare but Deadly Complication of Transcatheter Aortic Valve Replacement. Cureus 14(9): e29530.
- Jabbour RJ, Tanaka A, Finkelstein A, Mack M, Tamburino C, et al. (2018) Delayed Coronary Obstruction After Transcatheter Aortic Valve Replacement. Journal of the American College of Cardiology 71(14): 1513-1524.
- Neuss M, Kaneko H, Tambor G, Hoelschermann F, Butter C (2016) Fatal Thrombotic Occlusion of Left Main Trunk Due to Huge Thrombus on Prosthetic Aortic Valve After Transcatheter Aortic Valve Replacement. JACC: Cardiovascular Interventions 9(21): 2257-2258.
- Marchese A, Tarantini G, Tito A, Colombo A (2020) Transcatheter aortic valve-in-valve post-dilatation as an overlooked risk factor of delayed coronary obstruction: a case report. Eur Heart J Case Rep 4(5): 1-6.
- Yin WH, Lee YT, Tsao TP, Wei J (2020) Delayed Coronary Obstruction after Transcatheter Aortic Valve Replacement – An Uncommon but Serious Complication. Acta Cardiol Sin 36(5): 409-415.
- Ninomiya Y, Hamasaki S, Nomoto Y, Kawabata W, Fukumoto, et al. (2017) A case of acute coronary syndrome caused by delayed coronary ischemia after transcatheter aortic valve implantation. J Cardiol Cases 17(4): 107-110.
- Guimaron S, Kalavrouziotis D, Maranda-Robitaille M, Dumont Eric, Joubert P, et al. (2022) Macroscopic and microscopic features of surgically explanted transcatheter aortic valve prostheses. Journal of Cardiac Surgery 37(10): 3178-3187.
- Feldman D, Cao D, Sartori S, Zhang Z, Hengstenberg C, et al. (2023) Impact of coronary artery disease on clinical outcomes after TAVR: Insights from the BRAVO-3 randomized trial. Catheterization and Cardiovascular Interventions 101(6): 1134-1143.
- Mijares Rojas IA, Martinez EF, Leonor Lopez GL, De Marchena E, Alfonso CE (2023) A Case of Subacute Stent Thrombosis. Cureus 15(4): e37725.
- Teshima H, Hayashida N, Yano H, Nishimi M, Tayama E, et al. (2003) Obstruction of st jude medical valves in the aortic position: histology and immunohistochemistry of pannus. The Journal of Thoracic and Cardiovascular Surgery 126(2): 401-407.
- Catherine M Otto, Rick A Nishimura, Robert O Bonow, Blase A Carabello, John P Erwin, et al. (2020) ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines 143(5): e35-e71.
- Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, et al. (2022) ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Revista Española de Cardiología (English Edition) 75(6): 524.
- Hindi MN, Akodad M, Nestelberger T, Sathananthan J (2022) Antithrombotic Therapy After Transcatheter Aortic Valve Replacement: An Overview. Struct Heart 6(5): 100085.
- Koni E, Wanha W, Ratajczak J, Zhang Z, Podhajski P, et al. (2021) Five-Year Comparative Efficacy of Everolimus-Eluting vs. Resolute Zotarolimus-Eluting Stents in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. J Clin Med 10(6): 1278.
- Von Birgelen C, Van der Heijden LC, Basalus MWZ, Basalus MWZ, Kok MM, et al. (2017) Five-Year Outcome After Implantation of Zotarolimus- and Everolimus-Eluting Stents in Randomized Trial Participants and Nonenrolled Eligible Patients: A Secondary Analysis of a Randomized Clinical Trial. JAMA Cardiology 2(3): 268-276.
- Wilson GJ, Nakazawa G, Schwartz RS, Barbara Huibregtse, Bradley Poff, et al. (2009) Comparison of Inflammatory Response After Implantation of Sirolimus- and Paclitaxel-Eluting Stents in Porcine Coronary Arteries. Circulation 120(2): 141-149.
- Kolandaivelu K, Swaminathan R, Gibson WJ, Vijaya B Kolachalama, Kim-Lien Nguyen-Ehrenreich, et al. (2011) Stent Thrombogenicity Early in High-Risk Interventional Settings is Driven by Stent Design and Deployment, and Protected by Polymer-Drug Coatings. Circulation 123(13): 1400-1409.
- Mauri L, Hsieh W hua, Massaro JM, Ho KKL, Dagostino R, Cutlip DE (2007) Stent Thrombosis in Randomized Clinical Trials of Drug-Eluting Stents. N Engl J Med 356(10): 1020-1029.
- Li F, Wang S, Wang Y, Wei C, Wang Y, et al. (2023) Long-term safety of ultrathin bioabsorbable-polymer sirolimus-eluting stents versus thin durable-polymer drug-eluting stents in acute coronary syndrome: A systematic review and meta-analysis. Clinical Cardiology 46(12): 1465-1473.
-
Steven J Chen*, Daniel Mattimore, Jonathan Price and Jeremy Poppers. Pannus-Induced Delayed Coronary Obstruction After TAVR. Anaest & Sur Open Access J. 5(2): 2024. ASOAJ.MS.ID.000607.
-
Pannus formation, Delayed coronary obstruction, Sinus of Valsalva, Granulomatous inflammation, Stent thrombosis, Myocardial infarction, Pharmacologic therapy
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






