 Case Report
Case Report
Moyamoya disease in pregnancy: management during Pregnancy, Delivery, and Puerperium
Hicham Bennani1*, Ayoub Bouaiyda1, Nawfal Doghmi1 and A Tazi Saoud2
1Department of Anesthesia Resuscitation, Resuscitation Service, Mohammed V military hospital Rabat, Morocco
2Department of anesthesia and resuscitation, Ibn Sina Rabat University Hospital Center, Swissi maternity hospital, Morocco
Hicham Bennani, Department of Anesthesia Resuscitation, Resuscitation Service, Mohammed V military hospital Rabat, Morocco.
Received Date: March 30, 2023; Published Date: April 10, 2023
Abstract
Moyamoya disease (MMD) is characterized by severe stenosis of the circle of Willis arteries, which predisposes patients to vascular occlusion, hemorrhage, infarction, and, less commonly, to transient ischemic attacks (TIA), and seizures. Pregnancy is recognized as a risk factor for a cerebral vascular accident. MMD is more common in women and more prominent in the second or third decade of life, therefore it is not uncommon for it to occur during pregnancy. We report the case of a pregnant patient admitted to the maternity with a consciousness disorder related to a hemorrhagic stroke, whose postpartum evolution was marked by the occurrence of an ischemic stroke in the whole related to a moyamoya disease.
Keywords:Moyamoya; stroke; hemorrhage; infarction
Introduction
Moyamoya disease (MMD) is characterized by the association of an abnormal neovascular network and progressive steno-occlusive lesions of the bifurcation of the terminal internal carotid arteries (ICA). This arteriolar anastomotic network is visible near the carotid apices, where it looks like “swirls of smoke” (moya in Japanese) [1,2].
MMD is rare with a calculated prevalence of 3.16 per 100,000 and a calculated crude annual incidence of 0.35 per 100,000 in Japan, where the prevalence is highest. MMD primarily affects women, including those of childbearing age, therefore, several reports have described pregnancies associated with MMD [3,4]. The prevalence of pregnancy-associated strokes is approximately 0.03%, [5,6] of which only 2% are due to moyamoya disease. [7], The majority of its accidents are hemorrhagic and occur in the peripartum and early postpartum periods.
Observation
This is a 32-year-old patient without ATCD pregnant with 32 WA admitted to the maternity hospital with a disorder of consciousness. The history of his illness goes back to the day of his admission by installing two convulsive crises with 15 min intervals, the second of which is without regaining consciousness. An initial review found patient GCS 9/15 TA 15/9 FC 105 beats/min SaO2 96%. The patient was initially treated for eclampsia given the clinical context. Following acute fetal distress, a Caesarean section under general anesthesia was suggested. Giving birth to a newborn Apgar 3/10 dying in the following minute.
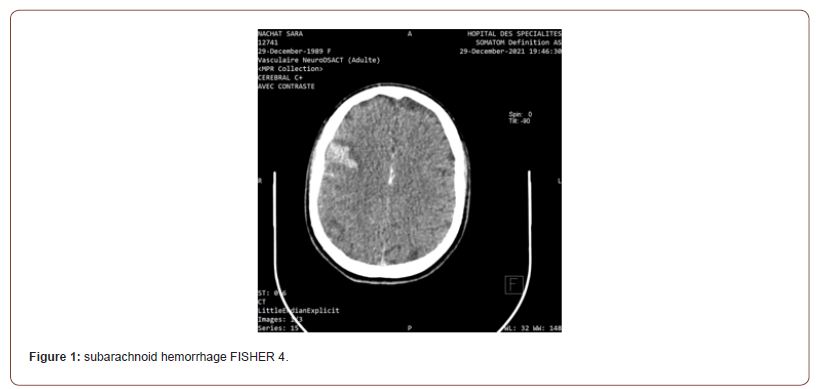
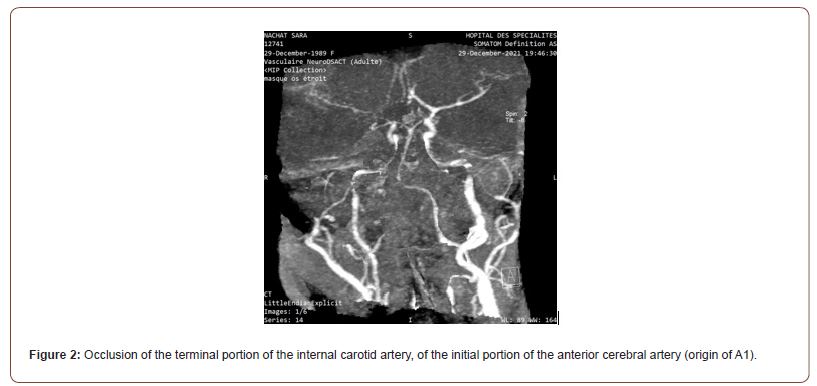
A cerebral scan was performed showing subarachnoid hemorrhage FISHER 4 (figure 1).Complemented by an angio-TDM showing a reduction in the caliber of the regular homogeneous right internal carotid extending over its entire course from its cervical segment to the supraclinoid proportion with complete occlusion at this level with the absence of visualization of the Q1 segment of the right ACA the termination of the ipsilateral internal carotid artery and the proximal part of ipsilateral M1 initially suggesting a moyamoya syndrome (figure 2).
The patient was subsequently admitted to intensive care where she developed in the immediate aftermath of postpartum a right hemiparesis related to a right ischemic cerebral Vascular Accident. The patient was subsequently transferred to a neurology department for additional PEC.
Discussion
Moyamoya disease is characterized by severe stenosis of the arteries in the circle of Willis, which predisposes patients to vascular occlusion, hemorrhage, and infarction and, less frequently, to transient ischemic attacks (TIA) and seizures. The diagnostic criteria are based on arteriography, MRI, or autopsy detection [7-10]:
• Stenosis or occlusion of the terminal portion of the internal carotid artery, of the initial portion of the anterior cerebral artery (origin of A1) or the initial portion of the middle cerebral artery (origin of M1)
• An abnormal supply arterial network.
• Bilateral arterial abnormalities.
The pathophysiology of MMD is unclear. Anatomopathological studies of affected arterial segments show fibro cellular thickening of the intima, thinning of the media, fragmentation of the internal elastic lamina, and luminal thrombosis [11]. This results in a reduction of the external diameter and the arterial lumen. The abnormalities observed are neither inflammatory nor atheromatous. During MMD, the progressive appearance of steno-occlusive lesions of the bifurcation of the termination of the internal carotid arteries is accompanied by the development of an abnormal vascular network, in particular near the carotid apices (network moyamoya). This network is particularly fragile and exposed to the risk of arterial rupture. It is made up of dilated deep perforating arteries and new vessels. Its development could be favored by the production of proangiogenic factors in brain regions suffering from insufficient perfusion. The recruitment of endothelial progenitor cells from bone marrow or peripheral blood also plays a role in the appearance of new vessels [12].
The involvement of genetic factors in the pathophysiology of MMD is strongly suspected due to the frequency of familial forms (9 to 15% of cases), differences in incidence between ethnic groups, and the high level of concordance among homozygous twins. Genome- wide association studies have recently demonstrated an association between MMD and localized polymorphisms in the RNF213 gene in the Japanese population. This association was not found in the Caucasian population. The exact role of the variants observed in the RNF213 gene in the occurrence of MMD remains undetermined to date [13]. Pregnancy is recognized as a risk factor for stroke. It is not uncommon for MMD to coincide with pregnancy, since it is more common in women and more prevalent in the second or third decade of life.
Patients with Moyamoya disease have a potential risk of hemorrhagic and ischemic stroke, and this risk may be increased by a variety of dynamic changes that occur during pregnancy. Estrogen and progesterone induce vascular dilation during pregnancy [14], and a marked increase in estrogen and progesterone can lead to weak blood vessels, which can lead to life-threatening complications [15,16]. renin-angiotensin-aldosterone, which is important for regulating sodium and water retention, is activated during pregnancy. This activation can lead to hypervolemia. Pregnant women at ≥ 30 weeks’ gestation have nearly 50% more blood volume than non-pregnant women. Also, coagulation will be increased but the fibrinolytic system will be reduced during pregnancy. The fibrinogen concentration at the end of pregnancy will increase by approximately 50% compared to that of the non-pregnant state. The concentration will have normalized only 2 weeks after delivery. During childbirth, hyperventilation can induce ischemic attacks by cerebral vasoconstriction. Additionally, the pain of labor and pushing can lead to elevated blood pressure, which, in turn, can induce intracranial hemorrhage, which can be fatal [17, 18].
Intracranial hemorrhage is the most serious complication. Maternal blood volume begins to increase during the first trimester but increases more rapidly during the second trimester. It then increases much slower during the third trimester, reaching a plateau. Additionally, blood pressure typically decreases to a nadir point between 24 and 26 weeks and increases thereafter [19]. These pregnancy-specific physiological changes could explain the fact that most cases reported in the literature of intracranial hemorrhage following unrecognized MMD occur during the antepartum period, which is the case for our patient.
Cerebral infarction is also a serious complication. The circulating blood volume returns almost to its non-pregnancy level one week after delivery [20]. Cardiac output will remain elevated for 24 to 48 hours after delivery and will decline to its non-pregnancy level after 10 days [20]. These physiological alterations of the circulatory system could lead to a decrease in cerebral perfusion and a subsequent cerebral infarction 3 to 7 days after delivery. The hypercoagulable state is at its maximum during childbirth and immediately after childbirth [21], and the coagulation system is more activated after cesarean section [21,22]. This could also explain why most cases of postpartum cerebral infarction have occurred after cesarean section. Nevertheless, excessive anticoagulant therapy may not have been routinely given because it has not been proven to be effective in preventing cerebral infarction in Moyamoya disease and poses a potential risk of causing a hemorrhagic stroke.
Several studies have been published on the choice of the most suitable method of childbirth for women with moyamoya. Cesarean section seems to be the preferred method of childbirth because hypertension and hyperventilation can be avoided, and childbirth is carefully planned and monitored. Vaginal birth is not contraindicated, however, and there is no evidence to support cesarean section over vaginal birth. In our context, the choice of method of childbirth was mainly based on the deterioration of the neurological state on admission and the acute fetal distress. The management of MMD requires multidisciplinary competence involving neurologists, neurosurgeons, and anesthesiologists.
Conclusion
MMD mainly affects women, including those of childbearing age, its coincidence with pregnancy represents a real challenge for the intensive care anesthetist as well as for the gynecologist and the neurologist, Intracranial hemorrhage is likely to occur during the antepartum period, particularly at or beyond 24 weeks, and cerebral infarction tends to occur after birth. Pregnant women with Moyamoya disease can give birth safely, regardless of the mode of birth. Provided that they must be followed in close collaboration with an experienced team with expertise in Moyamoya disease.
Conflict of interest
None.
Acknowledgments
None.
References
- Suwanwela NC (2012) Moyamoya disease: Prognosis and treatment. UpToDate website 1: 20.
2. Jennifer J Conn, Paul J Champion De Crespigny, Stephen M Davis, John D Laidlaw, Kevin Moriarty, et. al. (2008) Successful pregnancy outcome in a patient with moyamoya disease. Aust N Z J Obstet Gynaecol 48: 608-609.
3. K Wakai, A Tamakoshi, K Ikezaki, M Fukui, T Kawamura, et al. (1997) Epidemiologic features of moyamoya disease in Japan: findings from a nationwide survey. Clin Neurol Neurosurg 99: S1-S5.
4. Mariko L Ishimori, Stanley N Cohen, David S Hallegua, Franklin G Moser, Michael H Weisman (2006) Ischemic stroke in a postpartum patient: understanding the epidemiology, pathogenesis, and outcome of Moyamoya disease. Semin Arthritis Rheum 35: 250-259.
5. Richard H Swartz, Megan L Cayley, Norine Foley, Noor Niyar N Ladhani, Lisa Leffert, et al. (2017) The incidence of pregnancy-related stroke: a systematic review and meta-analysis. Int J Stroke 12: 687-697.
6. Andra H James, Cheryl D Bushnell, Margaret G Jamison, Evan R Myers (2005) Incidence and risk factors for stroke in pregnancy and the puerperium. Obstet Gynecol 106: 509-516.
7. Kazumichi Yoshida, Jun C Takahashi, Yohei Takenobu, Norihiro Suzuki, Akira Ogawa, et al. (2017) Strokes associated with pregnancy and puerperium: a nationwide study by the Japan Stroke Society. Stroke 48: 276-282.
8. Lu Ban, Nikola Sprigg, Alyshah Abdul Sultan, Catherine Nelson-Piercy, Philip M Bath, et al. (2017) Incidence of the first stroke in pregnant and nonpregnant women of childbearing age: a population-based cohort study from England. J Am Heart Assoc 6(4): e004601.
9. Lu Ban, Alyshah Abdul Sultan, Olof Stephansson, Laila J Tata, Nikola Sprigg, et al. (2017) The incidence of the first stroke in and around pregnancy: a population-based cohort study from Sweden. Eur Stroke J 2: 250-256.
10. (2012) Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir 52(5): 245-266.
11. Fukui M, Kono S, Sueishi K, Ikezaki K (2000) Moyamoya disease. Neuropathology 20 (Suppl.): S61-64.
12. Achal S Achrol, Raphael Guzman, Marco Lee, Gary K Steinberg (2009) Pathophysiology and genetic factors in moyamoya disease. Neurosurg Focus 26(4): E4.
13. Guey S, Tournier-Lasserve E, Hervé D (2015) Moyamoya disease and syndromes: from genetics to clinical manage¬ment. Appl Clin Genet 8: 49-68.
14. Khalil RA (2005) Sex hormones as potential modulators of vascular function in hypertension. Hypertension 46: 249-254.
15. Furlan A, Fakhran S, Federle MP (2009) Spontaneous abdominal hemorrhage: causes, CT findings, and clinical implications. AJR Am J Roentgenol 193: 1077-1087.
16. Pacini L, Digne F, Boumendil A, Muti C, Detaint D, et al. (2009) Maternal complication of pregnancy in Marfan syndrome. Int J Cardiol 136: 156-161.
17. Cunningham FG, Leveno KJ, Bloom SL, Spong CY, Dashe JS, et. al. (2014) Maternal physiology.Williams Obstetrics. McGraw-Hill Education New York: 46-77.
18. Gabbe SG, Niebyl JR, Simpson JL, Landon MB, Galan HL, et. al. (2012) Maternal physiology. Obstetrics: Normal and Problem Pregnancies. Elsevier Inc. Toronto, ON, Canada: 42-65.
19. Cunningham FG, Leveno KJ, Bloom SL, Spong CY, Dashe JS, et. al. (2014) Maternal physiology. Williams Obstetrics. McGraw-Hill EducationNew York, NY: 46-77.
20. Cunningham FG, Leveno KJ, Bloom SL, Spong CY, Dashe JS, et. al. (2014) The puerperium.Williams Obstetrics. McGraw-Hill EducationNew York, NY: 668-681.
21. Galambosi PJ, Gissler M, Kaaja RJ, Ulander VM (2017) Incidence and risk factors of venous thromboembolism during postpartum-period: a population-based cohort study. Acta Obstet Gynecol Scand 96: 852-861.
22. Kamel H, Navi BB, Sriram N, Hovsepian DA, Devereux RB, et al. (2014) Risk of a thrombotic event after the 6-week postpartum period. N Engl J Med 370: 1307-1315. - Koch A, Bouges S, Ziegler S, Dinkel H, Daures JP, et al. (1997) Low molecular weight heparin and unfractionated heparin in thrombosis prophylaxis after major surgical intervention: update of previous meta-analyses. Br J Surg 84: 750-759.
24. Stafford I, Dildy GA, Clark SL, Belfort MA (2008) Visually estimated and calculated blood loss in vaginal and cesarean delivery. Am J Obstet Gynecol 199: 519. e1-7.
-
Hicham Bennani*, Ayoub Bouaiyda, Nawfal Doghmi and A Tazi Saoud. Moyamoya disease in pregnancy: management during Pregnancy, Delivery, and Puerperium. Anaest & Sur Open Access J. 4(1): 2023. ASOAJ.MS.ID.000579.
-
Puerperium, stenosis, hemorrhage, infarction, internal carotid arteries (ICA), hemorrhagic stroke, Moyamoya disease
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






