 Case Report
Case Report
Lemierre Syndrome Secondary to Parotitis with Bacillus Brevis: A Forgotten Disease
Ilyass Masad*, Anass Elbouti, Walid Atmani, Nouredine kartite, Naoufal Doghmi and Hicham Bakkali
Department of Anesthesiology & intensive care, Military hospital, Mohammed V Rabat, Faculty of medicine, Mohammed V university, Rabat, Morocco
Ilyass Masad, Department of Anesthesiology & intensive care, Military hospital, Mohammed V Rabat, Faculty of medicine, Mohammed V university, Rabat, Morocco.
Received Date: June 03, 2021; Published Date:July 08, 2021
Abstract
Lemierre’s syndrome was described for the first time in 1900 by Courmont and Cade, It is a rare pathology that affects mainly adolescents or previously healthy young adults with a moderate male preponderance. The typical pathogen germ is Fusobacterium necrophorum and the organ most frequently affected is the lung where septic emboli cause a bilateral pleuro-pneumopathy. The diagnosis of Lemierre’s syndrome is primarily clinical, it evolves classically in three phases: oropharyngeal infection and then homolateral internal jugular septic thrombophlebitis by local diffusion and finally metastatic dissemination by blood and lymphatic systems in the next four to eight days. Management is primarily medical, sometimes surgical in the presence of deep abscess and / or cervical and mediastinal tissue necrosis.
We report the case of a patient suffering from necrotizing bacterial parotitis, responsible for cervicofacial cellulitis complicated by bilateral internal jugular thrombophlebitis and multifocal septic emboli performing a clinical picture of Lemierre syndrome. Our case is the only one described in the literature responsible for parotitis bacillus brevis to our knowledge.
The objective of our work is to recall the pathogenesis, the clinical and the treatment of Lemierre syndrome through this clinical case.
Keywords:Bacillus brevis; Lemierre syndrome; Parotitis
Introduction
Parotitis is the parotid gland inflammation. The most common form is punctual, of viral origin, and it is encountered especially during mumps. However, the cause may be a bacterial infection and its course may also be chronic.
Lemierre’s syndrome was described for the first time in 1900 by Courmont and Cade but was more deeply studied by Lemierre in 1936 [1]. It is a rare pathology associating a thrombosis of the internal jugular vein and secondary septic sites mainly pulmonary ones, because of an oropharyngeal infection, most often with anaerobic germs. This clinical picture is rarely complete, making diagnosis difficult and delaying treatment.
Case Report
We report the case of Mr. F.M, 55 years old, married and father of 3 children, having as antecedent unweaned chronic smoking. The patient was presented to the emergency department for a painful right-sided tumefaction [Figure1] associated with headache, and otalgia which appeared two days before. Everything evolves in a context of fever and deterioration of the general state.
The clinical examination at the time of the consultation found a conscious patient, feverish at 39°C, presenting a clinical picture of cervical cellulitis. Hemodynamically, blood pressure was at 120/70 mmHg tachycardia at 110 beats / minute. At the Respiratory level, he was polypnic at 24cycles / min with 96% ambient oxygen saturation, reporting right Basi thoracic pain. Examination of the abdomen noted a slight diffuse abdominal tenderness.
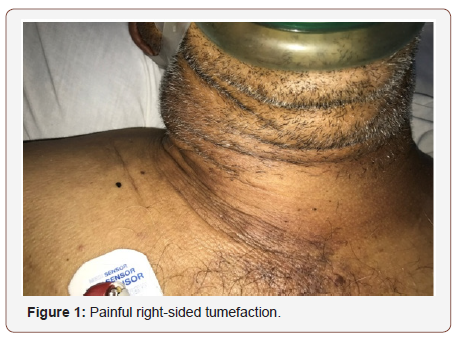
ENT examination also found swelling of the right parotid region extended at the ipsilateral cervical level and at the level of the right supraclavicular fossa. This sxelling is indurated, inflammatory, painful and uncollected appearance. As for the contralateral side, the examination found a swelling without inflammatory sign. With regard to the oral cavity, there was no dental foci or angina, and otoscopic examination was without peculiarities.
A chest x-ray showed multiple nodular opacities in the left pulmonary hemifield, mediastinal widening, and pleural effusion syndrome on the right. Cervical Doppler ultrasound revealed thrombosis of the right internal jugular vein. Thus, transthoracic echocardiography did not find images in favor of infective endocarditis. Cervicofacial and cerebral CT without injection revealed multiple bilateral cervical lymphadenopathy, right parotidomegaly, three tenteural brain lesions, and left cerebellar disease.
Complement CT with injection of contrast agent noted; the presence of multiple lesions sus and under tentorial fluid density enhanced on the periphery after injection of contrasting product evoking collections. There was a swelling of the right parotid lodge associated with multiple jugulo-carotid lymphadenopathy. The right and left internal jugular veins remained non-opacified evoking a thrombosis extended to the superior vena cava on the right and the left brachiocephalic venous trunk. The cervical fat seemed to have infiltrated without a clearly visible collection. At the thoracic level: multiple mediastinal and right axillary lymphadenopathies were noted. At the abdominopelvic level: Bilateral adrenal masses the largest part of which is the left one, a right pararenal nodular lesion of poorly limited tissue density, a hypo-dense lesion of segment VI of the liver and multiple latero-aortic and Coelio-mesenteric lymphadenopathy have been highlighted.
The initial biological assessment found an inflammatory syndrome with a C-reactive protein (CRP) at 228.1 mg / l, white blood cells at 24500 / mm3 predominantly neutrophils, and a procalcitonin at 1.20 ng / ml. The rest was unremarkable.
In front of this clinical picture (fever with infection of the ENT sphere), the presence of thrombosis of the two internal jugular veins, and multifocal septic emboli, the diagnosis of Lemierre syndrome was made.
After collection for bacteriological purposes, namely: oral swabs, blood cultures, lumbar puncture, cytobacteriological examination of the urine and finally a bone biopsy, the patient is put under ceftazidime 2g / 8h, metronidazole 500mg / 8h, gentamicin 5mg / kg / 24h and an effective heparin therapy.
The evolution was marked after 24 hours by the installation of septic shock and worsening of the neurological and respiratory state of the patient leading to admission to resuscitation, artificial ventilation, and the introduction of norepinephrine.
A chest X-ray of control found an aggravation of the pulmonary images. Thus, the biological assessment showed a rise in infectious parameters CRP 388.2 mg / l leukocyte at 20500 / mm3, procalcitonin at 6.39ng / ml, renal failure classed KDIGO 3 (creatinine 30 mg / l urea 1.92 g / l with diuresis less than 0.3ml / kg / hour over 24h), and biological cholestasis with a total bilirubin at 16mg / l predominantly conjugated bilirubin. However, the coagulation assessment has returned without particularity.
The bacteriological samples taken at the admission found Bacillus Brevis (48 hours positivity delay) in the peripheral venous and central venous catheter blood cultures. The same germ was isolated by oral swabbing with sensitivity to gentamicin, clindamycin, ciprofloxacin, and Fosfomycin in the antibiogram. Hence the addition of ciprofloxacin at a dose of 400mg / 12h. Moreover, the cytobacteriological examination of the urine and CSF were normal; the leukocytosis was 3 cells / mm3. Examination of the scapula biopsy and PCR screening of mycobacterium tuberculosis were negative. The search for viruses at the level of the ENT sphere, and at the level of the cerebrospinal fluid, as well as the serologies of HBV, HCV, HIV 1 and 2 and Ebstein-Barr virus were all negative. the cultures of the various specimens remained sterile. The distal bronchial-protected sampling performed after orotracheal intubation revealed the absence of bacterial flora on direct examination and after culture.
The evolution of the patient was marked by pneumonia acquired by mechanical ventilation with nosocomial germ at the 6th day of hospitalization. The culture isolated an acinetobacterbaumanii 10 * 7 CFU / ml sensitive to aminoglycosides and colimycin, and a pseudomonas 10 * 7 CFU / ml sensitive to imipenem, which pushed us to expand the spectrum of antibiotherapy. Following refractory septic shock requiring the use of high doses of epinephrine and multiorgan failure, the patient died on the 8th day of hospitalization in intensive care unit.
Discussion
The lemierre syndrome, also called pulmonary anginainfarction syndrome, was first described in 1936 by Lemierre [1]. It is a pathology that has become rare and affects mainly adolescents or previously healthy young adults with a moderate male preponderance [2,3,4,5]. The pathophysiology consists of a septic thrombosis of the peritonsillar veins in the aftermath of a tonsillitis infection that may go unnoticed. The thrombosis then extends to the internal jugular vein from which metastatic spread occurs [1,6]. Usually, the infectious starting point is oropharyngeal in 80% of cases, but this syndrome is described from sinusitis, otitis media, mastoiditis, dental abscess or parotitis [2]. Our case goes away usual cases, because a parotitis as an infectious starting point remains exceptional. Our case differs from usual cases, as parotitis as an infectious starting point remains exceptional. Only very rare cases of lemierre syndrome secondary to parotitis are found in the literature, but the germ was typical: Fusobacterium necrophorum.
In the old days frequent and most often fatal, its incidence has decreased with the advent of antibiotic therapy and described as a forgotten disease. Nevertheless, we are witnessing an upsurge in the last two decades, estimated at 0.8 cases per million per year from 1990 to 1995, and 3.6 cases per million per year from 1998 to 2001 [7]. All this is probably related to a current more restrictive antibiotic policy, an increasingly frequent prescription of antiinflammatory drugs for the treatment of angina, the appearance of antibiotic resistance or more and more rare indications of tonsillectomy. This syndrome most often affects young adults (16- 23 years old) without a notable history, not immunosuppressed and more particularly the male sex with a sex ratio of about 2/1 in the literature [8]. In our case the patient is male without history but does not obey the age range generally concerned by this pathology.
The diagnosis of Lemierre’s syndrome is primarily clinical, it evolves classically in three phases: oropharyngeal infection (pharyngitis, pain, inflammatory laterocervical swelling, sepsis) and then homolateral internal jugular septic thrombophlebitis by local diffusion and finally metastatic dissemination by blood and lymphatic systems in the next four to eight days [9]. However, and despite an initial suggestive symptomatology, it is difficult to assert the exact starting point given the numerous abscesses visualized on the imagery. In our patient, the starting point is parotitis.
The pathogen usually found in 92% of cases is Fusobacterium necrophorum. It is a strictly anaerobic Gram-negative bacillus belonging to the oropharyngeal flora (formerly known as Bacillus funduliformis) [10,11]. This germ secretes an endotoxin responsible for venous thrombosis [11,12]. Other germs are sometimes involved (Eikenellacorrodens, Prevotellabivia, Porphyromonasspp ...) [13,14]. In 10% of the cases, no germ is found and in a third of the cases a polymicrobial flora is found [10]. In our case, the germ in question is the bacillus brevis that was isolated hemoculture and Oral swabbing. No similar case has been described in the literature to our knowledge.
In this syndrome, the organ most frequently affected (97% of cases) is the lung where septic emboli cause a bilateral pleuropneumopathy with pulmonary infarction moving towards abcédation [4]. Other localizations are possible: arthritis, osteomyelitis, hepatic abscess, endocarditis, neuromeningeal involvement ... [1,3,4,6]. In our case, the patient also has, in addition to the above-mentioned lesions, mediastinitis secondary to cervical involvement, which seems to be associated with excess mortality [15].
Cervicothoracic computed tomography with injection of iodinated contrast is essential. It should be performed as soon as possible in search of thrombosis of the internal jugular vein or a facial vein often passing unnoticed on clinical examination. It also reveals the extent of the lesions and the severity of the infiltration of the tissues which contribute to the surgical indication of cervicotomy. In our case, there was no surgical indication after ENT opinion, nor neurosurgical indication.
The diagnosis is ultimately based on non-specific biological samples (Neutrophils), bacteriological samples with intraoperative samples, and especially blood cultures that isolate the germ in more than 80% of cases [16]. This is the case of our patient. In 23% of cases there is also a variable degree of Disseminated Intravascular Coagulation (DIC) and in 50% hepatic cytolysis with conjugated hyperbilirubinemia interpreted as consequence of bacterial endotoxins [17]. These last two biological abnormalities were absent in our case.
Management is primarily medical, sometimes surgical in the presence of deep abscess and / or cervical and mediastinal tissue necrosis. The patient will be admitted to intensive care in the presence of any organ failure. Our patient is transferred to intensive care unit after installation of septic shock without surgical indications of inaccessible deep abscesses.
Early antibiotic therapy is the key to treatment. Fusobacterium necrophorum is sensitive to penicillin, clindamycin, metronidazole and chloramphenicol [18]. There is natural resistance to aminoglycosides and glycopeptides. Macrolides should be avoided as they do not appear to prevent progression to severe sepsis [19]. After adaptation to the antimicrobial susceptibility testing, treatment will be maintained for four to six weeks due to the endovascular and deep tissue nature of the infection. The fever may persist for several days [17].
In our case, a probabilistic antibiotherapy based on ceftazidime and metronidazole is started pending the results of bacteriology, which isolated bacillus brevis sensitive, hence the addition of Ciprofloxacin. The worsening of the patient and the pneumopathy acquired by mechanical ventilation with nosocomial germ prompted us to widen the spectrum further by focusing on the results of the antibiogram.
Concerning internal jugular venous thrombosis, anticoagulants are recommended in effective doses only in case of retrograde extension of the thrombus towards intracranial venous drainage, and more precisely towards the cavernous sinus [20]. However, there is still controversy about the use of anticoagulants, but no controlled studies have been published [18]. Indeed, we can ask the question of their utility in isolated internal jugular thrombophlebitis in view of the positive gynecological experience of heparin therapy in pelvic septic thromboses [11]. The choice therefore remains free. In our case, the patient had bilateral internal jugular thrombosis and advocated for curative dose heparin therapy.
Mortality was estimated up to 90% in the first half of the twentieth century, it currently oscillates between 4 and 18% according to the publications [14,15]. Our patient died following a refractory septic shock with multi-organ failure on the eighth day after admission.
Conclusion
Lemierre’s syndrome is an infrequent and unknown disease. The diagnosis is clinical in the first place for young patient with oropharyngeal infection associated with signs of sepsis or pulmonary infiltration. It is even more difficult in our case, our patient not belonging to the usual population and the initial infection observed for the first time being a parotitis. In a second step, the key to diagnosis is microbiology with bacterial identification in blood cultures.
It should also be noted that any delay may have heavy consequences, with significant morbidity and mortality and sometimes an evolution towards mediastinitis due to the proximity of anatomical structures at the cervicothoracic level. Finally, the therapeutic attitude is nowadays well established, it essentially consists of medical treatment that can be added to surgery when abscessing. However, there remain some unknown points such as anticoagulation outside precise indications.
Acknowledgement
None
Conflicts of Interest
No conflict of interest
References
- Lemierre A (1936) On certain septicaemias due to anaerobic organisms. Lancet 1: 701-703.
- Hagelskjaer Kristensen L, Prag J (2000) Human necrobacillosis, withemphasis on Lemierre’s syndrome. Clin Infect Dis 31: 524-532.
- Hagelskjaer LH, Prag J, Malczynski J, Kristensen JH (1998) Incidence and clinical epidemiology of necrobacillosis, including Lemierre’s syndrome,in Denmark, 1990-1995. Eur J Clin Microbiol Infect Dis 17: 561-565.
- Sinave CP, Hardy GJ, Fardy PW (1989) The Lemierre Syndrome: Suppurativethrombophlebitis of the internal jugular vein secondary to oropharyngeal infection. Medicine 68: 85-94.
- Moreno S, Garcia Altozano J, Pinilla B, Lopez JC, Bernaldo dekQuiros, et al. (1989) Lemierre’s Disease: Post anginal bacteremia and pulmonary involvement caused by Fusobacterium necrophorum. Rev Infect Dis 11: 319-324.
- Yau PC, Norante JD (1980) Thrombophlebitis of the internal jugular vein secondary to pharyngitis. Arch Otolaryngol 106: 507-508.
- Hagelskjaer Kristensen L, Prag J (2008) Lemierre’s syndrome and other disseminated Fusobacterium necrophorum infections in Denmark: a prospective epidemiological and clinical survey. Eur J Clin Microbiol Infect Dis 27: 779-789.
- Brazier JS (2006) Human infections with Fusobacterium necrophorum. Anaerobe 12: 165-172.
- Courtin P, Toro A, Gazagnes M, Berrouba A, Gallardo M, et al. (2010) Syndromede Lemierre. Ann Fr Anesth Reanim 29: 799-802.
- Bouton F, Cotils M, Genard M, Hubert C (1998) Thrombophlebite septiquede la veine jugulaire interne et syndrome de Lemierre. RevMed Brux 1: 5-9.
- Langworth BF (1977) Fusobacterium necrophorum: its characteristics androle as an animal pathogen. Bact Rev 41: 373-390.
- Faussat JM, Coste A, Roger G, Page B, Marrek H, et al. (1993) Lesthrombophlébites septiques de la veine jugulaire interne a portedentree oropharyngee: a propos de 3 cas. Ann Oto-Laryng 110: 445-449.
- Celikel TH, Muthuswamy PP (1984) Septic pulmonary emboli secondary to internal jugular vein phlebitis (postanginal sepsis) caused by Eikenella corrodens. Am Rev Respir Dis 130: 510-513.
- Vandenbos F, Roth S, Girard Pipau F, Neri D, Boscagli Melaine A, et al. (2000) Syndrome de Lemierre a Porphyromonas spp. Chez un patient de 21 ans. Rev Med Interne 21: 521-522.
- Corsten MJ, Shamji FM, Odell PF, Frederico JA, Laframboise GG, et al. (1997) Optimal treatment of descending necrotising mediastinitis. Thorax 52: 702-708.
- Dool H, Soetekouw R, van Zanten M, Grooters E (2005) Lemierre’s syndrome: threecases and a review. Eur Arch Otorhinolaryngol 262: 651-654.
- Ridgway JM, Parikh DA, Wright R, Holden P, Armstrong W, et al. (2010) Lemierre syndrome: a pediatric case series and review of literature. Am J Otolaryngol 31: 38-45.
- Seidenfeld SM, Sutker WL, Luby JP (1982) Fusobacterium necrophorum septicemiafollowing oropharyngeal infection. JAMA 248: 1348-1350.
- Monge M, Aubry P, Dayen C, Ben Taarit I, Ducroix JP, et al. (2003) Lemierresyndrome: unusual, but still possible. Ann Med Interne (Paris) 154: 263-266.
- Williams A, Nagy M, Wingate J, Bailey L, Wax M (1998) Lemierre syndrome: acomplication of acute pharyngitis. Int J Pediatr Otorhinolaryngol 45: 51-57.
-
Ilyass Masad, Anass Elbouti, Walid Atmani, Nouredine kartite, Naoufal Doghmi, et al., Lemierre Syndrome Secondary to Parotitis with Bacillus Brevis: A Forgotten Disease. Anaest & Sur Open Access J. 2(5): 2020. ASOAJ.MS.ID.000546.
-
Inflammation, Bacillus Brevis, bacterial infection, Pneumopathy
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






