 Research Article
Research Article
Obstructive Sleep Apnoea in elective surgical patients admitted to a tertiary Intensive Care Unit: a 6-year Retrospective cohort study
Shaurya Jhamb1,2*, Venkat N Vangaveti2, Stephen Whebell3, Lachlan Young3, Alexander N Kippin4 and Melita J Trout3
1Department of Surgery, Townsville University Hospital (TUH), Townsville Hospital and Health Service, Queensland, Australia
2College of Medicine and Dentistry, James Cook University, Queensland, Australia
3Intensive Care Unit, Townsville University Hospital (TUH), Townsville Hospital and Health Service, Queensland, Australia
4Department of Anaesthesia, Townsville University Hospital (TUH), Townsville Hospital and Health Service, Queensland, Australia
Shaurya Jhamb, Department of Surgery, Townsville University Hospital (TUH), Townsville Hospital and Health Service, Queensland, Australia
Received Date: July 15, 2024; Published Date: December 12, 2024
Abstract
Background:Obstructive Sleep Apnoea (OSA) is a common sleep-related breathing disorder with increasing prevalence. Elective surgical patients with a background of OSA are at increased risk of peri-operative complications. Post operatively, patients often require a higher level of observation which may require admission to the Intensive Care Unit (ICU) although anecdotally these patients infrequently require ICU level intervention.
Aim: To identify patients with diagnosed or suspected OSA admitted to ICU post elective surgery for OSA monitoring, describe patient characteristics, interventions and compare this care with the literature.
Methodology: A retrospective cohort study of admissions to the Townsville University Hospital (TUH) Intensive Care Unit (ICU) from 2014 to 2019 inclusive was conducted. Patients with other indications for ICU admission were excluded.
Results: 1234 patients were screened; 105 total patients were included in the study. 87 patients (82.86%) had a documented sleep study (vs 18 patients without), 80 (76.2%) had been prescribed CPAP, and 45 patients (56.3%) used their machines regularly. Maximal respiratory management comprised of nasal prongs/mask oxygen for 61 patients (58.10%), High Flow Nasal Prongs (HFNP) for 11 patients (10.48%), and non-invasive ventilation for 11 patients (10.48%). No patients required escalation to invasive ventilation.
Conclusions: A risk stratified algorithm and multi-faceted management protocol for patients with OSA would help stratify perioperative requirements. A dedicated post-operative high acuity area is likely be safe and appropriate. A prospective study of the proposed alternative model of care to monitor patient outcomes, resource intensity, and impact on ICU capacity is required.
Keywords:Obstructive Sleep Apnoea (OSA); Elective Surgery; Intensive Care; Perioperative Medicine; Positive Airway Pressure (PAP)
Background
Obstructive Sleep Apnoea (OSA) is the most common sleeprelated breathing disorder with an estimated global prevalence of 9-38% [1] however, approximately 80-90% of patients with the condition have not been formally diagnosed [2,3]. It is characterised by repetitive episodes of apnoea or hypopnoea caused by partial or complete closure of the upper airway resulting in hypoxaemia [4-7]. The pathophysiological effects include hypoxaemia, hypercapnia, stimulation of the sympathetic nervous system, cortical arousal, fragmented sleep, and consequential impaired daytime functional status [8-11]. OSA is diagnosed using polysomnography (sleep study) [8] with severity quantified using the apnoea-hypopnea index (AHI, the average number of apnoeas and hypopnoeas per hour during sleep) with 5-15 being mild, 15-30 moderate and 30+ severe [9]. In the absence polysomnography, peri-operative screening and risk stratification can be performed with tools such as the STOP-BANG score (see Appendix) [12,13]. STOP-BANG is well validated and has a sensitivity of 90% and specificity 49% for any severity OSA (>5 AHIs) with higher scores correlating with more severe OSA [14,15].
Surgical patients with OSA are at increased risk of peri-operative complications [16,17] due to impacts of anaesthesia, analgesia, and surgery [15,18]. Generally, the peak of deteriorated sleep and increased severity of OSA occurs on the third post operative night with increased apnoea and hypopnoea events and desaturation of arterial oxygen18. Specific anaesthetic risks include difficulty with intubation, mask ventilation, maintenance of airway patency post extubation, and a higher likelihood of unplanned postoperative transfer to ICU [19]. Patients with OSA are more likely to experience post-operative cardiac events (cardiac arrhythmia, myocardial ischaemia, cardiac arrest) after non-cardiac surgery [20], transfer to ICU and pulmonary complications [21]. It remains difficult to interpret what proportion of postoperative complications are direct results of OSA or associated obesity or comorbidities [22,23].
In patients with established OSA, current literature suggests an association between continuation of CPAP or oral appliance therapy and a reduction in postoperative cardiopulmonary complications and recommend continuation of these post-operatively unless contraindications exist [24-26]. In patients with untreated or suspected OSA, the Society of Anaesthesia and Sleep Medicine suggests an extended period of postoperative observation [27]. Postoperative strategies include supplemental oxygen, multimodal postoperative analgesia (including regional techniques) with care to avoid respiratory depression, ensuring a non-supine position, strict fluid management and increased surveillance for cardiorespiratory events [5]. A recent narrative review published by Holt et al. in the Medical Journal of Australia (MJA) provides an algorithm (Figure 1) with risk stratification and subsequent management of elective post-surgical patients with confirmed or suspected OSA [8]. This algorithm includes previous recommendations of an extended postanaesthesia care unit assessment for upper airway compromise and ventilation vulnerability that would indicate a need for a monitored bed with continuous pulse oximetry on a high acuity ward with higher patient: nurse ratio [28,29].
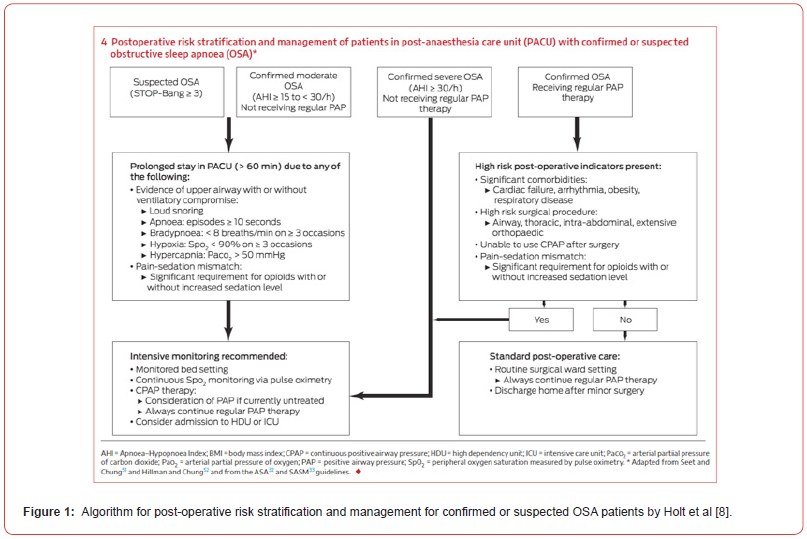
In our and many other facilities, the intensive care unit (ICU) remains the only area with such monitoring and staffing that can initiate non-invasive ventilation and patients are admitted directly to ICU from the operating room. There is very limited data about the therapeutics received by these patients whilst in ICU. ICU beds are a finite resource. It has been posited that whilst occupying an ICU bed, these patients infrequently require ICU level intervention. We objectively investigated this speculation through a retrospective study.
Methodology
A retrospective study of six years of post-operative ICU admissions (2014-2019) for OSA monitoring was conducted (LNR/QTS/60875). The key aims were to determine patient characteristics, identify interventions, and compare the standard of care provided with recommendations from the literature. To enable comparison to published recommendations, patients were stratified as per the algorithm detailed by Holt et al. in Figure 1.
The study was set in a tertiary hospital in North Queensland, Australia (Townsville University Hospital). All patients admitted to ICU post operatively were screened. Patients admitted to ICU for post operative OSA monitoring were included. Patients with an indication for ICU admission other than OSA were excluded; such as emergency surgery, neurosurgical, cardiothoracic and aortic surgery patients, and requirement for vasoactive medications or invasive ventilation.
Electronic medical records for ICU (MetaVision - iMDsoft, Tel Aviv, Israel) and the hospital (ieMR – Cerner, Kansas City, USA) were accessed. Data collected included demographics, risk factors, type of surgery, OSA details, CPAP compliance and prescription, specific ICU interventions (including head up at 30°, initiation of non-invasive ventilation (CPAP, BiPAP or High-Flow Nasal Prongs (HFNP)), continuous monitoring, etc), blood results, vital signs, arterial lines, scoring systems, opioid and sedating medications, fluid balance, use of regional anaesthesia, and complications. Data integrity was confirmed using cross checking and repeated collection.
Data Analysis
Data was collected on a secure Microsoft® Excel® file, cleaned and analysed using SPSS version 25. Descriptive data is presented as Mean ± Standard Deviation (SD), percentages where appropriate. Association between categorical variables was analysed using the Chi-square test.
Results
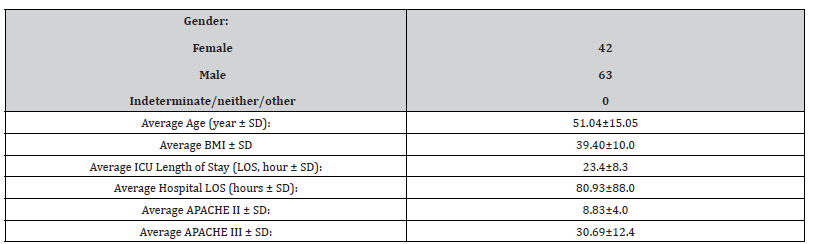
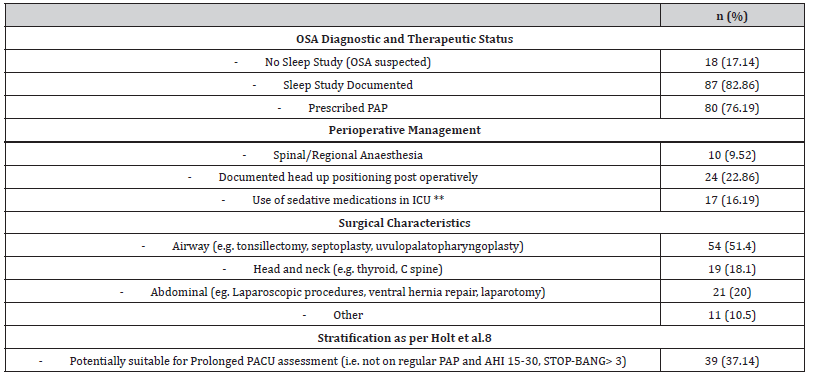
A total of 1234 patients who were admitted to ICU post elective surgery were screened. 105 patients admitted for suspected or diagnosed OSA were identified (Table 1 and 2).
Table 1:*SD= Standard Deviation. BMI = body mass index. LOS = length of stay. APACHE = acute physiology and chronic health evaluation.
Of the 105 patients, 82.86% (87) had a documented history of diagnostic sleep study (see Table 2). There were 41 patients that had a documented AHI with a mean AHI of 52.53 events (range 11 - 125). Of the 80 patients prescribed PAP, 45 (56.3%) used their machines regularly. 18 patients (17.14%) out of the total 105 had no history of diagnostic sleep study; one quarter (4) of those had a documented STOP-BANG score of 5 or greater.
Table 2:**non-opioid sedating medications including Benzodiazepines and anti-psychotic medications.
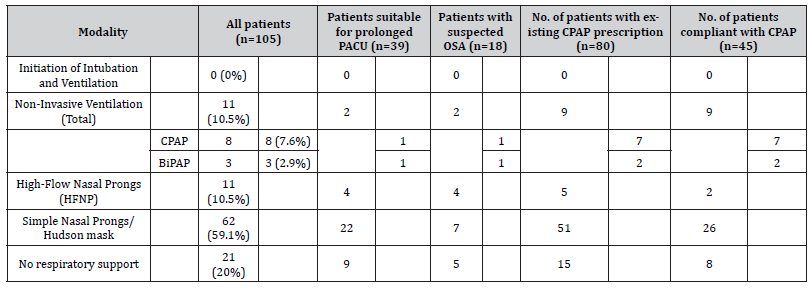
84 patients required some form of respiratory support, the maximal level of support required is shown in Table 3. The use of respiratory support beyond low flow oxygen was largely in response to low SpO2 recordings, rather than pre-planned. The lowest SpO2 by intervention (HFNP, CPAP, BiPAP) was 88%, 88% and 87% respectively. Of the 3 patients who received BiPAP in ICU, 2 had prescriptions for CPAP and both were post abdominal surgery. Of the 8 patients who received CPAP, 7 patients had existing CPAP prescriptions and 4 patients used their own device. No patients required escalation to invasive ventilation during their stay in ICU.
The total average oral morphine equivalent of opioid used per patient during their stay in ICU was 19.02mg. In proportion to weight, this was on average 0.1624mg/kg of oral morphine equivalent per patient. This did not include intraoperative opioid doses. Out of the total cohort 39 patients (37.14%) were identified as potentially suitable for a ‘Prolonged PACU Assessment’ as per the post-operative risk stratification criteria created by Holt et. al. (see Figure 1)8.
Table 3:
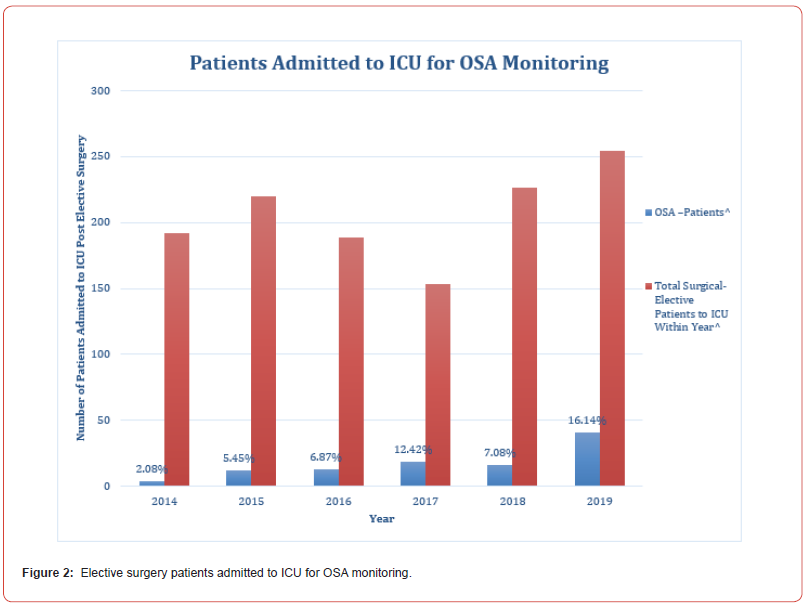
Over the 6-year study period the number of post elective surgery ICU admissions due to OSA dramatically increased, from 2% of all elective surgical admissions in 2014, to 16% in 2019 (see Figure 2).
Discussion
This retrospective cohort study identified rising rates of ICU admission, variable application of recommendations for management, and a low rate of respiratory intervention escalation for patients with suspected or proven OSA post elective surgery.
The rising number of admissions may be due to increased identification of patients, as well as rising prevalence in the community. The trend suggests a growing need to cater for this patient cohort and manage future resource allocation.
Application of current recommendations for post-operative OSA care were variably applied. Close post-operative monitoring, continuous pulse oximetry and high nursing staff ratios were well documented parameters and were received by every patient included in this study. Several patients received multimodal analgesia including regional techniques. This is anesthetist and procedure dependent and there is no strict protocol or guideline in our institution currently. Attention to patient positioning and ensuring non-supine position were lower than expected. Given that standard ICU care involves a 30 degree head up position, this low figure may be due to poor documentation, rather than representing the actual position of the patient.
A large subgroup of patients who regularly use CPAP at home did not receive this therapy in ICU, however contraindications to prescribed CPAP were not collected. Escalation of respiratory intervention was almost always in response to a decrease in peripheral oxygen saturations. Supplemental oxygen therapy was provided to the majority of patients. It is possible this was due to continuation of oxygen therapy from the operative theatre, institutional policy for nasal cannula oxygen with patient controlled opiate analgesia or institutional culture rather than true hypoxaemia requiring therapy.
Our results identified 39 patients who were potentially suitable for a prolonged PACU assessment (per Figure 1), rather than direct admission to intensive care. Admission to ICU would not necessarily have been prevented as occurrence of high-risk features in PACU would have resulted in an unplanned ICU admission.
Given that only two patients who did not previously have a PAP prescription went on to receive PAP therapy, it is likely that the entire cohort could have been managed in a dedicated area outside of the ICU. This area would require a higher nursing ratio than a standard ward (e.g., 1:2 or 1:3), continuous SpO2 monitoring, the ability to provide established PAP therapy, and to initiate PAP or escalate to an area that can initiate PAP when required. This would need requisite equipment, nursing expertise and medical input, yet would still be less resource intensive than a standard ICU bed. A dedicated non-ICU post-operative high acuity area may result in a reduction in day-of-surgery cancellations due to lack of ICU bed availability. However, if escalation to ICU was required following high risk features in PACU or escalation to PAP from the ward then these ICU admissions would be unplanned that would contribute to ICU capacity surges, albeit at a low frequency.
A multi-faceted management protocol for patients with OSA would be beneficial to create consistency and stratify requirements throughout the perioperative course. Preoperatively, this includes routine screening utilising the STOP-BANG score, documentation of sleep study results particularly AHI, counselling to patients to bring CPAP machines on day of surgery, and perioperative anaesthetic evaluation. Intraoperative management has not been a focus of this study but is an integral part of managing OSA, with an emphasis on regional anaesthesia, minimisation or avoidance of opiates and sedative medications, and judicious fluid management. From a post-operative perspective, this study identified that ICU level resources were not required for most patients. Patients who required escalation of respiratory intervention may have been able to be identified during an extended PACU stay. Application of a risk stratified algorithm to identify these patients based on their immediate post-operative phase in PACU may reduce planned ICU admissions but implemented alone, may lead to increased unplanned admissions. Further research is required to confirm this conclusion.
There are several limitations of this study. This study while insightful, was reflective of the current practice at a single centre, which limits its generalisability. It does provide a good perspective into the current deficit in the literature of therapeutics received by these patients in ICU. Retrospective studies rely on documentation which may be poor due to a variety of reasons, for example interventions may be made without documentation. Fluid management is an important part of post-operative OSA management however was difficult to track from intraoperative to post-operative phase across different medical records systems. This study did not examine intra-operative analgesia, anaesthetic technique, local anaesthetic use, and details of regional anaesthesia. Application of the Holt et al.8 algorithm was limited as not all patients had AHI values documented, and we included patients who were non-compliant with CPAP. It also assumed that all patients without a PAP prescription had at least suspected moderate OSA without a calculated STOP-BANG score. This study did not examine the outcomes of patients with a STOP-BANG >3 who were not admitted to the ICU. It is likely patients with undiagnosed OSA underwent surgery without being identified as the STOP-BANG is not applied to every elective surgical patient.
Conclusion
Our data suggests that the requirement for escalation to ICU level care in this single centre was low and an alternative model of care may be safe and appropriate. Whilst this study has highlighted some key areas of focus, further data collection and a prospective study is required to examine the impact of such a model of care, particularly the resource intensity, impact on ICU and elective surgery capacity, and the maintenance of a safe level of postoperative care for patients.
Appendix
STOP-BANG score which includes: Snoring, Tiredness (daytime), Observed apnoea, blood Pressure (hypertension), BMI)>35, Age >50, Neck circumference (>41cm for female, >43cm for male), Gender (male); with a score of 1 point for each question and a score of 3 or more raising concern for possible OSA.14, 27.
Acknowledgement
We would like to thank Holt et al. for their work in development of the algorithm for post-operative risk stratification and management for confirmed or suspected OSA patients which has been used in this study.
Conflict of Interest
No conflict of interest.
References
- Senaratna CV, Perret JL, Lodge CJ, Adrial JL, Brittany EC, et al. (2017) Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep medicine reviews 34: 70-81.
- Redline S, Sotres-Alvarez D, Loredo J, Hall M, Sanjay RP, et al. (2014) Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. American journal of respiratory and critical care medicine 189(3): 335-344.
- Young T, Peppard PE and Gottlieb DJ (2002) Epidemiology of obstructive sleep apnea: a population health perspective. American journal of respiratory and critical care medicine 165(9): 1217-1239.
- Remmers JE, DeGroot WJ, Sauerland EK, Anch AM (1978) Pathogenesis of upper airway occlusion during sleep. Journal of applied physiology: respiratory, environmental and exercise physiology 44(6): 931-938.
- Deflandre E, Gerdom A, Lamarque C, Berbard B (2018) Understanding Pathophysiological Concepts Leading to Obstructive Apnea. Obesity surgery 28(8): 2560-2571.
- Resta O, Foschino-Barbaro MP, Legari G, S Talamo, Bonfitto P, et al. (2001) Sleep-related breathing disorders, loud snoring and excessive daytime sleepiness in obese subjects. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity 25(5): 669-675.
- Franklin KA, Lindberg E (2015) Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis 7(8): 1311-1322.
- Holt NR, Downey G, Naughton MT (2019) Perioperative considerations in the management of obstructive sleep apnoea. Medical Journal of Australia 211(7): 326-332.
- (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 22(5): 667-689.
- Sateia MJ (2014) International classification of sleep disorders-third edition: highlights and modifications. Chest 146(5): 1387-1394.
- Drager LF, McEvoy RD, Barbe F, Geraldo LF, Susan R, et al. (2017) Sleep Apnea and Cardiovascular Disease: Lessons From Recent Trials and Need for Team Science. Circulation 136(19): 1840-1850.
- Chung F, Yegneswaran B, Liao P, Sharon A Shung, Santhira V, et al. (2008) STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 108(5): 812-821.
- Tan A, Yin JD, Tan LW, Rob Vem Dam, Yan Yi Cheung, et al. (2016) Predicting obstructive sleep apnea using the STOP-Bang questionnaire in the general population. Sleep Med 27-28: 66-71.
- Nagappa M, Liao P, Wong J, Auckley D, Satya Krishna Ramachandran, et al. (2015) Validation of the STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea among Different Populations: A Systematic Review and Meta-Analysis. Plos one 10(12): e0143697.
- Xará D, Mendonça J, Pereira H, Santos A, Fernando Jose Abelha (2015) Adverse respiratory events after general anesthesia in patients at high risk of obstructive sleep apnea syndrome. Braz J Anesthesiol 65(5): 359-366.
- Singh M, Liao P, Kobah S, Wijeysundera DN, F Chung (2013) Proportion of surgical patients with undiagnosed obstructive sleep apnoea. British journal of anaesthesia 110(4): 629-636.
- Hai F, Porhomayon J, Vermont L, Frydrych L, Jaoude P, et al. (2014) Postoperative complications in patients with obstructive sleep apnea: a meta-analysis. Journal of Clinical Anesthesia 26(8): 591-600.
- Chung F, Liao P, Yegneswaran B, Shapiro CM, Kang W (2014) Postoperative Changes in Sleep-disordered Breathing and Sleep Architecture in Patients with Obstructive Sleep Apnea. Anesthesiology 120(2): 287-298.
- Liao P, Yegneswaran B, Vairavanathan S, Paul Z, Chung F (2009) Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Canadian journal of anaesthesia = Journal canadien d'anesthesie 56(11): 819-828.
- Kaw R, Chung F, Pasupuleti V, PC Gay, AV Hernandez (2012) Meta-analysis of the association between obstructive sleep apnoea and postoperative outcome. British journal of anaesthesia 109(6): 897-906.
- Chan MTV, Wang CY, Seet E, Tam S, Lai HY, et al. (2019) Association of Unrecognized Obstructive Sleep Apnea with Postoperative Cardiovascular Events in Patients Undergoing Major Noncardiac Surgery. JAMA 321(8): 1788-1798.
- Sood A, Abdollah F, Sammon JD, Majumder K, Schimd M, et al. (2015) The Effect of Body Mass Index on Perioperative Outcomes After Major Surgery: Results from the National Surgical Quality Improvement Program (ACS-NSQIP) 2005-2011. World journal of surgery 39(10): 2376-2385.
- Ortiz VE, Kwo J (2015) Obesity: physiologic changes and implications for preoperative management. BMC Anesthesiol 15: 97-97.
- Tong S, Gower J, Morgan A, Kyle G, Wisbach G (2017) Noninvasive positive pressure ventilation in the immediate post-bariatric surgery care of patients with obstructive sleep apnea: a systematic review. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery 13(7): 1227-1233.
- Mutter TC, Chateau D, Moffatt M, Ramsey C, Ross LL, et al. (2015) A matched cohort study of postoperative outcomes in obstructive sleep apnea: could preoperative diagnosis and treatment prevent complications? Anesthesiology 121(4): 707-718.
- Abdelsattar ZM, Hendren S, Wong SL, Campbell DA, Ramachandra SC, et al. (2015) The Impact of Untreated Obstructive Sleep Apnea on Cardiopulmonary Complications in General and Vascular Surgery: A Cohort Study. Sleep 38(8): 1205-1210.
- Chung F, Memtsoudis SG, Ramachandran SK, Nagappa M, Mathias O, et al. (2016) Society of Anesthesia and Sleep Medicine Guidelines on Preoperative Screening and Assessment of Adult Patients with Obstructive Sleep Apnea. Anesthesia & Analgesia 123(2): 452-473.
- Gali B, Whalen FX, Schroeder DR, Peter C Gay, David J Plevak (2009) Identification of patients at risk for postoperative respiratory complications using a preoperative obstructive sleep apnea screening tool and postanesthesia care assessment. Anesthesiology 110(4): 869-877.
- Weingarten TN, Kor DJ, Gali B, Sprung J (2013) Predicting postoperative pulmonary complications in high-risk populations. Current opinion in anaesthesiology 26(2): 116-125.
-
Shaurya Jhamb*, Venkat N Vangaveti, Stephen Whebell, Lachlan Young, Alexander N Kippin and Melita J Trout. Obstructive Sleep Apnoea in elective surgical patients admitted to a tertiary Intensive Care Unit: a 6-year Retrospective cohort study. Anaest & Sur Open Access J. 6(1): 2024. ASOAJ.MS.ID.000626.
-
Obstructive Sleep Apnoea (OSA); Elective Surgery; Intensive Care; Perioperative Medicine; Positive Airway Pressure (PAP), Apnoea, Non-cardiac surgery
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






