 Case Report
Case Report
Extended, Iatrogenic Coronary Artery Dissection Post-Partum: Clinical Management of a Very Unusual Case
Ventsislav Sheytanov1*, Joerg Seeburger1, Georg Trummer2,3, Marie Thomas2,3
1Department of Cardiac Surgery, Herzzentrum Klinikum Stuttgart, Stuttgart, Germany
2Department of Cardiovascular Surgery, University Heart Center Freiburg, Freiburg, Germany
2Faculty of Medicine, University of Freiburg, Freiburg, Germany
Ventsislav Sheytanov, Department of Cardiac Surgery, Herzzentrum Klinikum Stuttgart, Stuttgart, Germany
Received Date:March 24, 2024; Published Date:April 08, 2024
Abstract
Spontaneous coronary artery dissection (SCAD) is a very uncommon cause of myocardial infarction, mostly reported to occur in women during or after pregnancy. This may be due to hormonal changes and subsequently increased hemodynamic stress. It is presumable, that due to these factors pregnant or early post-partum women are at greater risk of iatrogenic coronary dissection during diagnostic coronary angiography. We herein present an unusual case of an extensive iatrogenic coronary artery dissection of all coronary arteries leading to acute massive myocardial infarction in a young woman three days postpartum. Clinical course and therapeutic management are reported.
Case Presentation
An otherwise healthy 38-year-old female patient at 35 plus 4 weeks of gestation and thus far unremarkable pregnancy presented herself with chest pain and numbness in both arms. She had no risk factors for coronary artery disease. Primary examination led to the diagnosis of musculoskeletal disorder and hyperventilation as because of her symptoms. Three days later, new onset signs of ischemia on ECG as well as elevation of the troponin I levels up to 4900 ng/l were detected. Immediate Cesarean delivery of a healthy infant was performed.
Increasing creatine kinase and troponin levels on day two postpartum, led to a diagnostic coronary angiography. Initially a normal coronary artery status was found. However, during the course of the angiography, iatrogenic dissection of all coronary arteries including the left main (LM), mid – left anterior descending (LAD), ramus intermedius (RIM), left circumflex (LCX) and right coronary artery (RCA) occurred (figure 1).
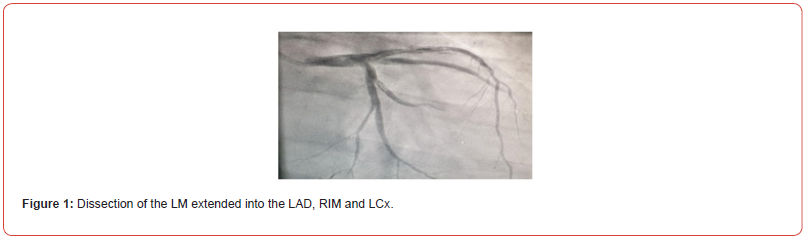
Emergency coronary artery bypass grafting (CABG) was performed. During induction of anesthesia cardiopulmonary resuscitation due to ventricular fibrillation was performed. Transesophageal echocardiography showed a severely reduced ventricular ejection fraction of 20%. Immediate installation of cardiopulmonary bypass (CPB) and retrograde coronary artery perfusion through the coronary vein sinus was performed.
Initially a left internal mammary artery (LIMA) to LAD and venous grafts to RCA, RIM and LCX were performed on-pump beating heart. Intimal layers of all coronary vessels were found to be completely torn with difficult to almost impossible identification of the true lumen.
Weaning of CPB was not possible, so standard ECLS circuit Extracorporeal Life Support circuit (ECLS) was initiated.
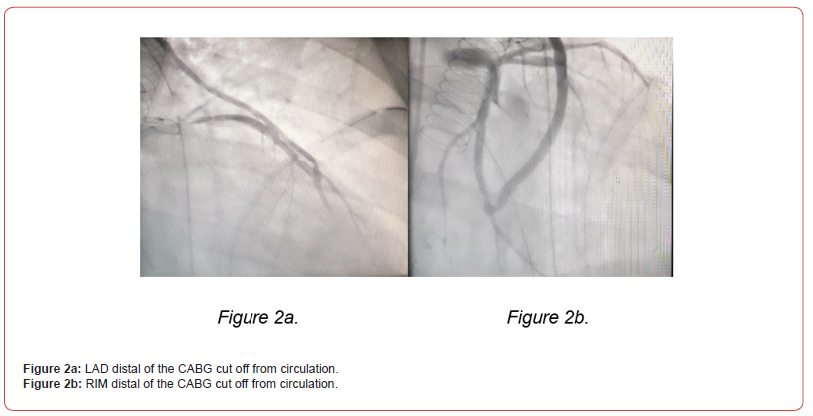
The extremely difficult coronary status with unpredictable bypass run-off, led to the intraoperative performance of a repeat coronary angiography. This examination showed the distal third of the LAD and the RIM to be cut off from circulation (figure 2. A and B), thus additional venous grafts were performed distally.
Finally, the patient was transferred with open chest and under full extracorporeal circulatory support to the intensive care unit.
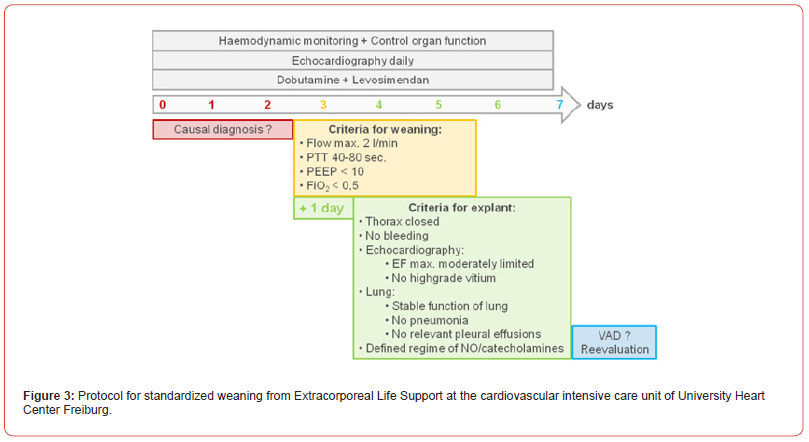
In a second step, the patient was transferred to a center offering long term circulatory support (VAD) and cardiac transplantation. Initially ECLS was switched from central to femoral cannulation. During the consecutive days, the weaning process from ECLS was attempted following the institutional SOP (figure 4). From day 0 to 2 (red stage), stabilization of hemodynamics under maximum ECLS of 4l/min flow was focused, accompanied by a switch of the inotropic support from epinephrine to dobutamine. On day 2 secondary closure of the chest was performed and the patient was loaded with levosimendan. Echocardiography was carried out every day to assess left ventricular function and myocardial recovery. On day 3, the patient showed stable hemodynamics under minimal support of dobutamine only, allowing her to enter the stage of weaning (figure 4, yellow stage). At this time, accompanied by echocardiographic monitoring, ECLS was reduced from 4l/min to 3l/min with a corresponding left ventricular ejection fraction (LVEF) of 25%. Finally, all listed criteria could be reached (day 3 to 5) and explanation was performed on day 6. Thereafter the patient was extubated one day later, and transferred awake and without neurological deficits or other organ failure to the referring hospital. Final discharge from the hospital was possible on the 22nd postoperative day with a LVEF of 40%. At one year follow up the patient reported to be completely recovered. LVEF was 46%.
Discussion
Such extent of coronary artery dissection is rarely seen and the presence of pregnancy further aggravated the situation. Periand intraoperative management including retrograde myocardial perfusion and the highly structured operation and weaning from the ECLS with consecutive full recovery of the patient is remarkable.
It is known, that pregnancy increases the risk of acute myocardial infarction (AMI) up to 3-4-fold compared with nonpregnant reproductive-age women [1]. Data on pregnancy-related AMI suggests that coronary artery dissection may account for 27% [2]. These findings raise concerns upon the vulnerability of coronary vessels during pregnancy, presumably related to hemodynamic changes and the hormonally mediated alterations of vascular walls.
These include an increase in stroke volume and heart rate as well as a significant compression of the abdominal aorta and the iliac arteries by the enlarged uterus. This leads to an increase in afterload as well as increased wall stress in the thoracic aorta and subsequent arteries such as the coronary arteries. In addition, histological changes in the arterial wall, increase vascular vulnerability suggesting a higher risk of spontaneous or catheter induced coronary dissection [3].
In the present case, intraoperative macroscopic examination showed extensive dissection of all coronary arteries from their origin to the vascular bed. Since Cath angiography only showed a dissection in the LCA, rapid and extensive progression of coronary dissection most likely occurred due to an increased vulnerability of the vessels in the puerperium.
Studies have shown the superiority of off-pump CABG and onpump beating heart strategies over conventional arrested heart CABG in patients with AMI [4,5]. In such patients, also complicated with severe cardiogenic shock and severely reduced cardiac output, initiation of cardiopulmonary bypass (CPB) support is inevitable. Especially due to the massive extent of coronary dissection in the present case, with antegrade perfusion being close to impossible, emergency initiation of CPB was needed. In similar settings, continuous retrograde warm blood coronary perfusion has shown to be effective in preventing myocardial damage [6]. Continuous retrograde coronary perfusion thus significantly increased the chance of perfusing the true lumen and therefore reducing ischemia. In addition, the presence of a retrograde blood flow was utilized to identify the true lumen while performing coronary anastomoses. Irrespective of the underlying diagnosis forcing ECLS treatment, the management of this short-term mechanical assist device and the process of weaning are complex issues. No official guidelines to lead the attending physician are available until now [7]. However, a corresponding protocol has recently been published with key aspects focusing on an integrated approach of intensive care, frequent echocardiographic monitoring and sophisticated inotropic support within a timeframe of three to seven days [8].
Conclusion
Pregnancy induced vascular vulnerability remains a potential risk for acute myocardial infarction as well as iatrogenic arterial dissections. However, massive coronary dissection can successfully be treated with coronary artery bypass graft surgery and retrograde coronary perfusion under extracorporeal life support.
Acknowledgement
None.
Conflict of interest
No conflict of interest.
References
- M R T Sadeghi, N Aslanabadi, K Aghdam, R Parizad, H Namdar (2014) Pregnancy related acute myocardial infarction: a review of epidemiology, diagnosis, medical and surgical management. Int J Women’s Health Reprod Sci 2(5): 272-277.
- Roth A, Elkayam U (2008) Acute myocardial infarction associated with pregnancy. J Am Coll Cardiol 52: 171-180.
- Manalo Estrella P, Barker AE (1967) Histopathologic findings in human aortic media associated with pregnancy. Arch Pathol 83(4): 336-341.
- Abbas Afrasiabirad, Naser Safaie, Hosein Montazergaem (2015) On-Pump Beating Coronary Artery Bypass in High-Risk Coronary Patients. Iran J Med Sci 40(1): 40-44.
- Khalil Fattouch, Francesco Guccione, Pietro Dioguardi, Roberta Sampognaro, Egle Corrado, et al. (2009) Off-pump versus on-pump myocardial revascularization in patients with ST-segment elevation myocardial infarction: A randomized trial. J Thorac Cardiovasc Surg 137(3): 650-656.
- Blase A Carabello, Gerald M Lemole, Kang W Lee, James F Spann (1976) Retrograde Coronary Capillary Perfusion for Prevention and Reversal of Cardiogenic Shock in Experimental Myocardial Infarction, The Annals of Thoracic Surgery 21(5): 405-411.
- Working Group of Scientific Medical Societies (AWMF). Guideline detail view. Registered guideline project. Use of extracorporeal circulation (ECLS / ECMO) in heart and circulatory failure.
- Thomas M, Kreibich M, Beyersdorf F, Benk C, Maier S, et al. (2020) Standardized Weaning from Temporary Extracorporeal Life Support in Cardiovascular Patients. Thorac Cardiovasc Surg 68(5): 425-432.
-
Ventsislav Sheytanov*, Joerg Seeburger, Georg Trummer, Marie Thomas. Extended, Iatrogenic Coronary Artery Dissection Post-Partum: Clinical Management of a Very Unusual Case. Anaest & Sur Open Access J. 4(5): 2024. ASOAJ.MS.ID.000599.
-
Anesthesia cardiopulmonary resuscitation, Coronary dissection, Anaesthesia, Cardio Surgery, Myocardial infarction, Bypass graft surgery, Coronary perfusion, Intensive care
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






