 Review Article
Review Article
A Brief History of Coronary Revascularization Surgery
Elizabeth J Bashian1*, Eleanor E Bashian2, Cameron Walker3, Mark Nelson4, Josue Chery1
1Department of Surgery, Virginia Commonwealth University Health, Richmond, USA
2Scripps Research Institute, San Diego, California
3Trinity Episcopal School, Richmond, USA
4Virginia Commonwealth University, Health Department of Anesthesia, USA
Elizabeth J Bashian, Department of Surgery, Virginia Commonwealth University Health, Richmond, USA
Received Date:March 29, 2024; Published Date:April 05, 2024
Abstract
The quest to treat coronary artery disease, the leading cause of death in the United States and worldwide, is fascinating and continues to evolve. Advances in coronary angiography including physiologic tests of coronary function, have paved the way for a greater understanding and more precise application of revascularization techniques. Development and application of improved medical treatments, goal directed medical therapy, add valuable treatment options. As management options have progressed, several studies have emerged as foundational for societal guidelines and have provided valuable and rational approaches for treatment of coronary artery disease. While debates persist on the treatment of coronary artery disease, overall, there has been a shift in paradigm to a team approach with treatment options tailored to anatomy, physiology, and patient wishes. Percutaneous coronary intervention, coronary artery bypass graft, and goal directed medical treatment are now viewed as tools that can be used in either tandem or in combination. As technology progresses and our understanding of the complex pathophysiology of coronary artery disease improves, our approach to coronary revascularization and preservation of cardiac function will continue to evolve.
Case Presentation
Coronary artery disease (CAD) continues to be a leading cause of death in the United States and the rest of the world [1]. Management of coronary artery disease includes goal directed medical therapy (GDMT), percutaneous intervention (PCI), and coronary artery bypass surgery (CABG). Since the 1980s, CABG has consistently been a reliable and effective treatment for severe CAD. This paper will provide an overarching history of coronary revascularization including landmark trials supporting its use, current practices of coronary revascularization, and patient factors, including preferences which affect treatment decisions.
History and development of coronary bypass surgery
Cardiac catheterization has been foundational to coronary revascularization, the origins of which can be traced back to the 1920s when a German urologist, Werner Forssmann [2], inserted a catheter into his own heart proving right heart catheterization was possible in humans. While others had managed to place catheters in a central vein before [3], Forssmann was the first to confirm the catheter position in the heart with a chest radiograph [2]. Right heart catheterization became utilized in humans for direct measurements of mixed venous blood oxygenation and pressure tracings, providing considerable information about cardiac and pulmonary physiology [4-6] which ushered in a new era for the possibility of treatment [7-9]. Forssman, along with Andre Cournand and Dickison Richards won the 1956 Nobel Prize in Physiology and Medicine for “their discoveries in concerning heart catheterization and pathological changes in the circulatory system.
As the understanding of coronary disease processes progressed, physicians began attempting various methods of surgically correcting CAD. Given the timing of publication differed from time of discoveries, the history is convoluted (Figure 1). In the early 1950s, the first attempts were published with the internal mammary artery (IMA) anastomosed directly to the myocardium [10]. Shortly thereafter, reports began to emerge of anastomosing the IMA to a coronary artery [11-13]. At the same time, Longmire attempted coronary endarterectomy for management of CAD, which was largely unsuccessful [14]. Patch grafting of the coronary arteries with pericardium was also attempted [15]. In general, early attempts at revascularization were less successful largely due to the lack of accurate imaging modalities which could locate areas of significant blockage. In 1958, Sones inadvertently injected contrast into the right coronary artery (RCA) while performing an aortogram. This led to direct coronary angiography, which significantly changed the nature of coronary revascularization [16,17]. With angiography and accurate anatomical localization of obstructions, coronary revascularization became much more feasible. Reports of successful CABG utilizing different conduits emerged: right IMA to RCA in 1965 [18], right saphenous vein graft (SVG) to coronary arteries in 1967 [19], and left IMA to left anterior descending artery in 1968 [20].
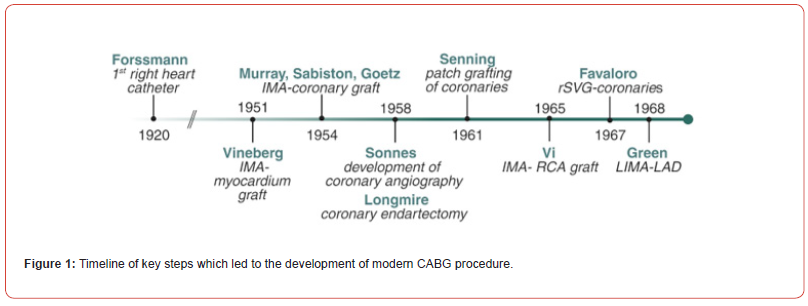
In the 1970s, three separate randomized controlled trials demonstrated the early efficacy of CABG in the treatment of CAD: Coronary-artery bypass surgery instable angina pectoris: Survival at two years- The European Coronary Surgery Study Group [21], National Heart, Lung, Blood Institute Coronary Artery Surgery Study (CASS) [22]; and Veterans Affairs Cooperative Study of Coronary Artery Bypass Surgery for Stable Angina [23]. The European study, published in 1979, randomized 768 males with angina to either medical or surgical treatment. There was no difference in two-year mortality between the groups, however subgroup analysis showed better survival with CABG in two vessel disease involving left anterior descending artery (LAD) or any three-vessel disease [24]. The Veterans Affairs Cooperative Study of Coronary Artery Bypass Surgery for Stable Angina, published in 1976 with final follow-up at twenty-two years, investigated benefits of SVG in a cohort of 686 patients randomized to either medical management or CABG. They reported a large majority (69%) of grafts were patent at one year and no statistically significant difference in survival between groups at 21 months [21]. There was a survival benefit from five to ten years in the CABG group, largely in those patients deemed high risk clinically or angiographically and no survival benefit observed in any group thereafter. These observations seemed to coincide with SVG patency [23]. In 1983, the CASS study randomized 780 patients to surgical or non-surgical treatment to evaluate effects of CABG compared to medical therapy alone in patients with stable coronary disease. At five years, mortality was similar in both groups (medical group 1.6% vs surgical group 1.1%); outcomes were also similar between single, double, and triple vessel disease. However, at 10-year follow-up, a survival benefit was noted for CABG in patients with reduced left ventricular ejection fraction (LVEF) <0.5 (78% vs 51%, p=0.01) [22]. In general, these three trials demonstrated CABG was no worse than medical management and in specific cases such as triple vessel or LAD disease, CABG resulted in better outcomes than medical therapy alone.
As surgical techniques continued to improve, several landmark trials demonstrated the value of CABG over other treatment options in severe CAD. The Bypass Angioplasty Revascularization Investigation (BARI) trial was a multicenter investigation across three years, which compared percutaneous transluminal coronary angioplasty (PTCA) to CABG. In this study, 1829 patients at 18 centers with clinically important lesions, defined as stenosis greater than 50 percent vessel diameter, were randomly assigned to CABG or PTCA and followed for five years. The primary end point was all cause mortality. Results showed no statistically significant difference in cumulative survival rates between groups (CABG 89.3%, PTCA 86.3%). PTCA patients were more likely to require early reintervention (12.8% reintervention during hospitalization, 6.3% required emergency CABG). In patients with diabetes, however, long-term survival rates were noted be significantly higher with CABG (CABG 57.8, PTCA 45.5%). CABG was also associated with higher long-term survival rates [25]. The BARI 2 trial was a 10-year follow-up with the same patients. Similar survival rates were noted between PTCA and CABG groups (71.0%, 73.5% respectively). Again, PTCA patients were more likely to require revascularization (76.8% vs 20.3% CABG). In patients with diabetes, CABG patients continued to have higher long term survival rates (CABG 57.8%, PTCA 45.5%) In all, investigators concluded that there was no disadvantage to initial PTCA compared to CABG; however, patients would be more likely to require repeat revascularization than with surgical intervention initially [26].
The subsequent Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial investigated whether revascularization with either PCI or CABG reduced the incident of cardiovascular events or death compared to optimal medical therapy (OMT) alone in patients with stable CAD and type 2 diabetes mellitus (T2DM), who are more likely to have diffuse multivessel CAD. Primary end points included rate of death, composite of death, MI, and stroke. Twenty-three hundred and sixty patients were randomized to OMT alone or in addition to revascularization and assigned to PCI or CABG by their physician. At five years, results demonstrated no difference in rates of survival between the revascularization group and the OMT group (88.3% vs 87.8%) or in freedom from major cardiovascular events (77.2% vs 75.9%). However, subgroup analysis demonstrated those randomized to CABG had lower rates of cardiovascular (CV) events than OMT alone (77.6% vs 69.5%) [27]. Similar to the BARI trial, the key takeaway from this study was that in patients with T2DM and CAD, CABG with OMT could reduce rate of adverse CV events, whereas there was no difference with PCI.
Coronary Bypass Surgery Today Conduit choices
Principles of bypass surgery dictate that inflow, outflow, and conduit choice can influence success of the bypass. Proximal and distal endpoints are largely dictated by lesion location and patient anatomy. There is slightly more flexibility with the choice of conduit, which can include venous or arterial grafts, the latter of which can be in-situ or free grafts. Multiple studies have investigated efficacy, advantages, and disadvantages of various conduit choices (Figure 2).
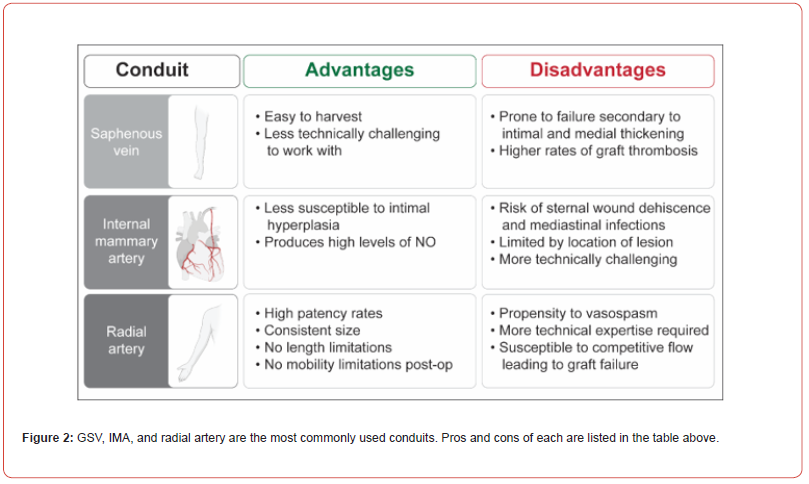
Saphenous venous grafts (SVG) were initially popularized by Favaloro, an Argentinian-born physician who trained with Sones at the Cleveland Clinic. One of the first successful bypasses was performed with an interposition SVG with end-to-end anastomosis in a patient with an occluded right coronary artery (RCA). Favaloro’s studies in the late 1960s showed successful outcomes in 150 patients who underwent CABG with SVG19. However, SVG are prone to failure secondary to intimal and medial thickening and higher rates of graft thrombosis.
Internal mammary artery grafts were first utilized in the 1980s and found to have improved outcomes at ten years, including increased survival, decreased rate of myocardial infarction, and decreased rates of repeat revascularization. SVG only, when compared to LIMA-LAD (left internal mammary artery to left anterior descending) either with or without SVG, had 1.61 times greater risk of death, 1.4 times risk of myocardial infarction (MI), 1.24 times risk of rehospitalization, 2.0 times risk of reoperation, and 1.27 times risk of late cardiac events [28]. IMA grafts have been found to have better long-term outcomes as well. IMA is an elastic artery, with 7-11 elastic layers. The intima is non-fenestrated and has endothelium with some intima. The media consists of circumferentially oriented smooth muscle complexes compared to those longitudinally oriented in SVG. These characteristics reduce graft susceptibility to intimal hyperplasia, the key factor for atherosclerosis. Additionally, IMA produces high levels of nitric oxide (NO) which decreases vasospasm and benefits the entire coronary system [29]. A 20-year follow-up study by Cameron et al. of patients who underwent CABG between 1970-1973 with either vein grafts alone or in addition to single IMA (SIMA) or bilateral IMA (BIMA) alone demonstrated increased survival, lower rates of MI and less angina for the first fifteen years in either the IMA or BIMA groups [30].
Given the success demonstrated with IMA grafts, researchers gained interest in efficacy of BIMA grafts. Rankin and Tuttle [31] investigated effects of SIMA vs BIMA grafts in 867 patients undergoing CABG at Duke University Medical Center between 1984- 1986 with 20-year follow-up. Four hundred and ninety patients were SIMA-LAD and SVG vs 377 patients who were BIMA (66% LAD/RCA and 33% LAD/Cx) and SVG. In general, multiple systems proved more effective, and the BIMA group had consistently better outcomes31. Complications of BIMA grafts include risks of sternal wound dehiscence and mediastinal infections. Risk factors to these complications include, but are not limited to, T2DM, obesity, chronic obstructive pulmonary disease and smoking [32]. Steps to mitigate these complications include skeletonization of the conduit, ligating branches close to IMA to maintain collateral circulation, and preserving venous drainage [28,29,31]. The Arterial Revascularization Trial (ART) [32] randomized 1548 patients with multivessel CAD to grafting with BIMA or SIMA; the primary outcome was all cause mortality at 10 years. Results demonstrated no significant mortality difference at 10 years (BIMA 20.3% vs SIMA 21.2%, p=0.62). There was an absolute 1.6 percent increase in sternal wound complications in the BIMA group. Overall, investigators reported no clear advantage to BIMA over SIMA but noted it could be used safely in the appropriate patient [32]. A significant limiting factor in use of pedicle right internal mammary artery (RIMA) grafts is the location of the lesion, as the graft must be able to reach distally without undue tension [33]; most data on efficacy of RIMA is in situ use. Additionally, the relatively small diameter of IMA can make it more technically challenging graft than a vein graft [34].
Radial artery grafts were first used by Carpentier in 1971, but initially had high rates of failure due to endothelial injury after mechanical dilation and/or skeletonization during harvest. Radial artery grafts were repopularized in 1993 by Acar using the “no touch” harvest method and pharmacologic vasodilators such as papaverine [35]. Vasodilators, most commonly calcium channel blockers or nitrates, can be used post-operatively to minimize vasospasm [36]. Radial artery grafts have a propensity to vasospasm, rendering them sensitive to competitive flow, which limits its use to grafting vessels with a very high degree of stenosis [34,37]. These remain frequently used today and are effective in the appropriate patient.
Indications and current guidelines
Until recently, indications for CABG included left main (LM) disease greater than 50 percent; three vessel disease greater than 70 percent with or without proximal LAD involvement or two vessel disease involving the proximal LAD and another major artery; stenosis greater than 70 percent in a major vessel plus anginal symptoms on maximal medical therapy; or one vessel disease greater than 70 percent in a survivor of sudden cardiac death with ischemia related ventricular tachycardia [38]. Contraindications to surgical revascularizations from a purely anatomical standpoint are relatively few: arteries incompatible with grafting or patients with no viable myocardium to which to graft. However, co-morbid conditions may make patients a poor candidate for surgical revascularizations. The Society of Thoracic Surgery operative risk calculator incorporates a patient’s demographic and physiologic data to calculate their overall risk for surgery.
In 2021, the American College of Cardiology/American Heart Association/Society of Cardiovascular Angiography and Intervention (ACC/AHA/ASCAI) released updated guidelines recommendations for surgical and medical intervention in CAD patients which were met with a mixture of fanfare and criticism. First, for patients with three vessel CAD and normal left ventricular function, CABG/PCI was downgraded from level 1 to level IIB. The 2020 ISCHEMIA trial [39] was cited to support this change. This study identified no difference in death from cardiac causes, MI, hospitalization, or heart failure in 5179 patients with stable coronary disease and moderate or severe ischemia randomized to revascularization with either PCI or CABG vs conservative treatment. However, critics state this trial was not designed to investigate long term survival benefits of CABG and in addition, utilizes a nonrepresentative patient population. Proponents of the former guidelines cite the 2009 Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) [40] trial which studied patients more appropriate for revascularization and found a 40 percent higher mortality rate in patients with three vessel disease treated with PCI compared to CABG. The 2018 European Society of Cardiology/European Association of Cardiothoracic Surgery (ESC/EACTS) guidelines have not been revised and continue class I recommendation for CABG with 3 vessel CAD and one or two vessel CAD with proximal LAD stenosis [41]. Another controversial change was the grouping of PCI and CABG as equivalent revascularization strategies supported by the 2019 EXCEL [42] and NOBLE 44 trials which compared PCI to CABG in left main disease, and the 2022 FAME 343 trial which compared PCI to CABG in three vessel disease. All three studies showed equipoise between PCI and CABG. While CABG failed to demonstrate a survival advantage over PCI, it has shown a reduced rate of repeat revascularization and decreased rates of postprocedural MI [43-46]. Finally, the updated guidelines assigned level I for the use of radial artery as a conduit, the same level as IMA and a stronger support than BIMA. The guidelines cited six small RCTs in which patients were judged to be good candidates for radial artery conduits but excluded patients with poor ventricular function and who would not have been good radial artery candidates. However, the official recommendation did not include any qualifiers for candidacy. In response, the Society for Thoracic Surgeons (STS) and the American Association of Thoracic Surgeons (AATS) stated they did not support the updated guidelines, citing the issues above [47]. Many other societies worldwide including ESC/EACTS, Latin-American Association had similar critiques. The new guidelines continue to support CABG over PCI for left main CAD with high complexity CAD and in multivessel CAD.
Integrating non-invasive revascularization modalities
In the early 2000s, drug eluting stents became increasingly utilized to treat complex coronary artery disease. The 2009 SYNTAX trial was a multi-center, randomized control trial comparing CABG to PCI with drug eluding stents in 1800 patients at 85 centers in patients with left main and or three vessel CAD. The primary outcome was any major CV event, which included death, stroke, MI, or repeat revascularization at one year and results of the trial demonstrated a higher incidence of events in the PCI groups (17.8% vs 12.4 % [RR 1.44; 95% CI, 1.15-1.81; p=0.002]). It was noted that there was a higher percentage of strokes in the CABG arm (0.6% vs 2.2%). Additionally, SYNTAX developed a score by which to classify patients according to severity of CAD46.
To further elucidate the impact of emerging technologies in stents, the use of coronary physiology, and the use of intravascular ultrasound with percutaneous revascularization, the SYNTAX II trial was performed with a revised version of the SYNTAX score. Patients with three vessel disease treated with these new modalities were compared with a similar cohort from the SYNTAX I trial. The primary endpoint was major adverse cardiac and cerebrovascular events (MACCE). At one and five years, MACCE rate was significantly lower for patients treated with the SYNTAX II strategy compared to SYNTAX I PCI cohort [48]. The EXCEL trial, published in 2016, randomized patients with left main coronary disease and revised SYNTAX scores ≤32 to treatment either with PCI with a secondgeneration drug eluting stent or CABG. The primary outcome was composite risk of death, stroke, or MI at 3 years. Results demonstrated non-inferiority of PCI with second-generation DES compared to CABG (PCI 15.4% vs CABG 14.4%, absolute difference +0.7 [95.7% CI <0.0 to –4.0]; however, investigators did note a nonsignificant trend towards increased mortality in PCI42. Overall, evolving technologies with PCI continue to push the boundaries and demonstrate excellent results. In general, the focus should be on how to incorporate these two different treatment modalities (surgery and PCI) to provide the best outcome.
Conclusion
Coronary artery disease is a complex medical entity that is heterogenous in its presentation, anatomical features, and patient population. Subsequently, treatment decisions are equally as complex and an individualized, tailored approach is now required to optimize outcomes. Current practices are not competitive as once seen, but rather represent a synergy of medical therapy, PCI, and surgical revascularizations. These modalities should be seen as tools in an armamentarium that can be used in isolation or in various combinations based on a patient’s specific anatomy, medical co-morbidity, preferences, and needs. Today’s treatment modalities have stemmed from various key developments, from the first cardiac catheterization to the development of coronary angiograms to the various attempts at revascularization, to current practices utilized today. CABG remains a gold standard for complex CAD and confers survival benefits in these high-risk populations. Current research suggests that arterial revascularizations with BIMA and radial artery confer outcome advantages over venous conduits. Decisions of conduits can vary between patients as their availability or suitability for the anatomical lesion must be taken into account. As technology progresses, societal recommendations on how surgical revascularization is best utilized to reestablish, preserve, and optimize blood flow to the myocardium will undoubtedly evolve as well.
Acknowledgement
None.
Conflict of interest
No conflict of interest.
References
- Ralapanawa U, Sivakanesan R (2021) Epidemiology and the Magnitude of Coronary Artery Disease and Acute Coronary Syndrome: A Narrative Review. J Epidemiol Glob Health 11(2): 169-177.
- Bourassa MG (2005) The history of cardiac catheterization. Can J Cardiol 21(12): 1011-1014.
- Zimmerman HA, Scott RW, Becker NO (1950) Catheterization of the left side of the heart in man. Circulation 1(3): 357-359.
- Cournand A, Lauson HD, Bloomfield RA, Breed ES, Baldwin EDF (1944) Recording of Right Heart Pressures in Man. Proc Soc Exp Biol Med 55(1): 34-36.
- Cournand A, Bloomfield RA, Lauson HD (1945) Double Lumen Catheter for Intravenous and Intracardiac Blood Sampling and Pressure Recording. Proc Soc Exp Biol Med 60(1): 73-75.
- Hilmert A Ranges (1941) Catheterization of the Right Auricle in Man-Andre Cournand. Accessed February 6(3).
- Nossaman BD, Scruggs BA, Nossaman VE, Murthy SN, Kadowitz PJ (2010) History of Right Heart Catheterization: 100 Years of Experimentation and Methodology Development. Cardiol Rev 18(2): 94-101.
- Forssmann Falck R (1997) Werner Forssmann: A Pioneer of Cardiology. Am J Cardiol 79(5): 651-660.
- Raju TN (1999) The Nobel chronicles. 1956: Werner Forssmann (1904-79); André Frédéric Cournand (1895-1988); and Dickinson Woodruff Richards, Jr (1895-1973). Lancet Lond Engl 353(9167): 1891.
- Vineberg A, Miller G (1951) Treatment of Coronary Insufficiency. Can Med Assoc J 64(3): 204-210.
- Murray G, Porcheron R, Hilario J, Roschlau W (1954) Anastomosis of systemic artery to the coronary. Can Med Assoc J 71(6): 594-597.
- Sabiston DC, Fauteux JP, Blalock A (1957) An experimental study of the fate of arterial implants in the left ventricular myocardium; with a comparison of similar implants in other organs. Ann Surg 145(6): 927-938.
- Goetz RH, Rohman M, Haller JD, Dee R, Rosenak SS (1961) Internal Mammary-Coronary Artery Anastomosis: A Nonsuture Method Employing Tantalum Rings. J Thorac Cardiovasc Surg 41(3): 378-386.
- Longmire WP, Cannon JA, Kattus AA (1958) Direct-Vision Coronary Endarterectomy for Angina Pectoris. N Engl J Med 259(21): 993-999.
- Senning A (1961) Strip grafting in coronary arteries. Report of a case. J Thorac Cardiovasc Surg 41: 542-549.
- Sones FM, Shirey EK (1962) Cine coronary arteriography. Mod Concepts Cardiovasc Dis 31: 735-738.
- Sandler H, Dodge HT (1968) The use of single plane angiocardiograms for the calculation of left ventricular volume in man. Am Heart J 75(3): 325-334.
- Vi K (1964) Operations on the coronary arteries. Exp Clin Anaesth 10: 3-8.
- Favaloro RG (1968) Saphenous Vein Autograft Replacement of Severe Segmental Coronary Artery Occlusion: Operative Technique. Ann Thorac Surg 5(4): 334-339.
- Green GE, Stertzer SH, Reppert EH (1968) Coronary arterial bypass grafts. Ann Thorac Surg 5(5): 443-450.
- (1979) Coronary-artery bypass surgery instable angina pectoris: Survival at two years. European Coronary Surgery Study Group. Lancet Lond Engl 1(8122): 889-893.
- (1983) Coronary artery surgery study (CASS): a randomized trial of coronary artery bypass surgery. Survival data 68(5): 939-950.
- Peduzzi P, Kamina A, Detre K (1998) Twenty-two-year follow-up in the VA Cooperative Study of Coronary Artery Bypass Surgery for Stable Angina. Am J Cardiol 81(12): 1393-1399.
- (1979) Coronary-Artery Bypass Surgery in Stable Angina Pectoris: Survival At Two Years: European Coronary Surgery Study Group. The Lancet 313(8122): 889-893.
- Willerson JT (1996) Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. Circulation 94(6): 1194.
- (2007) The Final 10-Year Follow-Up Results from the BARI Randomized Trial. J Am Coll Cardiol 49(15): 1600-1606.
- BARI 2D Study Group, Frye RL, August P, Maria Mori Brooks, Regina M Hardison, Sheryl F Kelsey, et al. (2009) A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 360(24): 2503-2515.
- Loop FD, Lytle BW, Cosgrove DM, R W Stewart, M Goormastic, et al. (1986) Influence of the Internal-Mammary-Artery Graft on 10-Year Survival and Other Cardiac Events. N Engl J Med 314(1): 1-6.
- Otsuka F, Yahagi K, Sakakura K, Virmani R (2013) Why is the mammary artery so special and what protects it from atherosclerosis? Ann Cardiothorac Surg 2(4): 51926-51526.
- Cameron AAC, Green GE, Brogno DA, Thornton J (1995) Internal thoracic artery grafts: 20-year clinical follow-up. J Am Coll Cardiol 25(1): 188-192.
- Rankin JS, Tuttle RH, Wechsler AS, Teichmann TL, Glower DD, et al. (2007) Techniques and Benefits of Multiple Internal Mammary Artery Bypass at 20 Years of Follow-Up. Ann Thorac Surg 83(3): 1008-1015.
- Taggart DP, Benedetto U, Gerry S, Doulgas G Altman, Alastair M Grayet, et al. (2019) Bilateral versus Single Internal-Thoracic-Artery Grafts at 10 Years. N Engl J Med 380(5): 437-446.
- Torregrossa G, Amabile A, Williams EE, Fonceva A, Hosseinian L, et al. (2020) Multi-arterial and total-arterial coronary revascularization: Past, present, and future perspective. J Card Surg 35(5): 1072-1081.
- Jegaden O, Bontemps L, De Gevigney G, et al. Does the extended use of arterial grafts compromise the myocardial recovery after coronary artery bypass grafting in left ventricular dysfunction? Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg 14(4): 353-359.
- Acar C, Jebara VA, Portoghese M, B Beyssen, J Y Pagny, et al. (1992) Revival of the radial artery for coronary artery bypass grafting. Ann Thorac Surg 54(4): 652-660.
- Verma S, Szmitko PE, Weisel RD, et al. (2004) Should radial arteries be used routinely for coronary artery bypass grafting? Circulation 110(5): e40-46.
- Cancelli G, Audisio K, Chadow D, Soletti GJ, Gaudino M (2021) The evidence for radial artery grafting: When and when not? JTCVS Tech 10: 114-119.
- (2011) ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery.
- Maron DJ, Hochman JS, Reynolds HR, Sripal Bangalore, Sean M Obrien, et al. (2020) Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med 382(15): 1395-1407.
- Serruys PW, Morice MC, Kappetein AP, Antonio Colombo, David R Holmes, et al. (2009) Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 360(10): 961-972.
- Neumann FJ, Sousa-Uva M, Ahlsson A, Fernando Alfonso, Adrain P Banning, et al. (2019) 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 40(2): 87-165.
- Stone GW, Sabik JF, Serruys PW, Charles A Simonton, Philippe Genereux, et al. (2016) Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. N Engl J Med 375(23): 2223-2235.
- Tonino PAL, De Bruyne B, Pijls NHJ, Uwe Siebert, Fumiaki Ikeno, et al. (2009) Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 360(3): 213-224.
- (2023) Percutaneous Coronary Intervention of Left Main Disease | Circulation: Cardiovascular Interventions. Accessed December 28.
- Jain SS, Li D, Dressler O, Lak Kotinkaduwa, Patrick W Serruys, et al. (2023) Impact of Periprocedural Adverse Events After PCI and CABG on 5-Year Mortality: The EXCEL Trial. JACC Cardiovasc Interv 16(3): 303-313.
- Farina P, Gaudino MFL, Taggart DP (2020) The Eternal Debate with a Consistent Answer: CABG vs PCI. Semin Thorac Cardiovasc Surg 32(1): 14-20.
- Sabik JF, Bakaeen FG, Ruel M (2022) The American Association for Thoracic Surgery and The Society of Thoracic Surgeons reasoning for not endorsing the 2021 ACC/AHA/SCAI Coronary Revascularization Guidelines. J Thorac Cardiovasc Surg 163(4): 1362-1365.
- Banning AP, Serruys P, De Maria GL, Nicola Ryan, Simon Walsh, et al. (2022) Five-year outcomes after state-of-the-art percutaneous coronary revascularization in patients with de novo three-vessel disease: final results of the SYNTAX II study. Eur Heart J 43(13): 1307-1316.
-
Elizabeth J Bashian*, Eleanor E Bashian, Cameron Walker, Mark Nelson, Josue Chery. A Brief History of Coronary Revascularization Surgery. Anaest & Sur Open Access J. 4(5): 2024. ASOAJ.MS.ID.000598.
-
Coronary artery disease, Physiologic tests, Bypass surgery, Coronary revascularization, Myocardium, Bypass Angioplasty Revascularization Investigation, Angioplasty, Arterial grafts
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






