Review Article
Review Article
The Role of Angiogenesis Inhibitors in The Treatment of Malignant Pleural Mesothelioma
Nightingale Syabbalo*
Professor of Physiology and Medicine, Nabanji Medical Centre, Zambia
Nightingale Syabbalo, Professor of Physiology and Medicine, Nabanji Medical Centre, Zambia.
Received Date: July 18, 2021; Published Date: August 27, 2021
Abstract
Malignant pleural mesothelioma (MPM) is a highly aggressive and incurable cancer that originates from the mesothelial cells of the pleural cavity. It is associated with a long latency period of inhalation of asbestos fibers of 20-40 years. The subtypes of MPM include epitheloid (60%), sarcomatoid (10%), and biphasic (30%), which comprise both epitheloid and sarcomatoid histological features. The sarcomatoid type has the poorest prognosis with a median overall survival (OS) of 3 months, whereas the epitheloid and biphasic have median survival of only 7% at 3 years. The mechanisms by which asbestosis fibers are carcinogenic are not fully understood. MPM is associated with several genetic mutations in DNA, and alterations in some oncogenes, and tumor suppressor genes; and the activation of signaling pathways involved in cell proliferation and apoptosis. MPM is usually diagnosed very late with advanced disease, and the treatment of unresectable disease is chemotherapy with cisplatin plus pemetrexed; which has a response rate of about 26.3%-41%, and modestly extends survival by 2-3 months. Vascular endothelial growth factor (VEGF) is the most potent endothelial specific mitogen for endothelial cells. It promotes angiogenesis in tumors and propagates cancer growth and metastasis. In pleural mesothelioma, VEGF directly acts as an autocrine mitogen for mesothelial cells, thus orchestrating the growth and local spread of the tumor. Bevacizumab is a humanized monoclonal murine antibody which blocks the immunopathological pathways of VEGF and its receptors on endothelial cells, and mesothelioma cells. Addition of bevacizumab a VEGF inhibitor to cisplatin plus pemetrexed regimen has been shown to extend the median OS for 16-22 months, versus 14-18 months with cisplatin plus pemetrexed. Other anti-angiogenesis agents have been studied for the treatment of MPM, such as nintedanib, and cidiranib, unfortunately, both biologics, resulted in insignificant improvement in OS and the progression-free survival (PFS). There is need to shift gear in the development of biologics for targeted treatment of MPM, such as immune checkpoints, in order to improve OS, PFS, and quality of life for the patients.
Keywords: Malignant pleural mesothelioma; Bevacizumab; Cisplatin; Pemetrexed; Vascular endothelial growth factor
Abbreviations: DVT-Deep Venous Thrombosis; TIA-Transient Ischaemic Attack
Introduction
Malignant pleural mesothelioma (MPM) is a highly aggressive and incurable cancer that originates from the mesothelial cells that line the serosal surface of the pleural cavity [1]. Malignant mesothelioma may arise from other serosal membranes, such as peritoneum (peritoneal mesothelioma, 10%), pericardium (pericardial mesothelioma, < 1%), and tunica vaginalis testis (tunica vaginalis mesothelioma, < 0.5%). However, MPM comprises about 80% of all malignant mesotheliomas [2]. MPM is associated with inhalation or exposure to asbestos fibres with a latency period of exposure of 20-40 years [3-7]. There are more than 400 types of asbestos fibres but only the five amphibole fibrous forms, such as actinolite, amosite, anthophyllite, tremolite, and crocidolite, are carcinogenic to humans, causing genetic mutations and DNA alterations, leading to malignant mesothelioma [2].
MPM is more common in men (84%) because of their occupational exposure, and men have the poorest prognosis [6]. The median age at diagnosis is 75 years, and the tumor has a poor overall survival of 38% at 1 year, and 7% at 3 years [6]. Despite a worldwide prohibition of asbestos production, and supply of asbestos products mortality due to MPM continues to rise [8,9], possibly related to the long latent period of developing the cancer.
There are three histopathological types of mesothelioma with different clinical presentation, response to treatment, oncogenic biomarker, and prognosis. The subtypes of MPM include “polygonal” epitheloid (50-70%), “spindle-shaped” sarcomatoid (10-20%), and biphasic (30%), which comprises both epitheloid and sarcomatoid histological features [2,10-13]. The sarcomatoid type has the poorest prognosis with a median overall survival (OS) of 3-4 months [14,15], whereas the epitheloid and biphasic have median overall survival of 19 months and 12 months, respectively [14].
Most patients with MPM are diagnosed late with advanced disease and are usually treated with systemic chemotherapy. The standard first-line chemotherapy consists of the doublet cisplatin plus pemetrexed. The regimen has a response rate ranging from 26.3% Santoro A, et al. [16] to 41% Vogelzang NJ, et al. [17] and extends the OS by 2-3 months. Addition of bevacizumab a vascular endothelial growth factor (VEGF) inhibitor to cisplatin plus pemetrexed has been shown to extend the median overall survival up to 19 months (16-22 months), versus 16 months (14- 18 months) with cisplatin plus pemetrexed [18]. Although the extension to the OS is not substantial, it is worthwhile in improving the health-related quality of life (HLQoL) of patients with this dreaded incurable disease.
Other anti-angiogenesis agents have been studied for the treatment of MPM, such as nintedanib, and cidiranib, unfortunately, both biologics, resulted in insignificant improvement in OS and PFS. There is need to shift gear in the development of biologics for targeted treatment of MPM, such as immune checkpoints, including, nivolumab, ipilimumab, and durvalumab in order to improve OS, and PFS in patients with malignant pleural mesothelioma [19,20]. Some of the immune checkpoint inhibitors, such as nivolumab, and ipilimumab have been approved by the Food and Drug Administration (FDA) as first line treatment for unresectable MPM [20].
Pathogenesis of Malignant Pleural Mesothelioma
The main cause of malignant pleural mesothelioma (MPM) is inhalation or exposure of asbestos fibres because a history of past exposure is documented in 80% of patients diagnosed with MPM [3-7,21,22]. The risks of developing MPM depend on the duration of exposure (20-40 years) [3-7], and in particularly the type of asbestos fiber [5]. There are two main types of asbestos fibres: amphibole (straight, needle-shaped, jagged fibres), and serpentine (short curly fibres, mainly consisting of chrysolite asbestos). Overall, there are more than 400 forms of asbestos fibres, but only the five amphibole type forms, such actinolite, amosite, anthophyllite, tremolite, and crocidolite, are carcinogenic to humans, causing mesothelioma [23-25]. Crocidolite (blue asbestos) is considered the most carcinogenic form of amphibole asbestos. Exposure to chrysolite (white asbestosis) which is the most common asbestos used industrially carries a less risk of mesothelioma [24,25]. However, the International Agency for Research on Cancer (IARC) has classified both asbestos fiber types equally as Class 1 carcinogens [26].
Erionite, an asbestos-like mineral found in the rocks of the Cappadocia region of Turkey (Tuzkoy, Karain, and Sarihidir), has been reported to cause familial forms of malignant mesothelioma with autosomal inheritance [27-29]. Other causes of Mesothelioma include simian virus 40 [30-32], and radiation [33,34].
The mechanisms of asbestos carcinogenesis are complex and multifactorial. Malignant pleural mesothelioma results from neoplastic transformation of mesothelial cells. It is associated with phenotypic modifications, and genetic mutations that alter cellcell, and cell-to extracellular matrix; and aberrant regulation of cell proliferation and apoptosis [35].
Inhaled asbestos fibres travel through the airways or the lymphatics to the lung parenchyma, and reach the visceral pleura, pleural space, and parietal pleural membrane, where they cause chronic irritation, tissue injury and repair [36,37]. Macrophage inflammatory responses lead to release of oxygen free radicals which cause intracellular DNA damage and abnormal repair [38]. The asbestos fibres also penetrate mesothelial cells, causing impaired mitosis, alteration of DNA structure, and generation of mutations in DNA [6]. Furthermore, asbestos fibers induce phosphorylation of several protein kinases, such as mitogenactivated protein, and extracellular signal-regulated kinases 1 and 2, which further increase expression of proto-oncogenes promoting cellular proliferation [38]. Once this vicious cycle of DNA damage, mutations, and generation of proto-oncogenes, mesothelioma cells proliferate, and the tumor grows profusely [37].
There are several genetic mutations in DNA, and alterations in some oncogenes, and tumor suppressor genes (TSG); and the activation of signaling pathways involved in cell proliferation and apoptosis linked to MPM [37,39]. The most frequent mutated genes associated with MPM include the cyclic-dependent kinase inhibitor 2A gene (CDKN2A), BRCA1 associated protein 1 gene (BAP1), and the neurofibromatosis type 2 (merlin) gene (NF2) [40-48]. P16/CDKN2A is a tumor suppressor gene associated with several tumors encoding a negative regulator of cell cycle progression and has several deletions in MPM [45-47]. Another TSG, neurofibromatosis type 2, has been found to be inactive in MPM [45-47]. The tumor suppressor NF2 which is detected in about 50% of MPM is associated with increased proliferation, and invasiveness of mesothelioma. Conversely, BAP1 loss which is present in 30-60% of MPM is associated with good prognosis [44,48,49]. Mutations in the NF2 and INK4A genes involved in apoptosis regulation, may be responsible for MPM resistance to most conventional chemotherapeutic agents, because cells are resistant to the induction of apoptosis [50].
Massive parallel sequencing (MPS) or next generation sequencing has revealed deletions and loss mutations of several other genes, such as TP53 tumor suppressor, ACTG2, CHEK2, CDKN2B, CFAP45, COL3A1, CUL1, DDX51, DPP10, DHFR, KDR, KMT2D, MAPK2K6, MLH1, NOD2, PALB2, PBRM1, PCBD2, POT1, domain containing 2 (SETDB1) and TXNRD1, unc-like autophagy activating kinase (ULK2), ryanodine receptor 2 (RR2), UQCRC1, and XRCC6 [51-59]. The lists of mutated genes which has been implicated in the pathogenesis of MPM is endless and are shown in references [60,61]. Because of these superfluous mutations, it is very difficult for targeted precision treatment for the tumor.
Angiogenesis in Malignant Tumors
Activated mesothelial cells and macrophages due to asbestos fiber injury, secrete a variety of inflammatory cytokines, and growth factors, such as tumor necrosis factor-α (TNFα), vascular endothelial factor-β (VEGFβ), platelet-derived growth factor (PDGF), interleukin-1β, and IL-8 [62] which promote neovascularization. Neovascularization which includes angiogenesis and lymph angiogenesis plays a very important role in tumor growth, local and metastatic spread of many cancers, including MPM [63]. Angiogenesis in tumors leads to abnormal vasculature, with tortuous vessels which can be either dilated or pruned, and deviate from the orderly morphology in normal tissues [64]. Increased vascular channels can facilitate local and metastatic spread of the cancer.
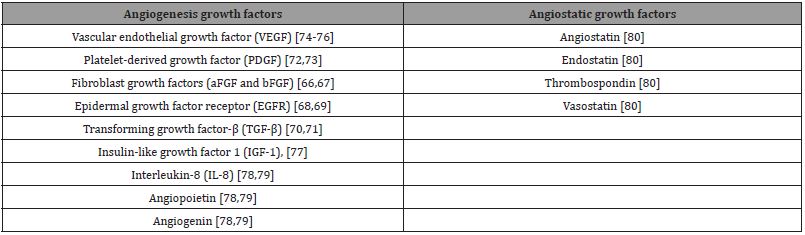
There are several growth factors which have been implicated in angiogenesis in solid tumors. Most of these growth factors are also mitogenic to mesothelioma cells, and promote tumor growth, survival; and merit targeting for the treatment of MPM. Mesothelioma cells exhibit increased expression of several growth factors and their receptors [64,65], such as fibroblast growth factors (aFGF and bFGF) [66,67], epidermal growth factor receptor (EGFR) [68,69], transforming growth factor-β (TGF-β) [70,71], platelet-derived growth factor (PDGF) [72,73], vascular endothelial growth factor (VEGF) [74-76], insulin-like growth factor 1 (IGF-1), [77], angiopoietin, angiogenin, and interleukin-8 (IL-8) [78,79]. These growth factors contribute directly or indirectly to endothelial proliferation, migration, vessel formation, and stabilization [80]. Conversely, angiogenesis is tightly regulated by anti-angiogenic factors, including thrombospondin, angiostatin, endostatin, and vasostatin [80]. Table 1 list the angiogenic and angiostatic growth factor for malignant tumors, including malignant pleural mesothelioma.
Table 1: Angiogenesis and Angiostatic Growth Factors in Malignant Mesothelioma.
Vascular Endothelial Growth Factor
Vascular endothelial growth factor is the most powerful and potent endothelial cell specific mitogen [81] and is produced by several malignant cells [82]. VEGF exists in more than 6 isoforms, including VEGF-A, B, C, D, E, and F. VEGF-A is the most studied isoform [20]. It exists in more than 20 splice isoforms, which vary according to their physiological function and to molecular weight, ranging from 121 to 206 kDa (121, 165, 189, and 206 amino acids) [74,80]. The VEGF165 isoform is quantitatively and qualitatively the most active tissue variant [44,80]. The VEGFs are potent mitogen and survival factors for endothelial cells [81- 83]. VEGF-A signaling is via two different receptors, designated Flt-1 (VEGFR-1), and KDR (VEGFR-2) [81]. Activation of VEGFR-2 leads to auto-phosphorylation and downstream signaling through several pathways, such as phosphatidylinositol 3’-OH kinase/Akt, and Src homology phosphate-2 [74,80,84].
In pleural mesothelioma, VEGF also directly acts as a powerful mitogen for mesothelial cells. Furthermore, mesothelial cell lines secrete VEGF-A, and VEGF-C, and express both VEGF, and VEGF-C receptors (VEGFR-1, VEGFR-2, and VEGFR-3), respectively [75,85,86]. Thus, VEGF signaling can induce mesothelial cell growth in an autocrine fashion [75,87]. VEGF-C and its receptor VEGF-R3 are primarily involved in lymph angiogenesis [74,88,89], and promote local spread of mesothelioma via the expanded lymphatic channels.
Increased expression of VEGF [79,88-90], and VEGF-C [76], and their receptors VEGFR-1, -2, and VEGFR-3 [88,89], respectively, have been demonstrated in mesothelioma tumor cells in primary cultures of samples, and biopsies from patients with MPM. The expression of VEGF and VEGF-C, and their receptors correlate with increased intratumoral micro vessel density, and inversely with survival in patients with MPM [89,91,92]. Demirag et al. [93] have shown a significant association between VEGF expression and the clinical stage, and prognosis of MPM. Thus VEGF, and VEGF-C and their receptors play an important role in mesothelioma growth, survival, and metastasis. Noteworthy, targeting VEGF and its receptors in the treatment of MPM may improve overall survival, from that achieved by the standard of care treatment with cisplatin plus pemetrexed.
Treatment
Most patients with MPM are diagnosed late with advanced, diffused disease unamenable to surgery, and are usually treated with systemic chemotherapy [94]. Treatment of MPM with chemotherapy is very difficult, and incurable; it only extends the median overall survival for few months [95]. The standard of care first-line regimen comprises of cisplatin 75 mg/m2 plus pemetrexed 500 mg/m2 ever 3 weeks [17,96]. This doublet of cisplatin plus pemetrexed has been shown to have a response rate of 41.3%, and a median overall survival of 12.1 months [17]. Gralla R, et al. [97], have reported that cisplatin plus pemetrexed improves the quality of life of the patients within the first three cycles of treatment.
Other antifolates, such as raltitrexed (3 mg/m2) have been used instead of pemetrexed for the treatment of MPM. The EORTC reported that cisplatin plus raltitrexed had an overall response rate of 24% compared with 14% for cisplatin alone [98]. The doublet improved the median OS by 11.4 months, however, there was no statistical difference in the health-related quality of life [98]. To date, there are no data to support a preference of pemetrexed over raltitrexed. However, an indirect comparison between cisplatin plus pemetrexed versus cisplatin and raltitrexed shows no significant differences in the overall response rate, overall survival, and safety profile between these two regimens [16,98].
Several clinical trials have substituted the platinum analog carboplatin for cisplatin [99-102]. Carboplatin plus pemetrexed resulted response rates of 6% to 22%, with a median time to progression of 6-5 to 7 months. The median overall survival ranged from 9.3 to 12.7 months [100-102]. The International Extended Access Program trial, consisting of 1,704 chemotherapy-naïve patients evaluated the therapeutic effects cisplatin plus pemetrexed versus carboplatin and pemetrexed [103]. This large, well conducted study showed no statistical difference in the endpoint between the two regimens [103]. The response rate for cisplatin plus pemetrexed was 26.3%, and the time to progression was 7 months. Whereas, for carboplatin and pemetrexed the response rate was 21-7%, and the time to progression was 6.9 months. In a parallel study, the International Extended Access Program compared the effects of pemetrexed alone in chemotherapy-naïve patients with pemetrexed pretreated patients [104]. Treatment with pemetrexed alone in chemo-naïve, and pretreated patients only achieved a response rate of 10.5% and 12.1%, respectively [104].
The above clinical trials suggest that combination of a platinum analog and an antifolate is superior and more effective in the treatment of MPM compared to single agent of a platinum compound or antifolate [105]. Cisplatin plus pemetrexed, and carboplatin and pemetrexed achieve almost similar efficacy, however, the combination chemotherapy with cisplastin plus pemetrexed is the most widely used regimen for patients with unresectable MPM [106]. This regimen was approved by the Food and Drug Administration (FDA) on the basis of the phase III EMPACIS trial by Vogelzang and colleagues [17].
The standard of care consisting of a platinum analogy and an antifolate achieves response rates of 24% to 41%; and improves the overall survival by 11.4 to 12.1 months [17]. There is an urgent need to investigate for novel targeted therapies for the treatment of MPM. Bevacizumab (Avastin) is an anti-VEGF recombinant humanized antibody derived from murine monoclonal antibody A4.6.1. Bevacizumab neutralizes all biologically active isoforms of VEGF, including its bioactive proteolytic fragments. It sterically prevents binding and activation of VEGFR-1 and VEGFR-2 on the surface of endothelial and mesothelial cells and inhibits VEGF-induced proliferation of endothelial cells [107]. Addition of bevacizumab to cisplatin plus pemetrexed regimen has been shown to significantly improve the PFS to 9.2 months compared with 7.3 months for patient treated with cisplatin and pemetrexed (P = 0.0001) [3]. Additionally, add-on bevacizumab has been demonstrated to significantly increase the median OS to 18.8 (15.9- 22.6) months compared with 16.1 (14.0-17.9) months for patients receiving cisplatin plus pemetrexed (P = 0.017). However, patients on bevacizumab experienced more Grade 3 and 4 side effects compared with patients treated with cisplatin plus pemetrexed [108].
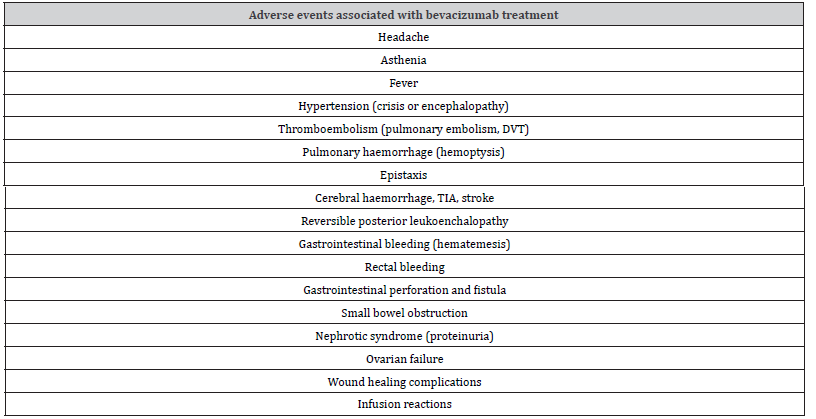
Bevacizumab is well tolerated, but it is associated with Grade 3 and 4 adverse events, such as hypertension [108,109], thromboembolism [110], pulmonary haemorrhage, haemoptysis, epistaxis, gastrointestinal haemorrage, haemetemesis [108,109,111,112], gastrointestinal perforation and fistula Kabbinavar FF, et al. [113], cerebral haemorrhage Latarte N, et al. [114], nephrotic syndrome (proteinuria), and ovarian failure [108,109]. Table 2 shows bevacizumab-associated toxicities.
Table 2: Adverse events associated with bevacizumab treatment.
Cediranib an oral pan-VEGFR1/2/3, PDGFRβ tyrosine kinase inhibitor, has been shown to have limited activity against MPM, and is associated with substantial toxicities, such as anorexia, diarrhoea, dehydration, and weight loss [115,116].
Nintedanib is an oral angiokinase inhibitor which blocks several growth factor receptor pathways, such as VEGFR1-3, PDGFRα/β, FGFR1-3, Flt3, RET, abl, and Src Ross GJ, et al. [117], it is safe and tolerable in combination with the standard of care [117]. Recently, Scagliotti et al. [118], have shown that adding nintedanib to cisplatin and pemetrexed does not improve progression-free survival in patients with advanced malignant pleural mesothelioma.
Several other multitargeted tyrosine kinase inhibitors, such as sunitinib malate Nowak AK, et al. [119], sorafenib Dubey S, et al. [120], semaxanib Kindler HL, et al. [121], and vatalanib Jahan TM, et al. [122] have been shown to have limited activity in advanced MPM in phase II clinical trials. However, none has met the primary endpoint, such as improvement in progression-free survival Jahan T, et al. [109], and are associated with severe adverse events, which outweighs their biotherapeutic benefits.
Currently, bevacizumab in combination with the standard of care has been shown to be the most effective antiangiogenic biologic in the treatment of advanced MPM [109,123]. The combination of bevacizumab and cisplatin plus pemetrexed is now the standard first-line treatment of advanced MPM in France, and other countries [109]. The National Comprehensive Cancer Network guidelines have also recommended this regimen as an option for standard front-line therapy [124]. The ERS/ESTS/EACTRO guideline suggest that bevacizumab, if available, be proposed in combination with cisplatin/pemetrexed as first-line treatment in patients fit for bevazucimab and cisplatin [125].
There is still unmet need for the development of novel anti-VEGF biologics, and other therapies, such as dual immune checkpoint inhibitors, including nivolumab (anti-PD1), and ipilimumab (anti- CTLA-4) [126-130].
Conclusion
Malignant pleural mesothelioma is a highly aggressive and incurable cancer that originates from the mesothelial cells of the pleural cavity. It is associated with inhalation of asbestos fibers and has a latency period of exposure of 20-40 years before presentation. MPM is usually diagnosed late with advanced, diffuse unresectable disease. The standard of care chemotherapy comprising of cisplatin plus pemetrexed has a response rate of about 26.3%-41%, and modestly improves median OS by 2-3 months. Vascular endothelial growth factor promotes angiogenesis in tumors, including MPM, and propagates cancer growth and metastasis. Bevacizumab is a humanized monoclonal murine antibody which blocks the immunopathological pathways of VEGF and its receptors on endothelial cells, and mesothelioma cells. Addition of bevacizumab a VEGF inhibitor to cisplatin plus pemetrexed regimen has been shown to extend the median OS for 16-22 months, versus 14-18 months with cisplatin plus pemetrexed. Other anti-angiogenesis biologics, such as nintedanib, and cidiranib, have resulted in insignificant improvement in median OS, and PFS, and have higher treatment-associated adverse effects. There is need to develop novel biologics for targeted treatment of MPM, such as immune checkpoints, in order to improve OS, PFS, and quality of life for the patients.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Asciak R, George V, Rahman NM (2012) Update on biology and management of mesothelioma. Eur Respir Rev 30(159): 200226.
- Rossini M, Rizzo P, Bonini I, Clementz A, Ferrari R, et al. (2018) New perspectives on diagnosis and therapy of malignant pleural mesothelioma. Front Oncol 8: 91.
- Lanphear BP, Buncher CR (1992) Latent period for malignant mesothelioma of occupational origin. J Occup Med 34(7): 718-721.
- Robinson BW, Musk AW, Lake RA (2005) Malignant mesothelioma. Lancet 366: 397-408.
- Royal College of Physicians (RCP) (2018) National Mesothelioma Audit report 2018 (audit period 2014-2016). London, RCP.
- National Cancer Institute (NCI) (2018) Thesaurus Version 18.11d.
- Selikoff IJ, Hammond EC, Seidman H (1980) Latency of asbestos disease among insulation workers in the United States and Canada. Cancer 46(12): 2736-2740.
- Tsao AS, Wistuba I, Roth JA, Kindler HL (2009) Malignant pleural mesothelioma. J Clin Oncol 27(12): 2081-2090.
- Røe OD, Stella GM (2015) Malignant pleural mesothelioma: history, controversy, and future of a manmade epidemic. Eur Respir Rev 24(135): 115-131.
- Attanoos RL, Gibbs AR (1997) Pathology of malignant mesothelioma. Histopathology 30(5): 403-418.
- Geltner C, Errhalt P, Baumgartner B, Ambrosch G, Machan B, et al. (2016) Management of malignant pleural mesothelioma – part 1: epidemiology, diagnosis, and staging: consensus of the Australian Mesothelioma Interest Group (AMIG). Wien Klin Wochenschr 128(17-18): 611-617.
- Hussain AN, Colby TV, Ordóñez NG, Timothy Craig Allen, Richard Luther Attanoos, et al. (2018) Guidelines for pathologic diagnosis of malignant mesothelioma 2017 update of the consensus statement from the international mesothelioma interest group. Arch Pathol Lab Med 142(1): 89-108.
- Van Zandwijk N, Clarke C, Henderson D, A William Musk, Kwun Fong, et al. (2013) Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis 5(6): E254-E307.
- Meyerhoff RR, Yang CFJ, Speicher PJ, Brian C Gulack, Matthew G Hartwig, et al. (2015) Impact of mesothelioma histological subtypes on outcomes in the Surveillance, Epidemiology, and End Results database. J Surg Res 196(1): 23-32.
- Beckett P, Edwards J, Fennell D, Hubbard R, Woolhouse I, et al. (2015) Demographics, management, and survival of patients with malignant pleural mesothelioma in the National lung cancer audit in England and Wales. Lung Cancer 88(3): 344-348.
- Santoro A, O Brien ME, Stahel RA, Kristiaan Nackaerts, Paul Baas, et al. (2008) Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaïve patients with malignant pleural mesothelioma: results of International Expanded Access Program. J Thorac Oncol 3(7): 756-763.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kauket E, et al. (2003) Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21(14): 2636-2644.
- Zalcman G, Mazieres J, Margery J, Greillier L, Audigier Valette C, et al. (2016) Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Studt (MAPS): a randomized, controlled, open-label, phase 3 trial. Lancet 387(10026): 1405-1414.
- Forde PM, Sun Z, Anagnostou V, Kindler HL, Purcell WT, et al. (2020) PrE0505: phase II multicenter study of anti-PD-L1, durvalumab, in combination with cisplatin and pemetrexed for first-line treatment of unresectable malignant pleural mesothelioma (MPM) – a PrECOG LLC study. J Clin Oncol 38(15): 9003-9003.
- Wright K (2020) FDA approves nivolumab plus ipilimumab for previously untreated unresecteable malignant pleural mesothelioma. Oncology (Williston Park) 34(11): 502-503.
- Wagner JC, Sleggs CA, Marchand P (1960) Diffuse pleural mesothelioma and asbestos exposure in the Northwestern Cape Province. Br J Ind Med 17(4): 260-271.
- Carbone M, Kratzke RA, Testa JR (2005) The pathogenesis of mesothelioma. Semin Oncol 29(1): 2-17.
- Robinson BW, Lake RA (2005) Advances in malignant mesothelioma. N Engl J Med 353(15): 1591-1603.
- Nicholson WJ (1991) Comparative dose-response relationships of asbestos fiber types: magnitudes and uncertainties. Ann NY Acad Sci 643: 74-84.
- Gilham C, Rake C, Burdett G, Andrew G Nicholson, Leslie Davison, et al. (2016) Pleural mesothelioma, and lung cancer risks in relation to occupational history and asbestos lung burden. Occup Environ Med 73(5): 290-299.
- IARC Working Group on the evaluation of carcinogenic risks of humans (2012) Arsenic, metals, fibers, and dusts. IARC Monogr Eval Carcinog Risks Hum 100(Pt C): 11-465.
- Roushdy Hammady I, Siegel J, Emri S, Testa JR, Carbone M (2001) Genetic-susceptibility factor, and malignant mesothelioma in the Cappadocian region of Turkey. Lancet 357(9254): 444-445.
- Dogan AU, Baris YI, Dogan M, Salih Emri, Ian Steele, et al. (2006) Genetic predisposition to fiber carcinogenesis causes a mesothelioma epidemic in Turkey. Cancer Res 66(10): 5063-5068.
- Carbone M, Emri S, Dogan AU, Ian Steele, Murat Tuncer, et al. (2007) A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer 7(2): 147-154.
- Galateau Salle F, Bidet P, Iwatsubo Y, Gennetay E, Renier A, et al. (1990) SV40-like DNA sequences in pleural mesothelioma, bronchopulmonary carcinoma, and non-malignant diseases. J Pathol 184(3): 252-257.
- Carbone M, Pass HI, Rizzo P, Marinetti M, Di Muzio M, et al. (1994) Simian virus 40-like DNA sequences in human pleural mesothelioma. Oncogene 9(6): 1781-1790.
- Testa JR, Carbone M, Hirvonen A, Khalili K, Krynska B, et al. (1998) A multi-institutional study confirms the presence and expression of simian virus 40 in human malignant mesotheliomas. Cancer Res 58(20): 4505-4509.
- Cavazza A, Travis LB, Travis WD, Wolfe JT 3rd, Foo ML, et al. (1996) post-irradiation malignant mesothelioma. Cancer 77(7): 1379-1385.
- Teta MJ, Lau E, Sceurman BK, Meghan E Wagner (2007) Therapeutic radiation for lymphoma: risk of malignant mesothelioma. Cancer 109(7): 1432-1438.
- Jaurand MC, Fleury Feith J (2005) Pathogenesis of malignant pleural mesothelioma. Respirology 10(1): 2-8.
- Miseroochi G, Sancini G, Mantegazza F, Chiappino G (2008) Translocation pathways for inhaled asbestos fibers. Environ Health 7: 4.
- Bibby C, Tsim S, Kanellakis N, Ball H, Talbot DC, et al. (2016) Malignant pleural mesothelioma: an update on investigation, diagnosis, and treatment. Eur Respir Rev 25(142): 472-486.
- Yates D, Corrin B, Stidolph P, Browne K (1997) Malignant mesothelioma in southeast England: clinicopathological experience of 272 cases. Thorax; 52(6): 507-512.
- Quetel L, Meiller C, Assie JB, Blum Y, Sandrine Imbeaud, et al. (2020) Genetic alterations of malignant pleural mesothelioma: association with tumor heterogeneity and overall survival. Mol Oncol 14(6): 1207-1223.
- Bueno R, Stawiski EW, Goldstein LD, Steffen Durinck, Assunta De Rienzo, et al. (2016) Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and slicing alterations. Nat Genet 48(4): 407-416.
- Guo G, Chmielecki J, Goparaju C, Adriana Heguy, Igor Dolgalev, et al. (2015) Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKNA, and CUL in malignant pleural mesothelioma. Cancer Res 75(2): 264-269.
- Bott M, Brevet M, Taylor BS, Shigeki Shimizu, Tatsuo Ito, et al. (2011) The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet 43(7): 668-672.
- Prins JB, Williamson KA, Kamp MM, Van Hezik EJ, Van der Kwast TH, et al. (1998) The gene for the cyclin-dependent-kinase-4 inhibitor, CDKN2A, is preferentially deleted in malignant mesothelioma. Int J Cancer 75(4): 649-653.
- Sekido Y, Pass HI, Bader S, Mew DJ, Christman MF, et al. (1995) Neurofibromatosis type 2 gene is somatically mutated in mesothelioma but not in lung cancer. Cancer Res 55(6): 1227-1231.
- Murphy SS, Testa JR (1999) Asbestos, chromosomal deletions, and tumor suppressor gene alterations in human malignant mesothelioma. J Cell Physiol 180(2): 150-157.
- De Rienzo A, Testa JR (2000) Recent advances in the molecular analysis of human malignant mesothelioma. Clin Ther 151(6): 433-438.
- Metcaff RA, Welsh JA, Bennett WP (1992) p53 and Kirsten-ras mutations in human mesothelioma cell lines. Cancer Res 52(9): 2610-2615.
- Carbone M, Gaudino G, Yang H (2015) Recent insights emerging from malignant mesothelioma genome sequencing. J Thorac Oncol 10(3): 409-411.
- Singhi AD, Krasinskas AM, Choudry HA, David L Bartlett, James F Pingpank, et al. (2016) The prognostic significance of BAP1, NF2, CDKN2A in malignant peritoneal mesothelioma. Modern Pathol 29(1): 14-24.
- Asciak R, George V, Rahman NM (2012) Update on biology and management of mesothelioma. Eur Respir Rev 30(159): 200226.
- Sugarbaker DJ, Richards WG, Gordon GJ, Lingsheng Dong, Assunta De Rienzo, et al. (2008) Transcriptome sequencing of malignant pleural mesothelioma tumors. Proc Natl Acad Sci 105(9): 3521-3526.
- Dong L, Jensen RV, De Rienzo A, Gavin J Gordon, Yanlong Xu, et al. (2009) Differentially expressed alternatively spliced genes in malignant pleural mesothelioma identified using massively parallel transcriptome sequencing. MMC Med Genet 10: 149.
- The AACR Project GENIE Consortium (2017) AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov 7(8): 818-831.
- Panou V, Gadiraju M, Wollin A, Caroline M Weipert, Emily Skarda, et al. (2018) Frequency of germline mutations in cancer susceptibility genes in malignant mesothelioma. J Clin Oncol 36(28): 2863-2871.
- Hida T, Matsumoto S, Hamasaki M, Kunimitsu Kawahara, Tohru Tsujimura, et al. (2015) Deletion status of p19 in effusion smear preparation correlates with that of underlying malignant pleural mesothelioma tissue. Cancer Sci 106(11): 1635-1641.
- HmeljaK J, Sanchez Vega F, Hoadley KA, Juliann Shih, Chip Stewart, et al. (2018) Integrative molecular characterization of malignant pleural mesothelioma. Cancer Discov 8(12): 1548-1565.
- Lo Iacono M, Monica V, Righi L, Federica Grosso, Roberta Libener, et al. (2015) Target next-generation sequencing of cancer genes in advanced stage malignant pleural mesothelioma: a retrospective study. J Thorac Oncol 10(3): 492-499.
- Nicolini F, Bocchini M, Bronte G, Delmonte A, Guidoboni, et al. (2020) Malignant pleural mesothelioma: state-of-the art on current therapies and promises for the future. Front Oncol 9: 1519.
- Abbot DM, Bortolotto C, Benvenuti S, Lancia A, Filippo AR, et al. (2020) Malignant pleural mesothelioma: genetic and microenvironmental heterogeneity as an unexpected reading frame and therapeutic challenge. Cancers (Basel)12(5): 1186.
- Hylebos M, van Camp G, van Meerbeeck JP, de Beck KO (2016) The genetic landscape of malignant pleural mesothelioma: results from massively parallel sequencing. J Thorac Oncol 11(10): 1615-1626.
- Panou V, Røe OD (2020) Inherited genetic mutations and polymorphisms in malignant mesothelioma: A Comprehensive review. Int J Mol Sci 21(12): 4327.
- Toyokuni S (2009) Mechanisms of asbestos-induced carcinogenesis. Nagoya J Med Sci 71(1-2): 1-10.
- Remon J, Lianes P, Martinez S, Velasco M, Querol R, et al. (2013) Malignant mesothelioma: new insights into a rare disease. Cancer Treat Rev 39(6): 584-591.
- Jain RK (2013) Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol 31(17): 2205-2218.
- Deroanne CF, Hajitou A, Calberg Bacq CM, Nusgens BV, Lapiere CM (1997) Angiogenesis by fibroblast growth factor 4 is mediated through an autocrine up-regulation of vascular endothelial growth factor expression. Cancer Res 57(24): 5590-5597.
- Gerwins P, Skoldenberg E, Claesson Welsh L (2000) Function of fibroblast growth factors and vascular endothelial growth factors and their receptors in angiogenesis. Crit Rev Oncol Hematol 34(3): 185-194.
- Schelch K, Hoda MA, Klikovits T, Munzker J, Ghanin B, et al. (2014) Fibroblast growth factor receptor inhibition is active against mesothelioma and synergizes with radio- and chemotherapy. Am J Respir Crit Care Med 190(7): 763-772.
- Betta PG, Libener R, Orecchia, Bottero G, Pagnuzzi M, et al. (2004) Epidermal growth factor in serum from patients with malignant pleural mesothelioma. T Clin Oncol 22(14 Suppl).
- Chia P, Scott AM, John T (2019) Epidermal growth factor receptor (EGFR) - targeted therapies in mesothelioma. Expert Opin Drug Deliv 16(4): 441-451.
- Suzuki E, Kim S, Cheung HK, Corbley MJ, Zhang X, et al. (2007) A novel small-molecule inhibitor of transforming growth factor β type 1 receptor kinase (SM16) inhibits murine mesothelioma tumor growth in vivo and prevents tumor recurrence after surgical resection. Cancer Res 67(5): 2351-2359.
- Stockhammer P, Ploenes T, Theegarten D, Martin Schuler, Sandra Maier, et al. (2020) Detection of TGF-β in pleural effusions for diagnosis and prognostic stratification of malignant pleural mesothelioma. Lung Cancer 139: 124-132.
- Langerak AW, De Laat PAJM, Van Der Linden Van Beurden CAJ, Delahaye M, Van Der Kwast TH, et al. (1996) Expression of platelet-derived growth factor (PDGF) and PDGF receptors in human malignant mesothelioma in vitro and in vivo. J Pathol 178(2): 151-160.
- Kothmaier H, Quehenberger F, Halbwed I Morbini P, Demirag F, Zeren H, et al. (2008) EGFR and PDGFR differentially promote growth in malignant epithelioid mesothelioma of short and long survivors. Thorax 63(4): 345-351.
- Ohta Y, Shridhar V, Bright RK, Kalemkerian GP, Du W, et al. (1999) VEGF and VEGF type C play an important role in angiogenesis and lymphangiogenesis in human malignant mesothelioma tumors. Br J Cancer 81(1): 54-61.
- Masood R, Kundra A, Zhu ST, Xia G, Scalia P, et al. (2003) Malignant mesothelioma growth inhibition by agents that target the VEGF and VEGF-C autocrine loop. Int J Cancer 104(5): 603-610.
- Shibuya M (2011) Vascular endothelial factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: a crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2(12): 1097-1105.
- Lee TC, Zhang Y, Aston C, Hintz R, Jagirdar J, et al. (1993) Normal human mesothelial cells and mesothelioma cell lines express insulin-like growth factor 1 and associated molecules. Cancer Res 53(12): 2858-2864.
- Antony VB, Hott JW, Godbey SW, Holm K (1996) Angiogenesis in mesothelioma. Role of mesothelial cell derived IL-8. Chest 103(3 Suppl): 21S-22S.
- Galffy G, Mohammed KA, Dowling PA, Nasreen N, Ward MJ, et al. (1999) Interleukin 8: an autocrine growth factor for malignant mesothelioma. Cancer Res 59(2): 367-371.
- Nowak AK, Brosseau S, Cook A, Zalcman G (2020) Antiangiogeneic strategies in mesothelioma. Front Oncol 10: 126.
- Konig JE, Tolnay E, Wiethege T, Muller KM (1999) Expression of vascular endothelial growth factor in diffuse malignant pleural mesothelioma. Vichows Arch 435(1): 8-12.
- Aoe K, Hiraki A, Tanaka T, Gemba KI, Murakami T, et al. (2006) Expression of vascular endothelial growth factor in malignant mesothelioma. Anticancer Res 26(6C): 4833-4836.
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z (1999) Vascular endothelial factor (VEGF) and its receptors. FASEB J 13(1): 9-22.
- Kowanetz M, Ferrara N (2006) Vascular endothelial growth factor signaling pathways: therapeutic perspectives. Clin Cancer Res 12(17): 5018-5022.
- Hoenen A, Landuyt B, Highley MS, Wilders H, van Oosterom AT, et al. (2004) Vascular endothelial growth factor and angiogenesis. Pharmacol Rev 56(4): 549-580.
- Konig J, Tolnay E, Wiethege T, Muller K (2000) Co-expression of vascular endothelial growth factor and its receptor flt-1 in malignant pleural mesothelioma. Respiration 67(1): 36-40.
- Strizzi L, Catalano A, Vianale G, Orecchia S, Casalini A, et al. (2001) Vascular endothelial growth factor is an autocrine growth factor in human malignant mesothelioma. J Pathol 193(4): 468-475.
- Pepper MS, Mandriota SJ, Jeltsch M, Kumar V, Alitalo K (1998) Vascular endothelial growth factor (VEGF)-C synergizes with basis fibroblast growth factor and VEGF in induction of angiogenesis in vitro and alters endothelial cell extracellular proteolytic activity. J Cell Physiol 177 (3): 439-452.
- Kumar Singh S, Weyler J, Martin MJ, Vermeulen PB, Van March E (1999) Angiogenic cytokines in mesothelioma: a study of VEGF, FGF-1 and -2, and TGF beta expression. I Pathol 189: 72-78.
- Kraft A, Weindel K, Ochs A, Marth C, Zmija J, et al. (1999) Vascular endothelial growth factor in sera and effusions of patients with malignant and nonmalignant disease. Cancer 85(1): 178-187.
- Edwards JG, Cox G, Andi A, Walker RA, Waller DA, et al. (2001) Angiogenesis is an independent prognostic factor in malignant mesothelioma. Br J Cancer 85(6): 863-868.
- Soini Y, Puhakka A, Kahlos K, Saily M, Paakko P, et al. (2001) Endothelial nitric synthase is strongly expressed in malignant mesothelioma but does not associate with vascular density or expression of VEGF, FLK1 or FLT1. Histopathology 39(2): 176-186.
- Demirag F, Unsal E, Yilmaz A, Caglar A (2005) Prognostic significance of vascular endothelial growth factor, tumor necrosis, and mitotic tumor index in malignant mesothelioma. Chest 128(5): 3382-3387.
- Saddoughi SA, Abdelsattar ZM, Blackmon SH (2018) National trends in the epidemiology of malignant pleural mesothelioma: a national cancer data base study. Ann Thorac Surg 105(2): 432-437.
- Tsao AS, Wistuba I, Roth JA, Kindler HL (2009) Malignant pleural mesothelioma. J Clin Oncol 27(12): 2081-2090.
- Kindler HL, Ismaila N, Armato SG 3rd, Bueno R, Hesdorffer M, et al. (2018) Treatment of malignant pleural mesothelioma: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 36(13): 1343-1373.
- Gralla R, Hollen PJ, Liepa AM, Symanowski JT, Boyer MJ, et al. (2003) Improving quality of life in patients with malignant pleural mesothelioma: Results of the randomized pemetrexed and cisplatin vs cisplatin trial using the LCSS-meso instrument. Proc Am Soc Clin Oncol 22: 621a.
- Van Meerbeeck JP, Gaafar R, Manegold C, Van Klaveren RJ, Van Marck EA, et al. (2005) Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: An intergroup study of the European Organization for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 23(28): 6881-6889.
- Ceresoli GL, Zucali PA, Favaretto AG, Francesco G, Paolo B, et al. (2006) Phase II study of pemetrexed plus carboplatin in malignant pleural mesothelioma. J Clin Oncol 24(9): 1443-1448.
- Castagneto B, Botta M, Aitini E, Spigno F, Degiovanni D, et al. (2008) Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM). Ann Oncol 19(2): 370-373.
- Bruno C, Luca C, Manlio M, et al. (2006) Pemetrexed (MTA) and carboplatin (CBDA) in advanced pleural mesothelioma (MPM): Evaluation of the activity and toxicity in a series of 178 chemonaive patients. Lung Cancer 54(suppl): S48.
- Papi M, Genestreti G, Tassinari D, et al. (2006) Pemetrexed in combination with carboplatin for the treatment of patients with malignant mesothelioma. Lung Cancer 54(suppl): S49.
- Santoro A, O Brien ME, Stahel RA, Kristiaan Nackaerts, Paul Baas, et al. (2008) Pemetrexed plus cisplatin or pemetrexed plus carboplatin for chemonaive patients with malignant pleural mesothelioma: Results of the International Expanded Access Program. J Thorac Oncol 3(7): 756-763.
- Taylor P, Castagneto B, Dark G, Maurizio Marangolo, Giorgio V Scagliotti, et al. (2008) Single-agent pemetrexed for chemonaive and pretreated patients with malignant pleural mesothelioma: Results of an International Extended Access Program. J Thorac Oncol 3(7): 764-771.
- Muers MF, Stephen RJ, Fisher P, Liz Darlison, Christopher MB Higgs, et al. (2008) Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicenter randomized trial. Lancet 371(9625): 1685-1694.
- Cinausero M, Rihawi K, Sperandi F, Melotti B, Ardizzoni A (2018) Chemotherapy treatment in malignant pleural mesothelioma. A difficult history. J Thorac Dis 10(Suppl 2): S304-S310.
- Levin PA, Dowell JE (2017) Spotlight on bevacizumab and its potential in the treatment of malignant pleural mesothelioma: the evidence to date. Onco Targets Ther 10: 2057-2066.
- Cassidy J, Saltz LB, Giantonio BJ, Kabbinavar FF, Hurwitz HI, et al. (2010) Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol 136(5): 737-743.
- Jahan T, Gu L, Kratzke R, Arkadiusz Dudek, Gregory A Otterson, et al. (2012) Vatalanib in malignant mesothelioma: a phase II trial by the Cancer and Leukemia Group B (CALGB 30107). Lung Cancer 76(3): 393-396.
- Scappaticci FA, Skillings JR, Holden SR, Gerber HP, Miller K, et al. (2007) Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst 99(16): 1232-1239.
- Hang XF, Xu WS, Wang JX, Wang L, Xin H, et al. (2011) Risk of high-grade bleeding in patients with cancer treated with bevacizumab: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 67(6): 613-623.
- Zhu X, Tian X, Yu C, Hong J, Fang J, et al. (2016) Increased risk of haemorrhage in metastatic colerectal cancer patients treated with bevacizumab: an update meta-analysis of 12 randomized controlled trials. Medicine 95(34): e4232.
- Kabbinavar FF, Flynn PJ, Kozloff M, Ashby MA, Sing A, et al. (2012) Gastrointestinal perforation associated with bevacizumab use in metastatic colorectal cancer: results from a large treatment observational cohort study. Eur J Cancer 48(8): 1126-1132.
- Latarte N, Bressler LR, Villano JL (2013) Bevacizumab and central nervous system (CNS) haemorrhage. Cancer Chemother Pharmacol 71(6): 1561-1565.
- Campbell NP, Kunnavakkam R, Leighl N, Vincent MD, Gandara DR, et al. (2012) Cediranib in patients with malignant mesothelioma: a phase II trial of the University of Chicago Phase II Consortium. Lung Cancer 78(1): 76-80.
- Tsao AS, Miao J, Wistuba II, Vogelzang NJ, Heymatch JV, et al. (2019) Phase II trial of cediranib in combination with cisplatin and pemetrexed in chemotherapy-naive patients with unresectable malignant pleural mesothelioma (SWOG S0905). J Clin Oncol 37(28): 2537-2547.
- Ross GJ, Binder R, Colbatzky F, Dallinger C, Schlenker Herceg R, et al. (2015) Nintedanib: from discovery to the clinic. J Med Chem 58(3): 1053-1063.
- Scagliotti GV, Gaafar R, Nowak A, Nakano T, van Meebeek J, et al. (2019) Nintedanib in combination with pemetrexed and cisplatin for chemotherapy-naive patients with advanced malignant pleural mesothelioma (LUME-Meso): a double-blind, randomized, placebo-controlled phase 3 trial. Lancet 7(7): 569-580.
- Nowak AK, Millward MJ, Creaney J, Francis RJ, Dick IM, et al. (2012) A phase II study of intermittent sunitinib malate as second-line therapy in progressive malignant pleural mesothelioma. J Thorac Oncol 7(9): 1449-1456.
- Dubey S, Janne PA, Krug L, Pang H, Wang X, et al. (2010) A phase II study of sorafenib in malignant mesothelioma: results of Cancer and Leukaemia Group B 30307. J Thorac Oncol 5(10): 1655-1661.
- Kindler HL, Vogelzang NJ, Chien K, Stadler WM, Karczmar G, et al. (2001) SU5416 in malignant mesothelioma: a University of Chicago phase II consortium study. Proc Am Soc Clin Oncol 20: 341.
- Jahan TM, Gu L, Wang X, Kratzke RA, Dudek AZ, et al. (2006) Vatalanib (V) for patients with previously untreated advanced malignant mesothelioma (MM): a phase II study by the Cancer and Leukemia Group B (CALGB 30107). J Clin Oncol 24(18 Suppl): Abstr 7081.
- Brosseau S, Assoun S, Naltel C, Steinmetz C, Gounant V, et al. (2017) Bevacizumab in the treatment of malignant pleural mesothelioma. Future Oncol 13(28): 2537-2546.
- Ettinger DS, Wood DE, Akerley W, Bazhenova LA, Borghaei H, et al. (2016) NCCN guidelines insights: malignant pleural mesothelioma, version 3.2016. Natl Compr Cancer Netw 14(7): 825-836.
- Scherpereel A, Opitz I, Berghamans T, Psallidas, Glatzer M, et al. (2020) ERS/ESTS/EACTRO guidelines for the management of malignant pleural mesothelioma. Eur Respir J 55(6): 1900953.
- Disselhorst MJ, Quispel Janssen J, Lalezari F, Monkhorst K, de Vries JF, et al. (2019) Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (UNITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Oncol 7(3): 260-270.
- Scherpereel A, Mazieres J, Greillier L, Lantuejoul S Do P, Bylicki O, et al. (2019) Nivolumab or nivolumab plus ipilimumab in patients with relapsed pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomized, non-comparative phase 2 trial. Lancet Oncol 20(2): 239-253.
- Bass P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, et al. (2021) First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (Checkmate 743): a multicentre, randomized, open-label, phase 3 trial. Lancet 397(10272): 375-386.
- Ceresoli GL, Pasello G (2021) Immune checkpoint inhibitors in mesothelioma: a turning point. Lancet 397(10272): 348-349.
- Gray SG (2021) Emerging avenues in immunotherapy for the management of malignant pleural mesothelioma. BMC Pulm Med 21: 148.
-
Nightingale Syabbalo. The Role of Angiogenesis Inhibitors in The Treatment of Malignant Pleural Mesothelioma. Archives in Respiratory & Pulmonary Medicine. 1(1): 2021. ARPM.MS.ID.000502.
-
Malignant pleural mesothelioma, Bevacizumab, Cisplatin, Pemetrexed, Vascular endothelial growth factor, Angiogenesis inhibitors, Cancer, Mesothelial cells
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.