Mini Review
Mini Review
Respiratory Physiology of Pregnancy and Functional Diagnosis in Asthma
Jusufovic Edin1,2*, Kopitovic Ivan3,4, and Flezar Matjaz5,6
1Center for Specific and Non-Specific Lung Diseases of Public Health and Educational Center “Dr. Mustafa Sehovic”, Bosnia, and Hercegovina
2Medical Faculty of University of Tuzla, Bosnia, and Herzegovina
3Institute of Pulmonary Diseases of Vojvodina, Serbia
4Medical Faculty of University of Novi Sada, Serbia
5University Clinic for Lung Diseases and Allergy in Golnik, Slovenia
6Faculty of Medicine of University of Ljubljana, Slovenia
Jusufovic Edin, Center for Specific and Non-Specific Lung Diseases of Public Health and Educational Center “Dr. Mustafa Sehovic”, Medical Faculty of University of Tuzla, Bosnia, and Herzegovina.
Received Date: February 09, 2023; Published Date: April 28, 2023
Abstract
The transport of oxygen through the placenta is a complex process and the greatest danger to the fetus of a pregnant woman with asthma is insufficient therapy and control of asthma. Most diagnostic procedures for evaluating the function of the respiratory system during pregnancy are not harmful to the fetus. Except for the forced expiratory volume in the first second, the other pulmonary function tests are reduced and decrease with the progress of pregnancy. Hyperventilation and dyspnea are normal phenomena, and pregnancy is not a contraindication for stopping exercise. However, in case of severe dyspnea, additional diagnostic tests should be performed. Bronchoprovocation tests, ventilation-perfusion scintigraphic scans and cardio-pulmonary stress tests should be avoided, and forced expiratory tests should also be avoided in the later stages of pregnancy. Daily measurement of peak expiratory flow is simple, safe, inexpensive, and very informative in determining the control and therapy of pregnant women’s asthma.
Keywords: Asthma; Pregnancy; Respiratory physiology; Functional diagnostics
Abbreviations: FRC-Functional residual capacity; ERV-Expiratory reserve volume; TLC-Total lung capacity; FVC-Forced vital capacity; FEV1%- Forced expiratory volume in 1 second; PEF-Peak expiratory flow; V′E-Minute ventilation; VT-Tidal volume; Pga-gastric pressure; Poes-oesophageal pressure; TLCO-transfer capacity of the lung for the uptake of carbon monoxide; DLCO-diffusing capacity of the lungs for carbon monoxide; PaO2- partial pressure of oxygen in arterial blood FEF25-75%-forced expiratory flow over the middle one-half of the forced vital capacity; PaCO2-partial pressure of carbon dioxide in arterial blood; DO2-oxygen delivery; sO2-oxygen saturation; Hgb-hemoglobin; SV-specific ventilation; VO2-oxygen consumption; C(a-v)O2-arterial-venous oxygen content difference; CO-carbon monoxide; SVO2-mixed venous oxygen saturation; 2,3 DPG-2,3 diphosphoglyceric acid; FeNO-fractioned exhaled nitric oxide; ppb-parts per billion
Introduction
Changes in respiratory function during pregnancy in physiological frameworks represent the biological adaptation mechanism of the pregnant woman’s organism. It should be emphasized that most diagnostic procedures for evaluating the function of the respiratory system during pregnancy are not harmful to the fetus. An exception is the use of bronchoprovocation testing with methacholine, which can pass into the fetus and therefore is not used during pregnancy, such as during breastfeeding. If we still want to perform this test on a nursing mother, she should refrain from breastfeeding for at least 48 hours and discard the milk by expressing the breasts.
Also, most of the drugs used to treat lung diseases can be used during pregnancy without any particular risk. Therefore, except in a few exceptional situations, the diagnosis and treatment of lung diseases in pregnant women do not differ significantly from the treatment of non-pregnant women.
Respiratory Physiology of Pregnancy
During a healthy pregnancy, lung function, ventilation pattern and gas exchange are influenced by both biochemical and mechanical mechanisms [1].
Chemical/Hormonal Changes
During pregnancy, the physiological change in hormonal patterns is the main cause of ventilatory changes in respiratory function. The main role is played by the level of progesterone, estrogen and proglandin in the blood. Progesterone increases gradually during pregnancy, from 25 ng/mL at 6 to 150 ng/mL at 37 weeks of pregnancy. Progesterone acts as a trigger of the primary respiratory center by increasing the sensitivity of the respiratory center to carbon dioxide, which shows a steeper slope of the ventilation curve in response to alveolar changes in carbon dioxide. Progesterone changes the tone of the smooth muscles of the airways resulting in a bronchodilator effect. It also mediates hyperemia and edema of mucous surfaces, causing nasal congestion. On the other hand, circulating estrogen levels increase during pregnancy, before or in parallel with progesterone levels. Estrogen is a mediator of progesterone receptors. It increases the number and sensitivity of progesterone receptors within the hypothalamus and medulla, the central neuronal areas associated with respiration. Furthermore, prostaglandins stimulate the smooth muscles of the uterus during labor and are present during all three trimesters of pregnancy. Thus, prostaglandin F2α increases airway resistance by tightening bronchial smooth muscles, while the bronchodilator effect may be a consequence of prostaglandins E1 and E2 [1].
Mechanical Changes
Progressive uterine distention is the main cause of lung and chest volume changes in the last trimester of pregnancy, which consist of diaphragm elevation and altered thoracic configuration. Namely, the enlarged uterus increases the abdominal (stomach) pressure at the end of exhalation, which causes the diaphragm to move upwards, with two consequences. First, negative esophageal pleural pressure and alveolar leakage in the lungs increase, leading to a decrease in functional residual capacity (FRC) and expiratory reserve volume (ERV). Because of this, early closure of the small airways occurs. Second, chest height decreases, but other chest dimensions increase to maintain a constant total lung capacity (TLC) [1].
However, pregnancy due to chest stiffness reduces TLC, and this reduction is greater the more advanced the pregnancy is [2]. Data from several antenatal clinics in Australia in the period 2004- 2017, which were prospectively collected from 4 groups of pregnant women, between the 12th and 22nd weeks of pregnancy and followed until delivery, suggest that the progression of pregnancy negatively affects FVC% and FEV1% [3].
During pregnancy, spirometry remains within normal limits, with FVC, FEV1 and PEF not changing or increasing modestly with an unchanged FEV1/FVC index. In contrast, lung volume undergoes large changes. Namely, the ERV gradually decreases during the second half of pregnancy (a decrease of 8-40% at term), because the residual volume decreases (by 7-22%). FRC then decreases (by 9.5-25%), while inspiratory capacity increases at the same rate to maintain a stable TLC [1].
At the same time, respiratory resistance increases while respiratory conduction decreases during pregnancy. Total lung and airway resistance tend to decrease in late pregnancy as a consequence of hormonally induced relaxation of the smooth muscles of the tracheobronchial tree. Pulmonary static and dynamic compliance, and static pulmonary recoil pressure do not change during pregnancy. Respiratory function does not differ between singleton and twin pregnancies [1,3].
Furthermore, chest geometry and displacement play an important role. Thus, the decrease in FRC caused by pregnancy is accompanied not only by an increase in the abdomen, but also by an increase in the dimensions of the ribs. Namely, the chest expands, because its transverse diameter and lower thoracic perimeter increase in the third trimester compared to postpartum. The average subcostal rib angle at the xiphoid level increases from 68.5° at the beginning of pregnancy to 103.5° at delivery. Such changes in the dimensions of the chest can be a consequence of the accumulation of fatty tissue and fluid, especially blood, because the volume of pulmonary blood is often increased during pregnancy. However, it is more likely to compensate for the shortening of the thorax due to the upward movement of the diaphragm to provide space for the lungs and preserve TLC. Likewise, the compliance of the chest wall decreases in late pregnancy due to increased abdominal contents [1].
Furthermore, minute ventilation (V′E) begins to increase significantly (up to 48%) during the first trimester of pregnancy, due to higher tidal volume (VT) with unchanged breathing rate. This ventilatory pattern is then maintained throughout pregnancy, and the VT/inspiratory time ratio and mouth occlusion pressure at 100 ms increase throughout pregnancy, indicating increases in ventilation and inspiration. Increased VT in pregnancy is achieved mainly by improved chest displacement without consistent changes in abdominal contribution as measured by magnetometers [1,2].
In addition to the above, the role of the respiratory muscles is very important. Peak inspiratory and expiratory pressures do not change during pregnancy or after delivery, indicating that although there is a change in chest wall geometry, respiratory muscle strength is preserved. With the progression of pregnancy, the resting position of the diaphragm moves 5 cm upwards with an increase in the size of the uterus, which is also shown by chest X-ray measurement. This causes the following changes in the diaphragm: its ability to generate tension increases secondary to the lengthening of the muscle fiber; its surface of apposition to the lower chest increases, the radius of curvature of which increases, due to the progressive increase of the lower ribs, in order to give space to the lungs. In addition, upward movement of the diaphragm causes the FRC to decrease. Inspiratory movements of the diaphragm are similar or even become wider than postpartum, and transdiaphragmatic pressure oscillations during breathing do not change. The work of the diaphragm may increase as a consequence of the contraction due to the greater load of the enlarged uterus and is represented by a higher Pga at the end of exhalation. Furthermore, during pregnancy, the expansion of the chest wall shifts due to the increased coupling between the abdominal pressure and the lower ribs.
Thanks to the increased area of apposition, the abdominal pressure created by the contraction of the diaphragm acts mainly on the lower ribs, which raises and expands the chest where the diaphragm is placed.
During pregnancy, two possible strategies of respiratory muscles can be considered: 1) greater recruitment of intercostal and accessory muscles in inspiration since increased displacement of thoracic volume and changes in pleural pressure may also be a consequence of their increased action; 2) a similar relative contribution between the diaphragm and the inspiratory intercostal muscles since the slope of the curve Pga versus Poes remains constant. The progressive increase in the anterior abdominal dimension leads to morphological adaptation of the abdominal muscles by lengthening their muscles up to 115%, changing the line of action, changing the angle of insertion, and reducing their thickness. The consequences are compromised functional ability, poor torque production and reduced ability to stabilize the pelvis against resistance. The latter may be associated with back pain during pregnancy [1].
Changes in Functional Tests During Pregnancy
A healthy pregnancy brings about clinically significant changes in the respiratory system, which can influence the assessment and treatment of asthma (Table 1).
Table 1: The most important changes in respiratory physiology during pregnancy.
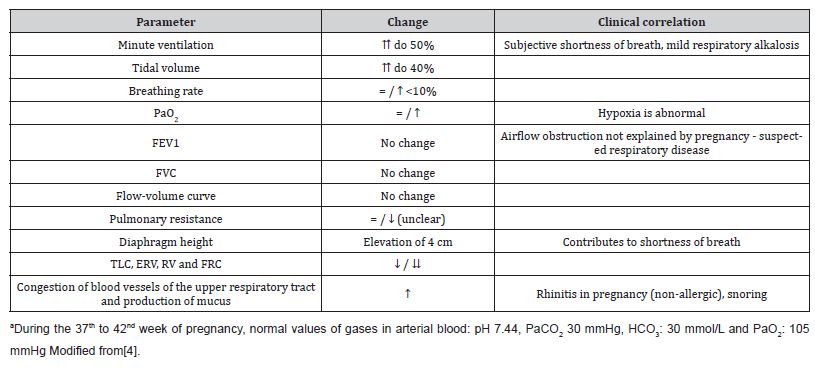
Although there is no significant decrease in dynamic spirometry values, airflow limitation (i.e., reduced FEV1 and FEV1/FVC ratio <0.75) in pregnancy is almost always abnormal (Table 1) [3].
The diffusion capacity of the lungs (TLCO or DLCO) increases progressively during pregnancy due to the increase in blood volume in the body and the increase in cardiac output. The monitoring of this parameter must be interpreted in the context of changes in the concentration of hemoglobin in the blood even after pregnancy. DLCO is a necessary test if a pregnant woman reports dyspnea and has normal spirometry, because then we can suspect anemia and thromboembolic complications in pregnancy [4].
During pregnancy, the mean value of respiratory parameters decreases as gestational age progresses from the first to the third trimester in healthy pregnant women. Except for FEV1% other pulmonary function tests including FVC, FEV1, PEF and FEF25-75% were significantly decreased. Thus, some of the pregnant women could show a tendency towards a restrictive pattern. These outcomes may arise from a combination of hormonal changes and mechanical adaptations of the enlarged uterus, which has a significant impact on the pulmonary physiology of the pregnant woman. Furthermore, an increased respiratory rate can occur as the gestational age increases. On the other hand, arterial blood oxygen saturation decreases as pregnancy progresses. These changes are required to meet the increased metabolic needs of the mother and fetus. Therefore, pregnancy at altitude may lead to compensatory changes in equilibrium with the changes that occur in dynamic pulmonary function tests. However, longitudinal studies may reveal better results with larger samples; therefore, future studies should be conducted on a large sample size (subjects) and longitudinal studies considering parity, breast size, type of pregnancy and other socioeconomic factors [5].
Exercise and Hyperventilation
Pregnant women maintain aerobic work capacity even in late pregnancy. The physiological response to elevated exercise in healthy pregnant women consists of an increase in both V′E and oxygen consumption, with a greater ventilatory equivalent (i.e., V′E/oxygen consumption). Hyperventilation occurs due to the recruitment of resting inspiratory capacity combined with pregnancy- induced bronchodilation, which allows an increase in VT to meet metabolic demands. Inspiratory capacity is recruited due to stable TLC and end-expiratory lung volume reduction before exercise. Because the ERV is reduced, the end-expiratory lung volume does not decrease further during exercise, to avoid mechanical deficiency of the lower part of the pressure-volume curve of the respiratory system. Otherwise, the presence of hyperventilation results in a drop in the partial pressure of carbon dioxide (PaCO2) below 35 mmHg, and a slight increase in the partial pressure of arterial blood oxygen (PaO2) on average from 101 to 104 mmHg in the third trimester of pregnancy. Observed changes occur early in pregnancy and are maintained until delivery [1].
change in the pH value of the blood is not manifested, because respiratory alkalosis is compensated by metabolic acidosis in terms of increased renal excretion of bicarbonates and their reduced level in the plasma to maintain the pH around 7.4 [1].
It has been proven that the main physiological mechanism of hyperventilation is the influence of progesterone on the respiratory center in terms of increasing its sensitivity to PaCO2. Namely, even in non-pregnant women, in the lutein (progesterone) phase of the menstrual cycle, transient hyperventilation occurs with a drop in PaCO2. This physiological mechanism is also used for therapeutic purposes in the treatment of hypoventilation syndromes, central sleep apnea and pulmonary emphysema. Pregnancy therefore does not affect symptoms of limited perception for cessation of exercise, as increased breathlessness is a normal consequence of increases in both V′E and work of breathing [1].
Oxygen Transport and Consumption in Pregnancy
All tissues at the level of cellular metabolism require oxygen for oxidative energy production. The supply of oxygen to all cells in the body (DO2) depends on the oxygenation of the venous blood in the lungs, the transport capacity of the blood for oxygen (CaO2) and the cardiac output. Therefore, DO2 takes place with the action of the cardiorespiratory system and with the help of blood, i.e., erythrocyte hemoglobin as a transport medium [6].
Under normal circumstances, delivery is 4 times higher than consumption, that is, consumption is about 25% of the content in arterial blood, so venous blood has an average saturation of 75%. The supply of oxygen to the cells can be calculated according to the formula:
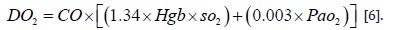
The concentration of dissolved oxygen in the blood is ignored, so the oxygen content in the blood is mostly related to the amount of hemoglobin present and its oxygen saturation. DO2 can be disturbed in any segment involved in the transport process. Thus, if there is anemia, which is a common occurrence in pregnancy, pO2 decreases, because the number of binding sites for O2 decreases. Furthermore, carbon monoxide poisoning also reduces oxygen binding sites. On the other hand, if there is hypoxemic respiratory insufficiency, the lungs are unable to expose the venous blood in the pulmonary capillaries to a sufficient amount of oxygen, so hemoglobin saturation decreases. Moreover, the affinity of hemoglobin for oxygen also decreases at lower pH value, at higher temperatures and with an increase in 2,3 DPG, which is a product of anaerobic glycolysis, whereby more oxygen is released in the tissues and vice versa. An increase in pH, a decrease in temperature and a decrease in 2.3 DPG lead to a higher affinity of O2 for hemoglobin and, therefore, less release in tissues. In addition to the above, the decrease in the cardiac output globally reduces the supply of oxygen to the tissues, as in the case of hypovolemia (dehydration of a pregnant woman!) or heart failure, but also in certain clinical situations, such as septic shock, where there is also a disturbed distribution of blood flow in peripheral structures, especially under the influence of vasoactive substances, which perform a dysfunctional redistribution of blood flow, so that certain tissues even have excessive blood flow while others are hypoperfused. It is especially dangerous if hypoperfusion is present at the level of the uterus [6].
On the other hand, oxygen consumption (VO2) is, logically, a product of the arterial-venous difference in oxygen content and cardiac output and is calculated according to the formula:
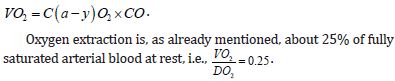
Thus, the increase in extraction or decrease in venous blood saturation is actually a compensatory mechanism when the supply of oxygen is not sufficient for the level of metabolic activity or may indicate a disorder of circulation distribution or functional shunts. Of course, the possibility of increasing the extraction of O2 acts as a kind of physiological buffer, in order to maintain the necessary consumption of oxygen in the peripheral tissues. All this is accompanied by a decrease in venous blood saturation that reaches a critical limit, where the extraction is maximal, and therefore there is no more space to cover the primary disorder, and anaerobic glycolysis is activated, which causes a shift in the pyruvate-lactate balance in the direction of the development of lactic metabolic acidosis. Mixed venous blood (SVO2) is on average saturated about 73% with a partial pressure of oxygen of about 40 mmHg. These parameters are determined exclusively by sampling blood from the right ventricle, that is, the pulmonary artery via a pulmonary arterial catheter or by modern types of catheters with continuous monitoring via a fiberoptic sensor on the tip of the catheter. High SVO2 values do not guarantee good tissue oxygenation. Thus, in septic shock, bacterial enzymes can block the mitochondrial respiratory chain and prevent oxidative metabolism, resulting in lactic acidosis with high SVO2 values. Also, the already mentioned poor flow distribution in septic shock contributes to reduced extraction in well-perfused tissues, which is reflected in the total mixed venous blood. Physiological anemia in pregnancy reduces the oxygen content in the arterial blood. However, due to the increase in cardiac output, up to 50% of DO2 is within normal limits, so it can be concluded that pregnant women are more dependent on the cardiac output in establishing good oxygenation than non-pregnant women. Oxygen consumption increases gradually during pregnancy with the development of the fetus with a maximum of an average of 330 ml/min at rest and 1170 ml/min during exertion. Childbirth itself increases oxygen consumption by as much as 40-60%, and cardiac output increases by about 22%. However, a healthy pregnant woman and the fetus tolerate these excesses in consumption during childbirth without any problems, which is not the case in a situation where oxygen supply is preferentially reduced during pregnancy, because reaching a critical level of oxygen supply, i.e., the buffering power due to increased exhaustion is reduced due to anemia and is already basically greater involvement of the cardiocirculatory system. The doctor involved in monitoring the delivery is obliged to prevent this potentially risky stage of pregnancy in terms of possible hypoxemic suffering of the fetus, and the pregnant women themselves, by optimizing DO2 before the very beginning of delivery [6].
Transport of oxygen through the placenta
Transport of oxygen through the placenta is a very complex process in which the partial pressure of oxygen (PaO2) in the maternal circulation is higher than in the fetal circulation. This is the reason that the fetus is in relative hypoxemia and in healthy pregnant women, which is compensated by a different hemoglobin of the fetus (fetal hemoglobin), which has a higher affinity for oxygen with a different dissociation curve of oxyhemoglobin, with faster blood flow. Despite the aforementioned compensatory mechanisms, it is clear that the greatest danger to the fetus and its damage and even death is hypoxemia of the mother, which most often occurs in pregnant women with asthma as a result of insufficient therapy of the underlying disease, and thus insufficient control of asthma [7].
Dyspnea in pregnancy
Anatomical changes in pregnancy and their responsibility for changes in lung volume have little consequence on the airways. However, changes in abdominal girth, pressure, diaphragm position, and chest wall size contribute to an increased incidence of physiological dyspnea in the later stages (Figure 1).
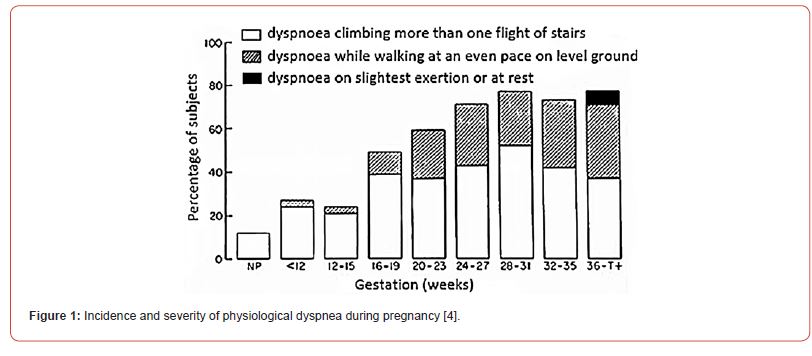
Dyspnea is a common symptom in normal pregnancy, so it does not always have to represent the presence of a certain cardiorespiratory disease. It is believed that a certain number of women (up to 50%) react more violently to the rise in progesterone levels and the fall in PaCO2, which is manifested by a feeling of difficulty breathing, sometimes even before the 20th week of pregnancy. It was observed that the maximum occurrence of dyspnea occurs between the 28th and 31st weeks of gestation. Dyspnea often occurs spontaneously, at rest, and is not associated with any physical exertion. It is certainly necessary to eliminate the suspicion of the presence of pulmonary thromboembolism, cardiomyopathy, interstitial lung disease or asthma with diagnostic procedures. Then it is necessary to carry out a careful clinical examination, rule out anemia as a possible cause of the complaints, perform an electrocardiogram and mandatory echocardiographic examination. Regarding lung function parameters, forced expiration tests should be avoided in the later stages of pregnancy and in all pregnancies with a risk of spontaneous abortion. Plethysmography of the whole body and pulse oscillometry can be used to assess the necessary ventilation parameters and especially the presence of obstruction and bronchial hyperreactivity. Bronchoprovocative tests should be avoided, and bronchodilator tests are always indicated when an increase in airway resistance is registered and are performed using β-2 agonists. Examination of diffusion disturbances in suspected lung fibrosis or pulmonary hypertension is less often necessary, as is the determination of gas exchange parameters in the arterial blood sample. It is mandatory to perform pulse oximetry before possible gas analysis, preferably from arterialized (hyperemia with antirheumatic ointment!) blood from the earlobe. Also, dosage of light to moderate load depending on the stage of pregnancy, allows a good evaluation of the cardiorespiratory system. If the saturation does not fall below 95% of the stress test, the existence of a more serious pathological condition that could threaten the course of pregnancy is unlikely. Ventilation- perfusion scintigraphic scans should be avoided [8].
Bronchoprovocation Tests
An asthma specialist can perform bronchoprovocation testing with exercise, histamine, or eucapnic voluntary hyperventilation. The results of these tests have a very high negative predictive value and are useful for excluding the diagnosis of asthma. The most common challenge is increasing the dose of inhaled methacholine. A decrease in FEV1 of 20% with a methacholine concentration of 8 mg/mL or less is considered a positive (abnormal) test result. This testing should be avoided during pregnancy (relative contraindication), due to the risk of precipitating an asthma attack and because methacholine is a class C drug (i.e., fetal risk detected in animal studies but not established or not tested in humans; it may be used if the benefit is greater than the risk to the fetus) [9].
Cardio-Pulmonary Stress Tests
Studies of physical efficiency and energy expenditure in pregnancy and childbirth have several goals. The main goal is to assess the pregnant woman’s ability to perform vaginal delivery safely. This is especially important for patients with cardiopulmonary disorders. Currently, the majority of such pregnant women qualify for a planned caesarean section based only on echocardiography without conducting a stress test with dobutamine, which is contraindicated in pregnancy. A study that included 22 healthy pregnant women with an uncomplicated pregnancy showed that submaximal cardiopulmonary load tests up to 80% HRmax with a cycle ergometer on the back are a safe and accurate method in women at term [10]. Despite this, cardiopulmonary exercise tests in pregnant women are still considered relatively contraindicated, as they may precipitate an asthma attack [4].
FENO
A healthy pregnancy does not affect the level of fractionated exhaled nitric oxide. However, in pregnant women with asthma, FENO is elevated compared to healthy pregnant women and correlates with the level of asthma control [11]. On the other hand, FENO values in pregnant women with asthma can be useful in facilitating therapy in terms of adjusting the dose of inhaled corticosteroid every 3-6 weeks, namely: increasing the dose when FENO is >29 parts ppb, decreasing doses when FENO is <19 ppb, and there is no change when FENO is between 19 and 29 ppb. Accordingly, a 2016 study indicated that FENO-based asthma therapy in pregnant women could significantly reduce the rate of adverse perinatal outcomes, namely preterm birth, intrauterine growth restriction, perinatal mortality, or neonatal hospitalization [12].
PEF in pregnancy
PEF in an asthmatic woman during pregnancy reflects well the variability of expiratory airflow and helps the medical monitoring of disease stability. Pregnancy thus represents one of the most important areas of use of PEF monitoring in asthmatics. In asthma, which was only mild and intermittent before pregnancy, about 13% of patients have an exacerbation of asthma during pregnancy, while in severe asthma this figure is even around 50% [13]. Women with asthma during pregnancy are also more motivated to measure PEF than ordinary patients with asthma. Telemedicine pregnancy monitoring and insight into the diary of PEF measurements at home is a useful tool for monitoring these patients.
PEF measurements are not necessary every day if asthma is stable and the pregnant woman’s breathing does not cause any difficulties. It is enough for the pregnant woman to know what her best PEF result is (it can be before pregnancy, although PEF decreases a little in the last trimester of pregnancy). If during the period when the pregnant woman has respiratory symptoms, PEF falls below 80% of her best value and if the pregnant woman notices that her PEF values are significantly lower in the morning (at least by 20%) than in the evening, she should react with an increase in the dose of control drugs for asthma or contact your doctor. Until the exacerbation of asthma is completely stabilized, it is necessary to measure PEF at least 2 times a day. If asthma worsens, we should always look for reasons for this, although we will only find them in 50% of cases. Worsening of asthma can also be related to other pregnancy problems (preeclampsia and the like) [13].
Many cases of worsening asthma during pregnancy are related to the discontinuation of asthma control drugs (inhaled corticosteroids), because the mother tries to protect the child due to the potential side effects of the drugs on the child. Despite medical advice not to do this, we will not be successful. Then PEF gives us some possibility of quickly detecting worsening of asthma before something serious happens to the pregnant woman or the child [13].
After the birth of the child, asthma in the mother usually stabilizes as a result of hormonal changes after childbirth. Due to the occupation of the mother with a newborn child and compliance with PEF measurements, it falls. And in that period, we advise mothers with asthma to start regular, twice-daily PEF measurement when any respiratory symptoms begin to appear [13].
Acknowledgement
We would like to thank all our colleagues at our workplaces, who gave us support and advice regarding the publication of this work. We would especially like to thank our colleagues, Zugic Vladimir, Jovancevic-Drvenica Mirjana and Hromis Sanja.
Conflicts of interest
No conflict of interest.
References
- Lo Mauro A, Aliverti A (2015) Respiratory physiology of pregnancy: Physiology masterclass. Breathe (Sheff) 11(4): 297-301.
- Pellegrino R, Antonelli A, de Jongh F (2019) Static and dynamic lung volumes. In: Palange P and Rohde G (eds), ERS Handbook, Respiratory Medicine, 3rd edn, European Respiratory Society, Latimer Trend & Co. Ltd, UK.
- Jensen ME, Robijn AL, Gibson PG, Oldmeadow C, Managing Asthma in Pregnancy study collaborative group, et al. (2021) Longitudinal Analysis of Lung Function in Pregnant Women with and without Asthma. J Allergy Clin Immunol Pract 9(4): 1578e3-1585e3.
- Couillard S, Connolly C, Borg C, Pavord I (2021) Asthma in pregnancy: An update. Obstet Med 14(3): 135-144.
- Amare YE, Haile D (2020) Evaluation of Pulmonary Function Tests Among Pregnant Women of Different Trimesters in Debre Berhan Referral Hospital, Shoa, Ethiopia. Int J Womens Health 12: 1135-1143.
- Gersh BJ (2022) Maternal adaptations to pregnancy: Cardiovascular and hemodynamic changes. In: Lockwood CJ and Gersh BJ(eds), Up to date.
- Burton GJ, Jauniaux E (2018) Pathophysiology of placental-derived fetal growth restriction. Am J Obstet Gynecol 218(2S): S745-S761.
- Lee SY, Chien DK, Huang CH, Shih SC, Lee WC, et al. (2017) Dyspnea in pregnancy. Taiwan J Obstet Gynecol 56(4):432-436.
- Oppenheimer JJ (2019) What is the role of bronchoprovocation in the workup of asthma?
- Jędrzejko M, Nowosielski K, Poręba R, Ulman Włodarz I, Bobiński R (2016) Physical efficiency, and activity energy expenditure in term pregnancy females measured during cardiopulmonary exercise tests with a supine cycle ergometer. J Matern Fetal Neonatal Med 29(23): 3800-3805.
- Tamási L, Bohács A, Bikov A, Andorka C, Rigó J Jr, et al. (2009) Exhaled nitric oxide in pregnant healthy and asthmatic women. J Asthma 46(8): 786-791.
- Murphy VE, Jensen ME, Mattes J, Hensley MJ, Giles WB, et al. (2016) The Breathing for Life Trial: a randomized controlled trial of fractional exhaled nitric oxide (FENO)-based management of asthma during pregnancy and its impact on perinatal outcomes and infant and childhood respiratory health. BMC Pregnancy Childbirth 16:111.
- Bonham CA, Patterson KC, Strek ME (2018) Asthma Outcomes and Management During Pregnancy. Chest 153(2): 515-527.
-
Jusufovic Edin*, Kopitovic Ivan, and Flezar Matjaz. Respiratory Physiology of Pregnancy and Functional Diagnosis in Asthma. Archives in Respiratory & Pulmonary Medicine. 1(2): 2023. ARPM.MS.ID.000507.
-
Asthma, Pregnancy, Respiratory physiology, Functional diagnostics, Severe dyspnea, Respiratory system, Lung diseases, Lung function, Ventilation pattern
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
- Abstract
- Introduction
- Respiratory Physiology of Pregnancy
- Chemical/Hormonal Changes
- Mechanical Changes
- Changes in Functional Tests During Pregnancy
- Exercise and Hyperventilation
- Oxygen Transport and Consumption in Pregnancy
- Dyspnea in pregnancy
- Bronchoprovocation Tests
- Cardio-Pulmonary Stress Tests
- FENO
- Allergens
- PEF in pregnancy
- Acknowledgments
- Conflict of Interest
- References