Research Article
Research Article
Implantation of a Hydrophilic Polymer Coated Silicone Airway Stent Improves Airway Injury and Mucostasis in a Porcine Model: A Pilot Study
Roy Joseph Cho1*, Koji Kadowaki2, Daniel E Glumac3, Leslie A Kent4, Ryan C Hunter4, Robroy H MacIver5, Gregory K Peterson6, Vidhu Pandey7, Davis Seelig8 and Kazuhiro Tanahashi2
1Section of Interventional Pulmonary, Division of pulmonary, allergy, critical care and sleep medicine, USA
2Advanced Materials Research Laboratories, Toray Industries, Inc., Japan
3University of Minnesota Bakken Medical Devices Center, USA
4Department of Microbiology & Immunology, University of Minnesota, USA
5Children’s Heart Clinic, Children’s Hospitals and Clinics of Minnesota. University of Minnesota, USA
6Earl E Bakken Medical Devices Center, University of Minnesota, USA
7Department of Internal Medicine and Pediatrics, University of Minnesota, USA
8College of Veterinary Medicine, Department of Veterinary Clinical Sciences, University of Minnesota, USA
Roy Joseph Cho, Department of pulmonary, allergy, critical care and sleep medicine, University of Minnesota, Minneapolis MN, USA.
Received Date: June 18, 2022; Published Date: July 06, 2022
Abstract
Background: Mucous buildup is a significant cause for stent exchange, with mucous being implicated as the reason for up to 25% of stent exchanges [1-3]. Furthermore, impaired mucous clearance is thought to lead to infections, which can cause airway inflammation, growth of granulation tissue, and further airway stenosis. Prevention of mucous buildup in airway stents may be an improvement that could have a significant impact on patient quality of life as well as both material cost of stent replacement, and procedural time.
Purpose: Our group has developed a hydrophilic coating for silicone stents which reduces mucous adhesion compared to non-coated stents in an in vitro model [4]. This reduction in mucous adhesion could be an opportunity to improve complications of stent placement. The aim of this pilot study is to investigate the degree of airway injury and mucostasis using silicone stents with and without this coating in a porcine model.
Methodology: We modified commercially available silicone stents with a hydrophilic polymer from Toray Industries. We used X-ray Photoelectron Spectroscopy (XPS) and Time-of-Flight Secondary Ion Mass Spectroscopy (TOF-SIMS) to analyze and confirm the hydrophilic polymer coating on the surface of the experimental stent. We conducted an in vivo survival study in a porcine model to compare the degree of airway injury and mucostasis between coated (Stent-X) vs non-coated (Stent-C) stented airways.
Results: We successfully implanted two 14x10mm silicone stents within the left (Stent-C) and right (Stent-X) mainstem bronchus in this porcine model. The animal survived to termination at 4-weeks. Both stents were intact without migration. Stent-X demonstrated reduced mucostasis (75mg vs 250mg), lower airway injury score (28 vs 44) and lower goblet cell hyperplasia on H&E staining compared to Stent-C.
Conclusion: Stent-X demonstrates promising anti-mucous adhesion capabilities resulting in less airway injury in this pilot study. Future work involving multiple randomized animal models will be needed to corroborate our findings.
Keywords: Interventional pulmonary, airway stent, bronchial stenosis, mucostasis, X-ray Photoelectron Spectroscopy (XPS), Time-of-Flight Secondary Ion Mass Spectroscopy (TOF-SIMS)
Introduction
Over the past 20 years, there has been significant advancement in the discipline of interventional pulmonary with parallel growth of medical devices in this space. In particular, there are a numerous selection of tracheobronchial stents available for the treatment of tracheobronchial disease. In severe or refractory tracheobronchial disease, airway stenting can be considered; however, the risk of stent complications including mucous impaction, granulation tissue formation, stent migration and infection must be weighed to the benefit of these devices [1-3]. Prevention of mucous buildup in airway stents could have a significant impact on patient quality of life and the cost of repeated bronchoscopies.
A novel hydrophilic coating was recently developed by Toray Industries that can be applicable to silicone-based materials. Our group has successfully incorporated this hydrophilic coating to the surfaces of silicone stents which reduced mucous adhesion by 80% compared to non-coated silicone stents in an in vitro study [4]. The modified surface exhibits not only high wettability but also high lubricity, and such super hydrophilicity generates an anti-adhesion property. We hypothesize that the proprietary hydrophilic surface coating could prevent mucous buildup compared to standard noncoated stents in a porcine model. The aim of this pilot study is to investigate the effect of the coated (Stent-X) vs non-coated stent (Stent-C) on mucostasis and airway injury in a survival study using a porcine model.
Methods
Preparation and surface analysis of the hydrophilic coating on silicone stents
Toray’s proprietary hydrophilic polymer was applied to a Novatech tracheobronchial silicone stent from Boston Medical Products (Shrewsbury, MA, USA). The preparation technique was previously described by our group [4]. Small sections were cut and tested using X-ray Photoelectron Spectroscopy (XPS) and Time-of- Flight Secondary Ion Mass Spectroscopy (TOF-SIMS) to confirm existence of the hydrophilic polymer and coating homogeneity, respectively.
In vivo porcine model preparation and stent deployment
The study assessed the short-term (4-weeks) performance of Stent-X and Stent-C in one female Yorkshire farm pig (body weight 45 kg). This model was selected on account of the similarity between porcine and adult human central bronchial diameters [5].
Inhalation and intravenous medications used for the induction and maintenance of general anesthesia followed our APIC laboratory and IUCAC guidelines. The stents were sterilized with ethylene oxide (ETO) using a low temperature recipe prior to implantation. Karl Storz (Tuttlingen, Germany) equipment including a 14-mm diameter adult rigid bronchoscope, rigid alligator forceps, and rigid peanut forceps were used for stent deployment and adjustment. Stent-X and Stent-C were planned a priori to be deployed in the right and left mainstem bronchus; respectively. The standard 14x40mm stents were cut to 10mm length to prevent inadvertent lobar obstruction and perceived morbidity during this 4-week study.
The stent performance was assessed by means of flexible bronchoscopy (Tele-Pak, Karl Storz) on a weekly basis for a duration of 4-weeks after initial stent placement. The performance evaluation involved the examination of the stents to assess migration, granulation tissue overgrowth at both ends, and degree of mucostasis. Any mucous build-up was suctioned clear with a flexible bronchoscope.
At four weeks from initial stent placement, the animal was euthanized, and the tracheobronchial tree was resected and placed in formalin for histological assessment. Any mucous adhering to the luminal surface was collected into a 1 mL syringe. The weight of the syringe was measured, and the weight of the mucous was calculated by subtracting the weight of the empty syringe from the weight of syringe containing mucous.
Assessment of airway injury
The insult to the airway was assessed using gross observation of mucostasis, granulation tissue formation and stent migration; mucous weight at termination of the study; and histological assessment of mucosal injury and application of an airway injury score adapted from Sinha R and colleagues [6]. Histological assessment and calculation of the airway injury score were performed along four sections of the airway that were in direct contact with the stent, this included proximal (section A), proximalmid (section B), mid-distal (section C) and distal (section D). The slides were randomized and then reviewed by a pathologist who was blinded to the animal group and subsection classification. The subsections on each slide were semi-quantitatively assessed and graded for inflammation, granulation tissue, and extent of tissue damage (i.e., ulceration, epithelial flattening, and cilia loss). Each variable was graded using a 4-point scale: 0=absent, 1=low, 2=medium, and 4=high. Absent and low grades for each variable were considered within normal physiological spectrum.
Statistical analysis
The null hypothesis regarding the performance (migration, granulation tissue formation, and mucous retention) in a porcine model was that there was no difference in the incidences between the two types of stents. Descriptive statistics were used to analyze the data.
Results
The porcine model survived to the 4-week termination endpoint. The two 14x10mm silicone stents were successfully deployed in the right (Stent-X) and left (Stent-C) mainstem bronchus without cardiopulmonary compromise.
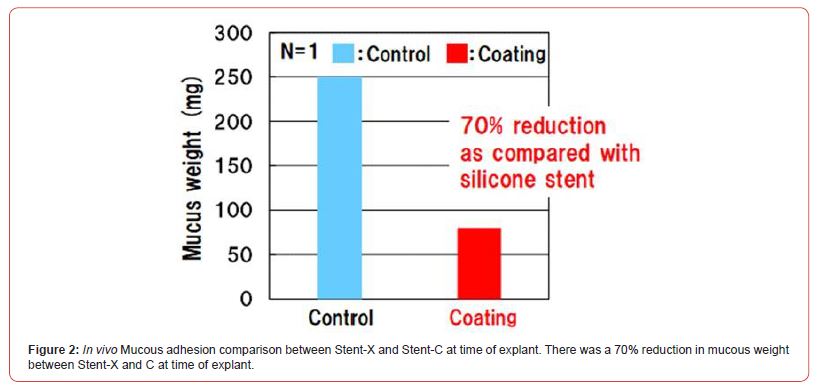
Gross comparison at the weekly surveillance bronchoscopies revealed that both stents remained intact with minimal migration at 4-weeks (Figure 1). At time of termination, there was more mucostasis in Stent-C compared to Stent-X. This was confirmed at time of explant where Stent-X demonstrated a lower mucous weight (75mg vs 250mg) compared to Stent-C (Figure 2).

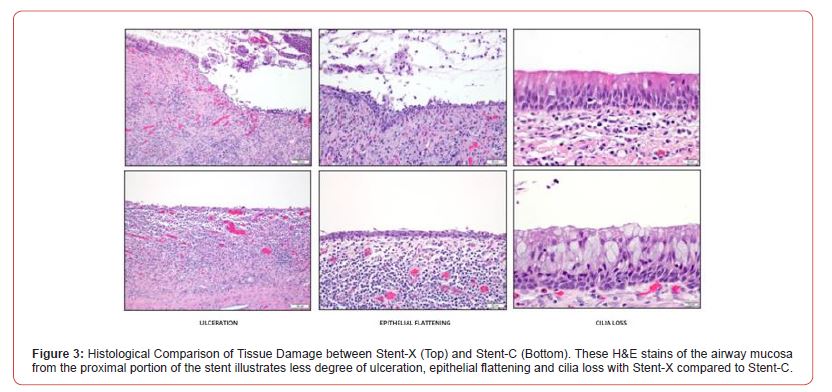
Histologically, the proximal portion of both stents demonstrated more tissue damage than the distal end (Figure 3). Stent-X demonstrated a lower degree of tissue damage demonstrated by degree of ulceration, epithelial flattening, and cilia loss. In addition, Stent-C showed a marked increase in goblet cell hyperplasia compared to Stent-X (Figure 4).
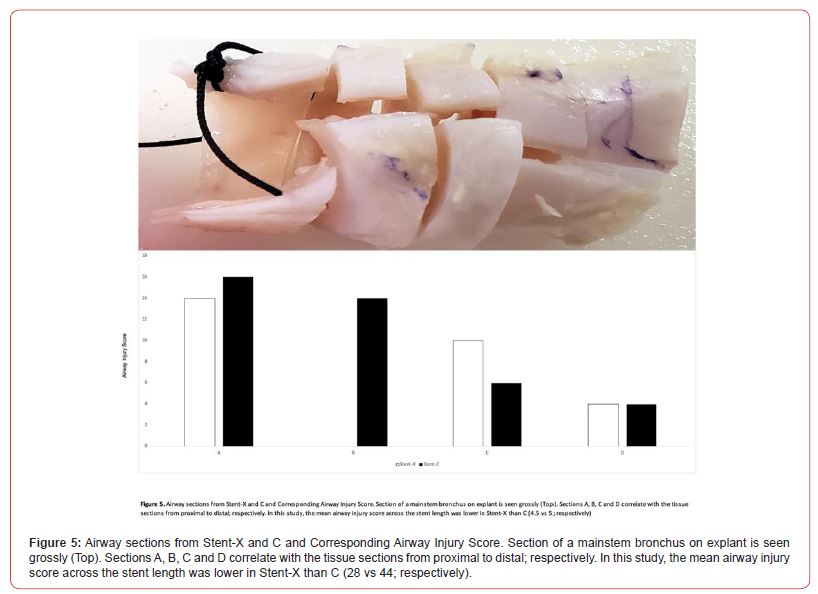
The airway injury score was lower across the length of Stent-X than in Stent-C (Table 1 and Figure 5). Along the four sections, Stent-X had a lower injury score than Stent-C (28 vs 44; respectively). This was most apparent in the proximal airway, where friction injury is most severe (Stent-X vs Stent-C: 14 vs 16, 0 vs 14; respectively).

Discussion
It has been suggested that hydrophilic surface modification could be effective in prevention of biological mucin adhesion onto the surface of silicone-based materials [7]. Lee S, et al. [8] revealed that the adsorption of porcine gastric mucin to polydimethyl siloxane surface was higher than hyaluronic acid and dextran and suggested that the unglycosylated polypeptide backbone segments of mucins act as binding sites through their hydrophobic interactions with the surface while hydrophilic carbohydrate chains may dangle out to interact with water [8]. This phenomenon has also been observed with other hydrophilic modification processes of siliconebased surfaces including hydrophilic polymer coating Jimenez Pardo I, et al. [9], plasma treatment Tan SH, et al. [10], surface polymerization of hydrophilic monomers Osawa S, et al. [11], and covalent bonding with hydrophilic polymers [12]. In our previous publication, we demonstrated an 80% reduction of purified saliva mucin adhesion with Stent-X compared to the control. In this study, we demonstrated a reduction in mucostasis within Stent-X and associated airway injury. Interestingly, there was less goblet cell hyperplasia associated with Stent-X which correlates well with the reduction in mucostasis we observed.
Although results from a multi-animal study would be more impactful, our group chose a single porcine model a priori. The rationale for this design was first, to understand the survivability of the animal with respect to our methodology given the lack of published literature; and second, to discover meaningful endpoints for a multi-randomized animal study. Moreover, the pathologist was blinded to stent-type; therefore, we feel that bias was minimized and our results, noteworthy. Like other studies evaluating degree of airway injury, there are no robust consistent scoring system to reflect injury related to an implantable device. The best injury score would encompass both depth of injury and type of cellular inflammation. This was best demonstrated using the composite airway injury scoring system by Sinha and colleagues [6]. Third, we found a remarkable contrast in the degree of goblet cell hyperplasia between Stent-X and C. Currently it is unknown whether this histological finding attributes to clinical outcomes. Fourth, we discovered that the proximal portion of the stents were where airway injury was more severe. This makes intuitive sense as friction from constant upward forces (i.e., coughing, mucociliary machine) would occur proximally than distally. Future studies investigating airway injury from implantable devices should incorporate goblet cell hyperplasia as a marker for airway injury; in addition, focus airway injury analysis on the proximal airway as opposed to distal portions.
Increasing number of devices have been developed for implantation within the airways including stents and intrabronchial valves. Although patient outcomes are improved, long-term effectiveness and sustainability of these implants are hindered by device-host reactions (e.g., mucostasis and formation of granulation tissue) that result in overall airway injury. In this study, we aimed to investigate the effect of a hydrophilic polymer coated silicone stent that could potentially disrupt the consequences of the host-device inflammatory response and airway injury. Stent-X demonstrated promising anti-mucous adhesion capabilities in our in vitro study and now in this in vivo preclinical study. Future work with multiple randomized animal models will be needed to corroborate our findings.
Conclusion
Stent-X demonstrates promising anti-mucous adhesion capabilities resulting in less airway injury in this pilot study. Future work involving multiple randomized animal models will be needed to corroborate our findings.
Statement of Ethics
The study was conducted at the University of Minnesota’s Advanced Preclinical Imaging Center (APIC) and Interventional Pulmonary Airway Lab. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Minnesota, Minneapolis MN (Protocol ID 2012-38723A).
Funding Sources
The study was funded by University of Minnesota in collaboration with Toray Industries.
Acknowledgement
We thank the University of Minnesota’s APIC lab for their expertise in the management and care of the animal in this study.
Conflict of Interest
Roy J Cho MD, MHA is a consultant for Toray. The other authors have no conflicts of interest to declare.
References
- Ost D, Shah A, Lei X, Godoy M, Jimenez C, et al. (2012) Respiratory Infections Increase the Risk of Granulation Tissue Formation Following Airway Stenting in Patients with Malignant Airway Obstruction. CHEST 141(6): 1473-1481.
- Folch E, Keyes C (2018) Airway stents. Ann Cardiothorac Surg 7(2): 273–283.
- Lee HJ, Labaki W, Yu DH, Salwen B, Gilbert C, et al. (2017) Airway stent complications: the role of follow-up bronchoscopy as a surveillance method. J Thorac Dis 9(11): 4651-4659.
- Glumac D, Kadowaki K, Cho RJ, Peterson G, Kazuhiro T, et al. (in press) An anti-fouling airway stent. Proceedings of the 2022 Design of Medical Devices Conference.
- Judge EP, Hughes JML, Egan JJ, Maguire M, Molloy EL, et al. (2014) Anatomy and bronchoscopy of the porcine lung. A model for translational respiratory medicine. Am J Respir Cell Mol Biol 51(3): 334-343.
- Sinha R, Correia R, Gardner D, Grau Roma L, de Brot S, et al. (2018) Mucosal injury following short-term tracheal intubation: A noval animal model and composite tracheal injury score. Laryngoscope Investigative Otolaryngology 3(4): 257-262.
- Altamash T, Esperanca JMSS, Tariq M (2021) Surface Coating and Treatments for Controlled Hydrate Formation: A Mini Review. Physchem 1(3): 272-287.
- Lee S, Muller M, Rezwan K, Spencer ND (2005) Porcine gastric mucin at the water/poly (dimethyl siloxane) interface: influence of pH and ionic strength on its conformation adsorption, and aqueous lubrication properties. Langmuir 21(18): 8344-8353.
- Jimenez Pardo I, Van der Ven LGJ, Van Benthem RATM, De With G, Esteves ACC (2018) Hydrophilic self-replenishing coatings with long-term water stability for anti-fouling applications. Coatings 8(5): 184.
- Tan SH, Nguyen NT, Chua YC, Kang TG (2010) Oxygen Plasma Treatment for Reducing Hydrophobicity of a sealed polydimethylsiloxane microchannel. Biomicrofluidics 4(3): 32204-32208.
- Osawa S, Kashiwakura M, Yamaguchi H, Otsuka H (2020) Influence of Anchoring segments on Methacrylate-based come-type hydrophilic polymer segments coated on silica surfaces using the grafting-to method. Colloid and Interface Science Communications 36: 100258.
- Litwinowicz M, Rogers S, Caruana A, Kinane C, Tellam J, et al. (2021) Tuning the Bulk and Surface Properties of PDMS Networks through Cross-linker and Surfactant Concentration. Macromolecules 54: 9636-9648.
-
Roy Joseph Cho, Koji Kadowaki, Daniel E Glumac, Leslie A Kent, Ryan C Hunter, et al., Implantation of a Hydrophilic Polymer Coated Silicone Airway Stent Improves Airway Injury and Mucostasis in a Porcine Model: A Pilot Study. Archives in Respiratory & Pulmonary Medicine. 1(1): 2022. ARPM.MS.ID.000504.
-
Interventional pulmonary, Airway stent, Bronchial stenosis, Mucostasis, X-ray Photoelectron Spectroscopy (XPS), Time-of-Flight Secondary Ion Mass Spectroscopy (TOF-SIMS), Airway injury, Hydrophilic polymer
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.