Case Report
Case Report
From Relief to Risk: BC Powder’s Impact on Chronic Salicylate Toxicity and Acute Respiratory Distress Syndrome
Joshua Clark, Kunal Jakharia* and Ashley Henderson
Pulmonary and Critical Care Medicine, University of North Carolina, Chapel Hill, NC
Kunal Jakharia, Assistant Professor, University of North Carolina, Chapel Hill, North Carolina, USA.
Received Date: July 19, 2023; Published Date: July 26, 2023
Abstract
The transport of oxygen through the placenta is a complex process and the greatest danger to the fetus of a pregnant woman with asthma is insufficient therapy and control of asthma. Most diagnostic procedures for evaluating the function of the respiratory system during pregnancy are not harmful to the fetus. Except for the forced expiratory volume in the first second, the other pulmonary function tests are reduced and decrease with the progress of pregnancy. Hyperventilation and dyspnea are normal phenomena, and pregnancy is not a contraindication for stopping exercise. However, in case of severe dyspnea, additional diagnostic tests should be performed. Bronchoprovocation tests, ventilation-perfusion scintigraphic scans and cardio-pulmonary stress tests should be avoided, and forced expiratory tests should also be avoided in the later stages of pregnancy. Daily measurement of peak expiratory flow is simple, safe, inexpensive, and very informative in determining the control and therapy of pregnant women’s asthma.
Keywords: Salicylate; Acute Respiratory Distress Syndrome; BC Powder; Toxicology; Drug Toxicity
Introduction
Salicylate toxicity encompasses acute manifestations following intentional ingestion, while chronic toxicity poses a more challenging diagnostic scenario. Often, chronic toxicity arises from inadvertent, prolonged exposure to various salicylate-containing products. Notably, Goody’s Powder and BC Powder are widely used pain relievers in the Southern United States. Goody’s Powder contains acetaminophen, acetylsalicylic acid (aspirin), and caffeine, with 520mg of aspirin per packet. BC Powder contains 845mg of aspirin and caffeine. Accumulated exposure to multiple doses of these products, both short and long-term, can result in unintentional toxicity. In this case report, we present a young, relatively healthy female who experienced repeated hospitalizations due to chronic salicylate intoxication from prolonged BC Powder use. The case emphasizes the diagnostic challenges of identifying chronic salicylate toxicity, while also highlighting various features such as acute respiratory distress syndrome (ARDS).
Case Presentation
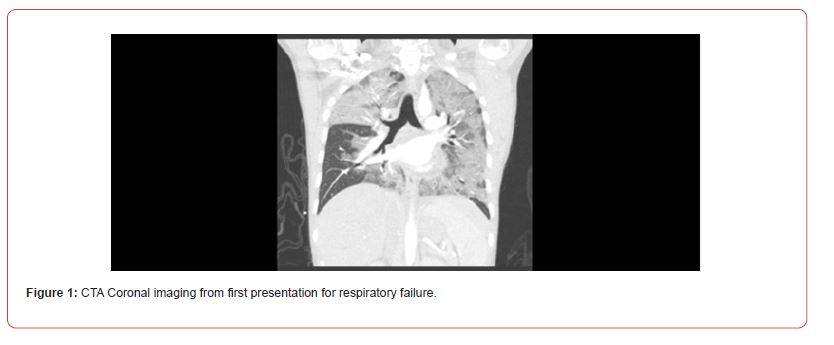
A 29-year-old female with a history of anxiety, depression, and migraines presented to the emergency department with several weeks of worsening dyspnea. She also had chronic sinus congestion associated with ear fullness, postnasal drip and cough producing thick, white sputum. She had no known sick contacts. She was a current smoker, smoking 1.5 packs per day and had been smoking for the last 10 years. She denied any vaping, alcohol, or other recreational drug use. In the emergency department, the patient was presented with evident respiratory distress characterized by tachypnea, nasal flaring, inspiratory crackles, and hypoxemia. Vital signs revealed a blood pressure of 107/60 mmHg, no fever, a pulse rate of 86 beats per minute, and a respiratory rate of 30 breaths per minute. She had rapidly escalating oxygen requirements eventually necessitating 80% FiO2 delivered via high-flow nasal cannula. Chest X-ray showed dense patchy airspace opacities in left perihilar and retrocardiac regions. Chest CTA (Figure 1) showed diffuse multifocal dense airspace opacification involving portions of all pulmonary lobes, most prominently in the left lung and right upper lobe. Labs were significant for decreased serum bicarbonate at 19 mmol/L, creatinine 0.79 mg/dl, hemoglobin 9.7 g/dl, WBC 7.8 109/L, pro-BNP 3,800 pg/ml, and ABG 7.42/28/89/18 on 100% FiO2. Urinalysis showed 30mg/dL protein, trace ketones and moderate blood. Follow-up urine protein creatinine ratio was 2.04 mg/dL. COVID PCR was negative. She was started on ceftriaxone and azithromycin for community acquired pneumonia coverage and admitted to the ICU (Figure 1).
In the ICU, extensive testing was performed to identify the cause of the patient’s respiratory symptoms. The respiratory pathogen panel and urine legionella screen yielded negative results. Despite this, the patient remained tachypneic with elevated oxygen requirements, leading to the decision intubate and perform diagnostic bronchoscopy with bronchoalveolar lavage (BAL). During the bronchoscopy, the airways appeared normal with minimal secretions, and no significant findings were observed. The bronchial culture demonstrated minimal polymorphonuclear leukocytes on stain, but no bacterial growth was detected. Analysis of the BAL fluid revealed 850 red blood cells and 525 nucleated cells, consisting of 62% neutrophils, 1% lymphocytes, 30% monocytes, and 7% other cells. Eosinophils were absent. Cytology results showed the presence of alveolar macrophages and acute inflammation, but no malignant cells were detected. Various additional tests were performed on the BAL fluid, including AFB culture, aspergillus galactomannan antigen, pneumocystis DFA, fungal culture, legionella culture, respiratory pathogen panel, and COVID PCR, all of which yielded negative results.
On hospital day 2, the patient was successfully extubated, and methylprednisolone was initiated due to a suspected inflammatory cause for her condition. Following the treatment, her oxygen requirements improved rapidly, accompanied by positive changes in radiographic findings observed on the chest X-ray. She completed her prescribed course of ceftriaxone and azithromycin. Throughout her hospital stay, occasional episodes of bradycardia were noted, with heart rates as low as the 30s and intermittent 2:1 atrioventricular block that self-resolved. A transthoracic echocardiogram revealed normal biventricular function and no significant valvular abnormalities. During the post-extubation discussion with the patient, it was revealed that she had been regularly consuming BC powder since the age of 18 as a treatment for her migraine headaches. She reported using up to four packets of BC powder per day during her most symptomatic episodes. Further workup completed during hospital stay included negative ANCA, HIV, ENA, ANA, myositis panel, anti-CCP, anti-GBM, cryoglobulin, hypersensitivity pneumonitis panel (drawn on steroids) and hepatitis panel. She had a mildly positive RF at 22.3 IU/mL, mildly decreased C4 (12.5 mg/DL) but normal C3 (98 mg/dL). SPEP with no monoclonal protein.
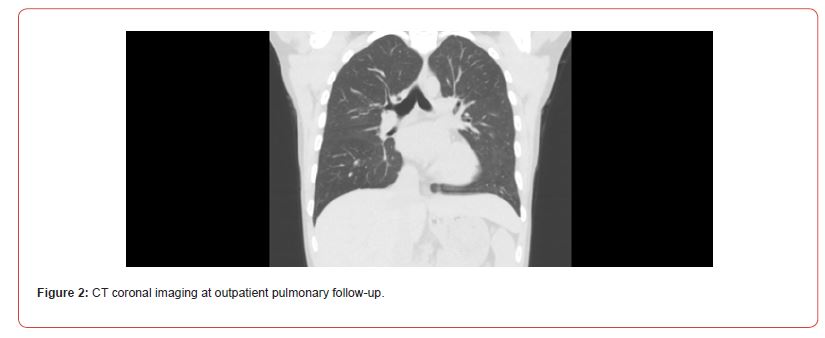
Given the presence of proteinuria and hematuria, a 24-hour urine protein test was conducted and revealed an elevated level of 576mg/dL. As a result, the patient underwent a renal biopsy, which indicated focal and segmental glomerulosclerosis with a tip variant. The nephrology team suspected that this finding was secondary to chronic salicylate use. Unfortunately, no salicylate level was obtained during her initial hospitalization. Upon discharge, the patient was prescribed a prolonged steroid taper, initiated on lisinopril, and strongly advised to refrain from further use of BC powder or salicylates due to the belief that they were contributing to her pulmonary toxicity. During her follow-up appointment with the pulmonology clinic, nearly 3 months later, the patient reported that her previous breathing concerns had completely resolved. She had completed her course of steroids one month prior to the appointment. Her exposure history was unremarkable. The patient acknowledged continued use of BC powder but in reduced amounts. A high-resolution non-contrast chest CT scan (Figure 2) showed an improvement with the resolution of previously observed bilateral ground-glass pulmonary opacities, with no new pulmonary parenchymal abnormalities detected (Figure 2).
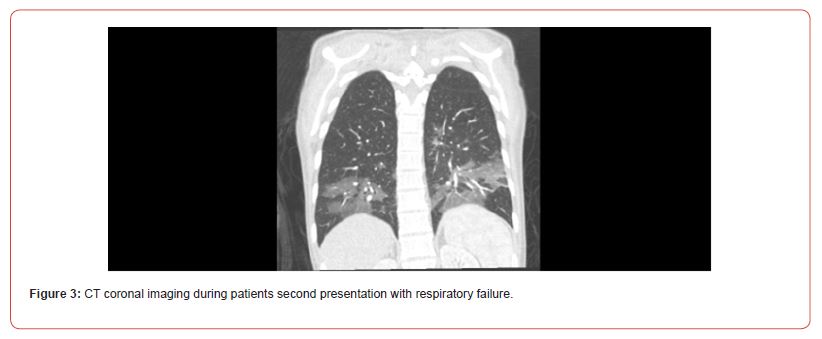
Twelve days later, the patient presented to the emergency room with pronounced shortness of breath and tachypnea. Her shortness of breath had worsened over the week leading up to her presentation. The patient reiterated her persistent sinus congestion, postnasal drip, and sporadic productive cough. She also reported chronic tinnitus, which had intensified in the last few days. Furthermore, she experienced ongoing headaches and admitted to consuming 2-6 BC Powders daily, with an escalated intake over the past few weeks. Although she continued to smoke, her smoking frequency had decreased compared to earlier periods. Vitals in the ER showed BP 98/77 mmHg, HR 118 beats/min, RR 30-35 breaths/min, temperature 36.7 C saturating 99% on RA. Exam showed significant tachypnea and difficulty speaking but clear lungs bilaterally. Chest x-ray showed mild bibasilar and retrocardiac streaky and patchy opacities. Follow-up chest CTA (Figure 3) showed bilateral lower lobe predominant mosaic pattern ground-glass opacities centered around the peribronchovascular region. Findings were noted to have a similar morphology as compared to prior CTA chest but were less severe. No PE was identified (Figure 3).
Labs demonstrated a serum bicarbonate of 11 mmol/L, creatinine of 0.80 mg/dl, WBC 11.4 109/L, Hgb 13.2 gm/dl, pro-BNP 42 pg/mL, troponin negative and negative lactate. ABG on room air was 7.5/11/177/9. UA was significant for continued proteinuria, positive ketones, and hematuria. COVID/influenza/RSV negative. INR elevated to 2.39 Salicylate level was obtained and elevated at 548.3 ug/mL. A hypersensitivity panel collected on admission was negative. The patient received an intravenous bolus of sodium bicarbonate and was initiated on a bicarbonate drip. Poison control and nephrology were consulted and recommended urgent hemodialysis. The patient was admitted to the intensive care unit (ICU), where hemodialysis was promptly initiated. An intravenous bicarbonate infusion was maintained alongside hemodialysis. Following her initial 4-hour hemodialysis session, the patient transitioned to continuous renal replacement therapy (CRRT) for approximately 24 hours. On hospital day 2, her salicylate level was undetectable, and serum bicarbonate levels normalized, resulting in the resolution of her shortness of breath and tachypnea. To address coagulation abnormalities, the patient received oral vitamin K, which successfully normalized her INR levels. Migraine prophylaxis was resumed using topiramate, and she was emphatically advised to abstain from using BC or Goody’s powder.
Discussion
Patients suffering from salicylate toxicity can present with tachypnea, hyperpnea and tinnitus. Patients develop tachypnea secondary to direct stimulation of the respiratory center in the medulla by toxic levels of salicylate. A consequential metabolic derangement includes respiratory alkalosis from continued tachypnea and reduction of carbon dioxide. As toxicity persists, salicylates can cause a concomitant anion gap metabolic acidosis through the accumulation of lactate and ketone bodies [1]. In the final stages, respiratory acidosis can occur secondary to exhaustion, co-ingestion (and resultant hypoventilation) or lung injury as patients are not able to maintain the needed respiratory rate to overcome the metabolic acidosis. As the pH falls, a larger amount of serum salicylate becomes non-ionized enabling increased tissue permeability. Salicylates can then more readily cross the bloodbrain- barrier and cause worsening altered mental status thus decreasing the ability to maintain an adequate respiratory drive. This further worsens pH allowing even more non-ionized drug to be present [1,2] Therapeutic serum salicylate levels range from 10- 30mg/dL with toxic levels usually being greater than 40-50mg/dL. Caution should be taken when interpreting serum salicylate levels as chronic toxicity can occur even at therapeutic levels of salicylate [3]. Salicylate toxicity is generally treated with serum and urinary alkalization with hemodialysis reserved for severe cases. Prompt consultation with a toxicologist is recommended when salicylate toxicity is suspected [1,2].
In addition to the above-mentioned clinical findings, patients with acute or chronic toxicity can have numerous other multiorgan system findings. One of note, and particularly relevant to our case, includes acute respiratory distress syndrome (ARDS) or noncardiogenic pulmonary edema. ARDS in salicylate toxicity presents in approximately 7% of cases [4]. The exact mechanism behind the development of ARDS and non-cardiogenic edema is not known but there are several proposed theories. One theory postulates that salicylates generate pulmonary edema by directly increasing alveolar capillary membrane permeability [5]. Another hypothesis involves a neurogenic pathway by inducing adrenergic excess, wherein the hypothalamus may become injured promoting a shift from systemic to pulmonary circulation. Subsequently, resultant hypoxia can lead to pulmonary artery hypertension, resulting in an increase in local vasoactive substances that worsen ARDS [6]. An alternative potential mechanism for ARDS in salicylate toxicity includes the alteration of prostaglandin synthesis through the known mechanisms of salicylates on cyclooxygenase. By shifting and influencing arachidonic acid pathways, salicylates may decrease the production of prostaglandins that normally limit fluid conductance and prevent membrane permeability [5,7]. Finally, hyperventilation may increase the risk of developing ARDS secondary to mechanical stress [8]. Risk factors for developing ARDS in salicylate toxicity include history of cigarette smoking, chronic overdose, metabolic acidosis, and neurological symptoms on presentation [7, 9]. Proteinuria is commonly accompanied with pulmonary edema suggesting that salicylates could potentially increase membrane permeability in many different organ systems [5]. Definitive treatment is accomplished through treatment of the salicylate toxicity through therapies such as alkalization of the urine or hemodialysis. The Extracorporeal Treatments in Poisoning Workgroup (EXTRIP) recommends initiating hemodialysis for any salicylate toxicity associated with noncardiogenic pulmonary edema requiring supplemental oxygen.
Other findings in salicylate poisoning include tinnitus, neuronal dysfunction, cerebral edema, gastritis, and hypoprothrombinemia among others [1]. Additionally, there are case reports of cardiac conduction abnormalities in patients with salicylate toxicity including complete heart block [10]. Usually, these conduction abnormalities are the result of acidemia or electrolyte disturbances [10-13] but studies by Neto [11] demonstrated concentrationdepended decrease in the rate of sinus node firing in isolated rabbit heart preparations. This could theoretically result in decreased conduction in sinus and AV nodes leading to bradyarrhythmias [12], as seen in our patient. This patient’s medical journey spanned three months, involving two distinct hospitalizations, during which she exhibited multiple signs and symptoms consistent with salicylate toxicity. Regrettably, a salicylate level was not obtained during her initial presentation, making a definitive diagnosis challenging. Nevertheless, this circumstance offers us an opportunity to retrospectively analyze data, leading us to believe that her initial presentation aligns with the characteristics of chronic salicylate toxicity.
The patient improved rapidly after starting on steroids which was a befuddling feature of her case. One possible explanation is that the two were entirely unrelated and rather it was the natural temporal response of her body clearing salicylate without any further intake while receiving optimized supportive care and fluid removal/diuresis. Additionally, while not being the definitive treatment for salicylate induced non-cardiogenic pulmonary edema, steroids may have expedited her recovery but not through the expected anti-inflammatory mechanism. There are several previous pharmacokinetic studies and case reports (mostly in pediatrics; [14,15]) that show with the addition of corticosteroids, there was a reduction in serum salicylate levels and conversely with the withdrawal of steroids there is a noted increase in serum salicylate levels. Although the mechanism is not clear, there seems to be increased clearance of salicylates when taken in conjunction with corticosteroids [14-15]. The patient’s findings of 2nd degree AV block, proteinuria, ketonuria, tachypnea and tinnitus are all features that can present with salicylate toxicity. We suspect that if a salicylate level had been drawn during her first hospitalization, it would have been elevated and she may have improved with standard salicylate toxicity treatments.
Chronic salicylate toxicity is a complex condition that may not always be present with the classic respiratory alkalosis and metabolic acidosis. It can lead to various systemic disturbances, including non-cardiogenic pulmonary edema and acute respiratory distress syndrome. Detecting potential sources of long-term exposure is crucial, especially in patients who unknowingly consume salicylate-containing products. In regions where the use of products like “Goody’s Powder” and “BC Powder” is common, explicitly inquiring about these medications is vital, as many patients may be unaware of their active ingredients. Serum salicylate levels should be obtained in any suspected salicylate intoxication, and consultation with local poison control or toxicology experts is recommended for further guidance. Hemodialysis is the preferred treatment for patients with salicylate toxicity and ARDS requiring supplemental oxygen. Healthcare providers should be mindful of potential alterations in serum salicylate levels when initiating or tapering steroids.
Acknowledgement
None.
Conflict of interest
No conflict of interest.
References
- Lugassy DM (2019) Salicylates. In: Nelson LS, Howland M, Lewin NA, Smith SW, Gold frank LR, et al. (eds.) Salicylate Toxicity Goldfrank's Toxicologic Emergencies, 11e. McGraw-Hill; New York City, United States.
- Palmer BF, Clegg DJ (2020) Salicylate Toxicity. N Engl J Med 382(26): 2544-2555.
- Hahn IH, J Chu, R S Hoffman, L S Nelson (2000) Errors in reporting salicylate levels. Acad Emerg Med 7(11): 1336-1337.
- Reed CR, Glauser FL (1991) Drug-induced noncardiogenic pulmonary edema. Chest 100(4): 1120-1124.
- Heffner JE, Sahn SA (1981) Salicylate-induced pulmonary edema. Clinical features and prognosis. Ann Intern Med 95(4): 405-409.
- Hrnicek G, James Skelton, Warren C Miller (1974) Pulmonary edema and salicylate intoxication. JAMA 230: 866-867.
- Glisson JK, Vesa TS, Bowling MR (2011) Current management of salicylate-induced pulmonary edema. South Med J 104(3): 225-232.
- Mascheroni D, Kolobow T, Fumagalli R, Moretti MP, Chen V, et al. (1988) Acute respiratory failure following pharmacologically induced hyperventilation: an experimental animal study. Intensive Care Med 15: 8-14.
- Niehoff JM, Baltatzis PA (1985) Adult respiratory distress syndrome induced by salicylate toxicity. Postgrad Med 78:117-119, 123.
- Aggarwal N, Kupfer Y, Chawla K, Tessler S (2013) Altered mental status and complete heart block: an unusual presentation of aspirin toxicity. BMJ Case Rep bcr2013010083.
- Riccioppo Neto F (1982 ) Electrophysiological effects of the salicylates on isolated atrial muscle of the rabbit. Br J Pharmacol 77(2): 285-292.
- Mukerji V, Alpert MA, Flaker GC, Beach CL, Weber RD (1986 ) Cardiac conduction abnormalities and atrial arrhythmias associated with salicylate toxicity. Pharmacotherapy 6(1): 41-43.
- Kent K, Ganetsky M, Cohen J, Bird S (2008) Non-fatal ventricular dysrhythmias associated with severe salicylate toxicity. Clin Toxicol (Phila) 46(4):297-299.
- Klinenberg JR, Miller F (1965) Effect of corticosteroids on blood salicylate concentration. JAMA 194(6): 601-604.
- Koren G, Roifman C, Gelfand E, Lavi S, Suria D, et al. (1987) Corticosteroids-salicylate interaction in a case of juvenile rheumatoid arthritis. Ther Drug Monit 9(2):177179.
-
Joshua Clark, Kunal Jakharia* and Ashley Henderson. From Relief to Risk: BC Powder’s Impact on Chronic Salicylate Toxicity and Acute Respiratory Distress Syndrome. Archives in Respiratory & Pulmonary Medicine. 1(2): 2023. ARPM.MS.ID.000509.
-
Salicylate toxicity, Goody's Powder, COVID PCR, Urine legionella, Cytology, azithromycin, Ceftriaxone, Tachypnea
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.