Case Report
Case Report
Reopening 8 Weeks Collapsed Lungs with Advanced Bronchoscopy due to Bronchial Amyloidosis with Refractory Bleeding
Yaniv Dotan¹a, Maria Zaharan¹b*, Marina Levi-Potral¹, Hanna Ammury², Amichai Gutgold², Haitham Nasrallah³, Abdah-Bortnyak Roxolyana3 and Asaf Miller²
1a,bPulmonary Institute, Rambam Health Care Campus, Ruth and Bruce Rappaport Faculty of Medicine, Haifa, Israel
2Department of Critical Care Medicine, Rambam Health Care Campus, Ruth and Bruce Rappaport Faculty of Medicine, Haifa, Israel
3Oncology Center, Rambam Health Care Campus, Ruth and Bruce Rappaport Faculty of Medicine, Haifa, Israel
aThese authors contributed equally to this work.
bThese authors contributed equally to this work.
Maria Zahran, Pulmonary Institute, Rambam Health Care Campus, Ruth and Bruce Rappaport Faculty of Medicine, Haifa, Israel.
Received Date: August 08, 2024; Published Date: August 20, 2024
Abstract
Amyloidosis is characterized by the deposition of misfolded proteins as amyloid fibrils in various tissues, including the lungs. Tracheobronchial amyloidosis, a rare form of pulmonary involvement, represents approximately 1% of amyloidosis cases. Symptoms include cough, wheezing, and hemoptysis.
We report a case of a 42-year-old female with a history of bronchial amyloidosis, previously managed conservatively. The patient presented with mild hemoptysis and was admitted for bronchoscopy, which revealed fresh blood and abnormal mucosa in the bronchi. During the procedure, the patient experienced sudden desaturation, leading to emergent intubation and subsequently to VV-ECMO due to refractory hypoxemia and complete atelectasis of the left lung. Despite extensive interventions including radiation therapy and multiple bronchoscopies, her condition deteriorated with persistent airway obstruction and bleeding.
After eight weeks of complete lung atelectasis, cryo-probe and argon plasma coagulation successfully managed the airway bleeding, allowing gradual lung re-inflation and weaning from mechanical ventilation and ECMO. However, the patient experienced significant complications, including digital ischemia requiring amputation, but ultimately recovered, with no further hemoptysis noted over 18 months.
This case highlights the potential for long-term lung re-inflation following complete atelectasis and underscores the importance of early intervention with radiation therapy for refractory tracheobronchial bleeding in bronchial amyloidosis. It illustrates the value of a multidisciplinary approach and innovative treatments such as cryo-probe and argon plasma coagulation. Continued research and awareness are essential to improve outcomes for patients with this rare condition.
Keywords:Tracheobronchial amyloidosis; Pulmonary amyloidosis; Bronchial bleeding; Lung atelectasis; VV-ECMO (Venous-Venous Extracorporeal Membrane Oxygenation); Cryo-Probe; Argon plasma coagulation; Radiation Therapy
Abbreviations:AL Amyloidosis-Amyloid light-chain (AL) amyloidosis; Venovenous (VV) extracorporeal membrane oxygenation (ECMO)
Introduction
Amyloidosis develops when one of more than 30 different proteins with an unstable structure misfolds and aggregates as insoluble amyloid fibrils that deposit in the extracellular space of organs and soft tissues [1]. These fibrils have a predominantly antiparallel beta-pleated sheet configuration, and can be identified on biopsy specimens both by their characteristic appearance on electron microscopy and by their ability to bind Congo red. Pulmonary amyloidosis results from plasma cell production of monoclonal free immunoglobulin light chains, which are produced in the bone marrow and deposited in the lungs (systemic amyloid light chain (AL) disease), or generated in the lungs and deposited locally (localized AL disease). AL amyloidosis is a rare disease with an incidence of about 10 cases per million [2]. Tracheobronchial amyloidosis is a very rare and under-recognized form of lung amyloid involvement that constitutes approximately 1% of amyloidosis cases [3]. Symptoms include cough, wheezing, dyspnea, hoarseness, and hemoptysis.
Case Presentation
In this case report, we present a 42-year-old female with previous medical history of bronchial amyloidosis, initially diagnosed through bronchial biopsy 3 years earlier. Since her diagnosis, the patient had multiple events of mild hemoptysis that were treated conservatively with bronchoscopy and local control of bleeding. In this event, the patient presented to our emergency department again with mild and non-life-threatening hemoptysis. She was transferred to the bronchoscopy suit stable hemodynamically with normal saturation on room air. Bronchoscopy revealed fresh blood in the left main bronchus and abnormal nodular mucosa in the right and left main bronchi. Uneventful and non-bleeding forceps biopsy was performed from a protruding suspicious lesion in the right main bronchus.
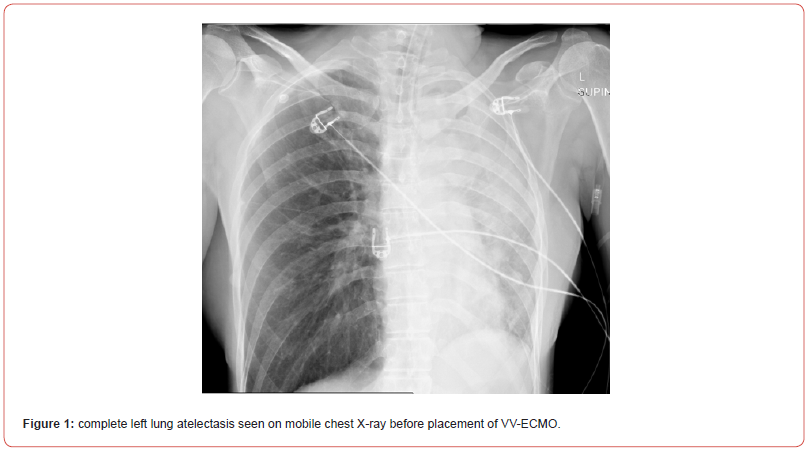
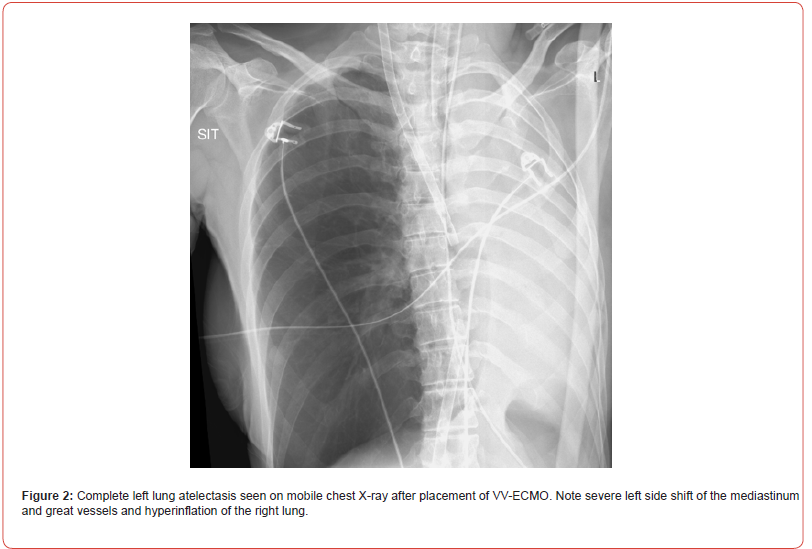
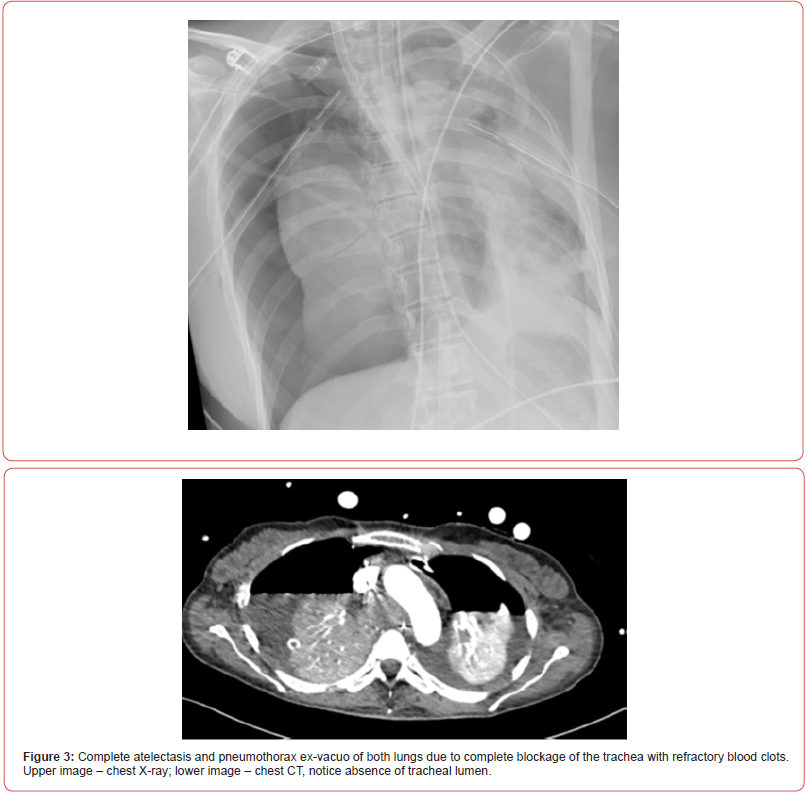
Shortly after, the situation escalated rapidly; the patient experienced a sudden desaturation to 70% and was emergently intubated. Mobile chest x-ray revealed complete atelectasis of the left lung (figure 1). Immediate bronchoscopy showed large amount of blood and blood clots in the left main bronchus and despite ongoing suctioning, the left lung did not re-inflate and the patient remained severely hypoxic. Few hours later a venous-venous extra-corporal membrane oxygenation (VV-ECMO) was emergently placed due to refractory hypoxemia (figure 2). In the following days, the patient’s lungs did not re-inflate despite multiple bronchoscopies performed due to ongoing bleeding that filled the airways with blood clots up to the upper trachea (figure 3). Tracheostomy was placed but blood clots filled also the tracheostomy tube that needed to be capped to prevent ongoing blood loss. The patient was treated with full VV-ECMO support, disconnected from the ventilator due to zero tidal volume secondary to the blocked trachea, and in the following weeks developed septic shock and acute renal failure requiring hemodialysis. In attempt to stop the ongoing bronchial bleeding the patient was transferred almost daily to the radiation oncology unit to receive radiation while on VV-ECMO (20 Gy in 10 fractions over 2 weeks) to the proximal tracheobronchial tree [4]. During and after completion of the radiation, multiple bronchoscopies aimed at controlling bronchial bleeding and clearing airway obstructions were conducted, unfortunately unsuccessfully.
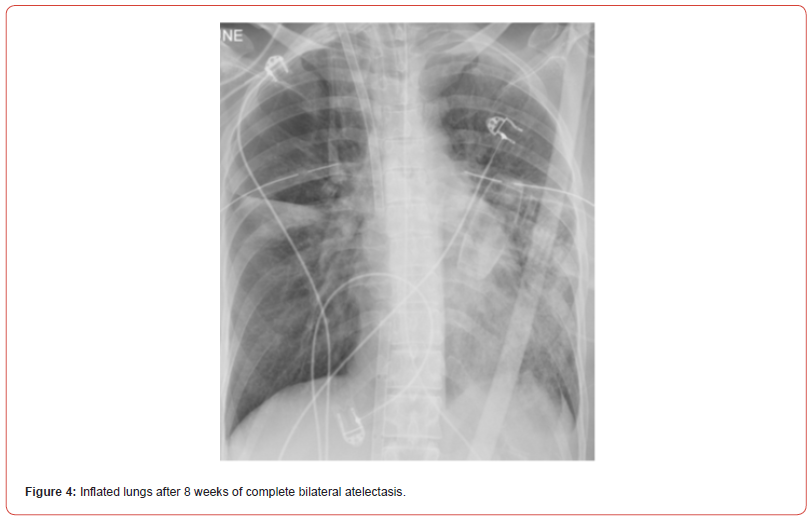
Eight weeks after the patient’s crisis, bronchoscopy with cryoprobe to freeze and pull blood clots from the airways followed by immediate argon plasma coagulation (APC) of remaining bleeding mucosal vessels were used. Following this procedure, the patient was ventilated with gradual increase of tidal volumes, and after 8 weeks of complete bilateral complete atelectasis, chest X-ray revealed both lungs inflation (figure 4). In the following days, the patient’s kidneys recovered and she was gradually weaned off the VV-ECMO and mechanical ventilation. Unfortunately, she suffered from severe upper and lower digital ischemia, presumably due to multiple etiologies: prolonged septic shock, prolonged VV-ECMO with no anti-coagulation due to ongoing bleeding, and probably from usage of intravenous and inhalational hexacaprone that was given as a salvage therapy to control the ongoing bleeding. Eventually, the digital ischemia required amputation of distal phalanx in both hands and anterior soles. Other than that, the patient recovered with no need of supplemental oxygen and preserved cognitive function and was discharged to a rehabilitation institute. Since then, there were no new events of hemoptysis so far (18 months).
Discussion
We conclude that long lasting completely collapsed lungs (8 weeks) can re-inflate and function normally and that radiation therapy for refractory tracheobronchial bleeding in patients with bronchial amyloidosis should be considered earlier in their clinical course in cases of refractory bleeding. In addition, this case provides valuable insights into the complexities of managing pulmonary amyloidosis. First, it underscores the necessity of early intervention, the potential for innovative treatment strategies, and the importance of a multidisciplinary approach to care. Second, it demonstrates the efficiency of using cryo-probe to freeze and pull blood clots and immediate APC to control local mucosal bleeding secondary to micro-trauma that occurs due to pulling these attached blood clots to the airway fragile mucosa. And finally, it highlights the need for continued research and awareness of amyloidosis to improve outcomes for patients affected by this rare and challenging disease.
Acknowledgements
Dr. Yaniv Dotan, Pulmonary Institute, Rambam Health Care Campus, Haifa, Israel. Dr. Dotan performed all invasive pulmonological procedures. He also wrote and edited the manuscript.
Dr. Maria Zahran, Pulmonary Institute, Rambam Health Care Campus, Haifa, Israel. Dr. Zahran contributed to writing and editing the manuscript.
Marina Levi-Portal, Registered nurse, Pulmonary Institute, Rambam Health Care Campus, Haifa, Israel. Mrs. Levi-Portal assisted with performing pulmonological procedures and contributed to editing the manuscript.
Dr. Hanna Ammury, Intensive Care Unit, Rambam Health Care Campus, Haifa, Israel. Dr. Ammury provided patient treatment and contributed to editing the manuscript.
Dr. Amichai Gutgold, Intensive Care Unit, Rambam Health Care Campus, Haifa, Israel. Dr. Gutgold treated the patient and contributed to editing the manuscript.
Dr. Haitham Nasrallah, Radiation Oncology, Rambam Health Care Campus, Haifa, Israel. Dr. Nasrallah treated the patients via radiation therapy and contributed to editing the manuscript.
Dr. Abdah-Bortnyak Roxolyana, Radiation Oncology, Rambam Health Care Campus, Haifa, Israel.
Dr Abdah-Bortnyak treated the patient via radiation therapy and contributed to editing the manuscript.
Dr. Asaf Miller, Intensive Care Unit, Rambam Health Care Campus, Haifa, Israel. Dr. Miller treated the patients and contributed to editing the manuscript.
Conflict of Interest
None.
References
- Wechalekar AD, Gillmore JD, Hawkins PN (2016) Systemic amyloidosis. Lancet 387(10038): 2641-2654.
- Quock TP, Yan T, Chang E, Guthrie S, Broder MS (2018) Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv 2(10): 1046-1053.
- O'Regan A, Fenlon HM, Beamis JF Jr, et al. (2000) Tracheobronchial amyloidosis. The Boston University experience from 1984 to 1999. Medicine 79(2): 69-79.
- Neben-Wittich MA, Foote RL, Kalra S (2007) External beam radiation therapy for tracheobronchial amyloidosis. Chest. 2007 132(1): 262-267.
-
Yaniv Dotan, Maria Zaharan*, Marina Levi-Potral, Hanna Ammury, Amichai Gutgold, Haitham Nasrallah, Abdah-Bortnyak Roxolyana and Asaf Miller. Reopening 8 Weeks Collapsed Lungs with Advanced Bronchoscopy due to Bronchial Amyloidosis with Refractory Bleeding. Archives in Respiratory & Pulmonary Medicine. 1(3): 2024. ARPM.MS.ID.000514.
-
Respiratory syndrome (SARS); COVID-19; Multisystem inflammatory Syndrome; Macrophages; Dendritic cells; Lymphocytes; Blood; Monocytes; Procalcitonin; Hepatosplenomegaly; Lymphadenopathy; Ascites; Colitis
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.