Research Article
Research Article
Is Endometrial Receptivity Assessment a First Line Study in Our Mexican Population? A Retrospective Study in Private Clinic
Luján Irastorza Jesús Estuardo1*, Durand Montaño Carlos1, Pacheco Pineda Josué Giovani1, Hernández Ramos Roberto1, Ávila Pérez Felipe de Jesús1, Ávila Rebollar Daniela1, Tomás Chávez Héctor1, Loof Esquivel Mónica Stéphanie1, Valdez Chávez Teresita de Jesús1, Gómez del Ángel Iván Francisco1, Regueyra Edelman Claudio1, Villa Jiménez Catalina1, Lemus Huerta Angel1, Angulo Rujano Francis Erika1, Arcos Hernández Héctor1, Tlapanco Crisostomo Sheyla Valeria1, Velasco Leyva Thania Patricia1, Serrano Trujillo Daniel Emmanuel1 and Vargas Hernández Víctor Manuel2
1Clínica de PRONATAL (Hospital Bité Médica). Prolongación Paseo de la Reforma 19, Santa Fe, Paseo de las Lomas, Cuajimalpa de Morelos, 01330 Ciudad de México, CDMX.
2Clínica de Salud Femenina. Insurgentes Sur 03810 Ciudad de México, México.
Luján-Irastorza Jesús Estuardo, Clínica de PRONATAL (Hospital Bité Médica). Prolongación Paseo de la Reforma 19, Santa Fe, Paseo de las Lomas, Cuajimalpa de Morelos, 01330 Ciudad de México, CDMX.
Received Date: February 10, 2023; Published Date: March 09, 2023
Annotation
Objective: To analyze whether endometrial receptivity evaluation, prior to euploid embryo transfer, improves the success rate in patients with recurrent implantation failure (RIF).
Methods: Retrospective, observational, cross-sectional study, which included couples undergoing assisted reproduction techniques (2021), who experienced RIF after transfer of good quality, euploid blastocysts (analyzed with PGT-A). Three groups were formed, which depended on the result of ERA: 1) Receptive, 2) Pre-receptive and 3) Early receptive.
Results: Of the 100% of the patients, 43% presented a normal window of implantation. However, 61.5% showed a higher prevalence of natural killer alterations and 42.8% of thrombophilia’s. In the case of patients with altered endometrial receptivity, they presented a cumulative implantation rate higher than 70% when correcting controlled ovarian stimulation.
Conclusions: More than half of the patients with RIF have a displaced implantation window and may benefit from a personalized adjustment of the endometrial stimulation protocol. Thus, couples with idiopathic RIF or associated with thrombophilia’s and immunological factors, benefit from the study of endometrial receptivity.
Keywords: Recurrent implantation failure; Natural killers; ERA, PGT-A; Thrombophilia’s
Introduction
Recurrent Implantation Failure (RIF) is an event that many obstetricians and assisted reproductive specialists may face. Currently, RIF has several definitions; some authors describe it as the impossibility of achieving a clinical pregnancy after the transfer of two good quality embryos in at least three In Vitro Fertilization (IVF) cycles, where the transfers can be performed with fresh or frozen embryos (total, 6 embryos), or in at least two oocyte donations (total, 4 embryos) [1]. It is also defined as the impossibility of achieving a clinical pregnancy after the transfer of at least 4 good quality embryos in three or more embryo transfers in women under 40 years of age [2]. The implantation process depends on 2 main components: 1) healthy embryos with implantation potential, and 2) a receptive endometrium suitable for implantation [3-6]. In order for implantation to be successful, both the embryo and the endometrium produce mediators (integrins, MUC 1, COX-2, HOXA 10, LIF, calcitonin, etc.) that, together with cytokines produced by lymphocytes [T, B, macrophages and Natural Killer (NK) cells] of the maternal immune system, promote this process [3-6]. Factors associated with implantation failure include anatomical factors (uterine anatomical abnormalities and thin endometrium), pelvic factors (altered expression of adhesive proteins, hypercoagulable state, immunological alterations), embryonic factors (genetic abnormalities, alterations in hatching (zona pellucida), embryo culture and transfer), energy deficiency and male factors [6-8].
The endometrium is a dynamic tissue that undergoes multiple changes during the menstrual cycle, for example it responds to hormones produced in the ovary as well as to paracrine secretions. In this regard, paracrine and endocrine secretions control the gene expression of endometrial cells. The proliferative phase is controlled by estrogens, allowing the proliferation of stromal cells and glands, as well as the elongation of the spiral artery. Post ovulatory progesterone (P4) causes secretory changes and, therefore, the endometrium acquires a receptive phenotype that allows blastocyst implantation. This period of endometrial receptivity (ER) is known as the “window of implantation (WOI)” and occurs between day 19 and 20 of the menstrual cycle [9, 10]. Currently, there are no objective and accurate methods to evaluate the ER, which, together with its lack of evaluation in infertile patients undergoing assisted reproductive techniques (ART), could lead to a decrease in the implantation rate because the focus is mainly directed to embryo development and embryo quality [11]. For this reason, in recent years techniques have been developed that more accurately indicate the WOI, thereby improving the success rate of assisted reproduction clinics. Transcriptomics or RNA sequencing emerged as a powerful tool for the clinical diagnosis of cancer, cardiovascular pathologies, and neurodegenerative diseases, among others [12]. However, in the case of infertility, it focuses on improving the implantation rate, which is based on the genetic information obtained from the human endometrium and generated during the last 18 years, allowing the development of endometrial receptivity analysis (ERA), which is composed of the evaluation of 248 genes analyzed by next generation sequencing (NGS) and coupled to a computational predictor that allows to appreciate the ER status to identify the WOI [13].
Therefore, the aim of this study is to analyze whether the evaluation of endometrial receptivity prior to embryo transfer improves the success rate in patients with recurrent implantation failure, to whom embryos with good embryo development, good quality and without aneuploidy [Preimplantation Genetic Testing for Aneuploidies) PGT-A] have been transferred.
Material and Method
Retrospective, observational, cross-sectional study that evaluated the results of ART in couples with recurrent implantation failure, obtained in 2021 at the Pronatal clinic located inside the Hospital Bité Médica in Mexico City. Thirty-seven women over 18 years of age, who experienced RIF after transfer of good quality, euploid blastocysts (analyzed with PGT-A), were included. Patients who failed to have a clinical pregnancy after transferring three good quality embryos in different single embryo transfers, either own or donated, were diagnosed with RIF. Three groups were formed which depended on the outcome of ERA: 1) Receptive, gene expression profile is compatible with normal receptive endometrium, it was recommended to perform blastocyst(s) transfer following the same endometrial preparation protocol used during the same ERA analysis, 2) Pre-receptive, gene expression profile could indicate a displacement of WOI, it is recommended to delay the transfer of blastocyst(s) with respect to the time when the endometrial biopsy was taken and 3) Early receptive, this gene expression means that the endometrium is at the beginning of the receptive stage, it is recommended to delay the transfer of blastocyst(s) with respect to the time when the endometrial biopsy was taken.
From the first consultation, the medical and nursing areas collected age, weight, height, and BMI. In addition, data such as Recurrent Pregnancy Loss (RPL), Repeated implantation failure (RIF), obesity, Premature Ovarian Insufficiency (POI), endometriosis, hypothyroidism, Natural killer (NK) were also collected from the clinical history, Inherite Thrombophilia’s [IT (MTHFR-C677T and PAI-1 4G>5G, TNF-α G238A, TNFα G308A and LT-α A252G)] homozygous and heterozygous, Insulin Resistance (IR), Frozen Embryo Transfer (FET), implantation rate and clinical pregnancy. In all the alterations found, the patients received treatment.
Endometrial Preparation: In order to carry out the endometrial preparation, hormone substitution was performedwhich has been used in failed transfers prior to ERA- the administration, in general, was as follows: 4 mg of oral estradiol (Primogyn), starting on day 3 of the menstrual cycle, by day 6 it was increased to 6 mg and by day 11 it reached a maximum of 8 mg daily. Transvaginal ultrasound was used in order to evaluate the pattern and thickness of the endometrium approximately 14 days after menstruation and 800 mg progesterone (Geslutin) was administered, when a trilaminar pattern with a thickness between 8 and 14mm was reached. The initial day of progesterone administration was considered “P+0”, and the biopsy was performed after 5 full days of progesterone administration “P+5”.
Endometrial receptivity analysis: All sample were sent to the Igenomix laboratory, there were DNAse treated, and cDNA was obtained by retro- transcription and analyzed by targeted RNA-Seq assay on IonTorrent Next Generation Sequencing, for 248 ERA genes in an Ion S5 system. Sequencing files were used as the input of the ERA predictor (Diaz-Gimeno et al., 2011) to quantify the expression of the ERA genes and to assess the endometrial receptivity status of each sample. Briefly, the reads were mapped to the hg19 human genome transcriptome using the STAR read aligner (Dobin et al., 2013). To count the number of reads that could be assigned to each gene, we used the HTSeq tool (Anders et al., 2015) with the union option. The ERA gene counts were used by the prediction model to classify each sample in an endometrial receptivity class: proliferative, pre-receptive, receptive, or post-receptive [14].
Preimplantation Genetic Testing for Aneuploidies (PGT-A): A trophectoderm biopsy was performed in the assisted reproduction laboratory, which was processed and sent to the Igenomix laboratory, where PGT-A was performed using massive sequencing technology (NGS). The Ion ReproSeqTM PGS kit was used for library preparation and the Ion ChefTM System (Thermo Fisher Scientific, USA) was used for 24-chromosome aneuploidy analysis. Sequencing of the libraries was performed with the Ion S5 System sequencer (Thermo Fisher Scientific, USA). For data analysis, the Ion Reporter software is used, which performs the alignment of the reads with respect to the latest version of the human reference genome (hg19) (Thermo Fisher Scientific, USA) [15]. All patients were informed about the use and handling of the collected data, allowing their inclusion in this study. In addition, their anonymity is maintained, as no reference is made to the origin of the information, therefore, only numerical and statistical data (according to each case) are disclosed.
• Inclusion criteria: euploid blastocyst transfer, patients with idiopathic recurrent implantation failure, women of reproductive age, endometrial thickness ≥7.
• Exclusion criteria: known causes of implantation failure.
Statistical analysis: Patients’ age, weight, height, and BMI are reported with mean ± standard deviation (SD) and the presence of significant difference between groups was evaluated using Student’s T (p≤0.05). For their part, RPL, RIF, obesity, POI, endometriosis, hypothyroidism, NK, IT and IR were expressed as percentages and the difference between groups was evaluated by performing the Chi-squared test (p≤0.05). In both cases, the SPSS statistical package, version 25, was used.
Results
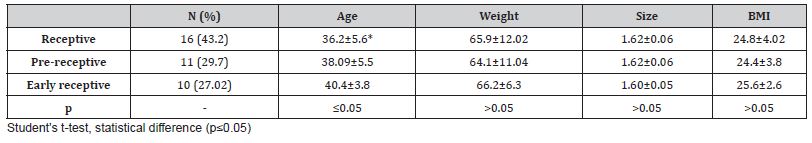
Thirty-seven patients with ERA were included in this study, and the results showed that 16 had a receptive, 11 pre-receptive and 10 early receptive gene profile. When analyzing the anthropometric data, it was observed that only the receptive group presented a statistically significant decrease in maternal age, when compared to the pre-receptive and early receptive groups (36.2±5.6 vs. 38.09±5.5 and 40.4±3.8, p≤0.05) (Table 1).
Table 1: Maternal anthropometric data
Regarding the background of the patients, we have that the receptive group presented a significant increase of NK, compared to pre-receptive and early receptive (61.53 vs. 18.18 and 14.2%, p≤005). In parallel, early receptive (42.8%) showed higher prevalence of TI, followed by receptive (25%) and prereceptive (9.09). In the case of insulin resistance (6.25, 0 and 0%), endometriosis (6.25, 0 and 0%) and obesity (6.25, 0 and 0%), these were only present in the receptive group. In contrast, three groups presented similar prevalence of hypothyroidism (18.75, 18.18 and 14.2%) and POI (12.5, 18.8 and 14.2) (Graph 1).
After performing the first embryo transfer, following the recommendations described in the ERA results sheet, it was found that both “Receptive” (56.3%), “pre-receptive” (63.6%) and “Early receptive” (57.1) achieved an implantation rate that exceeded 50%. After embryo implantation, the evaluation of the clinical pregnancy rate in this first embryo transfer was maintained in “pre-receptive” at 63.6% and decreased non-significantly to 50% in “Receptive” and 42.8% in “Early receptive” (Graph 2). When a second embryo transfer was carried out in patients who did not achieve embryo implantation in the first transfer, taking as a reference the total number of patients included in the first embryo transfer, the implantation rate was 27.2% in “Receptive”, 9.09% in “pre-receptive” and 28.5% in “Early receptive”. These percentages were maintained in “Early receptive” (28.5%) and decreased in “Receptive” (12.5%) and “pre-receptive” (9.09%) (Graph 2). Finally, the evaluation of the cumulative implantation rate (results of first + second embryo transfer), was 83.4% in “receptive”, 72.69% in “prereceptive” and 85% in “Early receptive” (Graph 2). The cumulative clinical pregnancy rate was 62.5% in “Receptive”, 72.69% in “prereceptive” and 71.3% in “Early-receptive” (Graph 2).
Discussion
In Mexico, 17% of women of reproductive age have infertility problems, which is equivalent to 1.4 million couples in need of assisted reproductive techniques [16,17]. One of the main causes of infertility is implantation failure, which has been associated with embryos with chromosomal alterations. Currently in assisted reproduction clinics, studies have been implemented to identify euploid embryos (without chromosomal alterations) and eliminate most of the genetically abnormal embryos to improve implantation rates and, therefore, the clinical pregnancy rate, such is the case of PGT-A, which in its latest versions uses NGS [18]. Despite this, a proportion of euploid embryos fail to implant, even if no structural pathology is identified in the uterus, which is why in recent years ERA is being implemented [19-21]. In this work, “Receptive” the group where patients with the correct WOI and, therefore, receptive endometrial gene expression present high prevalence of patients with NK≥12% in peripheral blood (61.53%), together with the high prevalence of thrombophilia’s (25%) (Graph 1), this could be the cause of implantation failure, as shown by Luján et al., 2022, in a study that included 54 women with RIF in which 66. 6% presented increased NK in peripheral blood (≥12%) compared to 20% of women in the control group [22]. Similarly, Sacks et al., 2012, the study that included 171 women with RIF, report significant increase of pNK concentrations in mid-luteal phase in RIF group compared to control (11.3 vs 8.7%) [23]. Santillan et al., 2015, in 73 patients with RIF found statistical increase in pNK concentration measured during the mid-luteal phase compared to control (13.4 vs 8.4%) [24]. On the other hand, several authors have associated RIF with the tendency to hypercoagulable states, showing that the mechanisms by which thrombophilia’s can generate RIF are the alteration of blood flow which decreases endometrial receptivity. In addition, implantation failure has a multifactorial factor and although in a very low proportion in this study the RIF patients presented obesity, endometriosis, hypothyroidism, and insulin resistance, all of these have been associated with an increase in implantation failure according to several studies (Figure 1) [25-28].
As for “Pre-receptive” and “Early-receptive” present statistical decrease in the prevalence of pNK≥12% compared to “Receptive”, ruling it out as the main cause of RIF in these two groups. Similarly, the prevalence of IT in “Pre-receptive” is too low to be associated as the main cause of RIF in this group. Contrary to the aforementioned, TI in “Early-receptive” presented a prevalence of 42.8 %, probably being one of the main causes of RIF. In the case of hypothyroidism, the 3 groups also showed low prevalence (Graph 1). As can be seen in the previous paragraphs, a higher prevalence of alterations was observed in the history of patients with normal WOI (“Receptive”), which was probably the origin of RIF, and when corrected with personalized treatments, without modifying the endometrial stimulation protocol, a higher implantation rate was achieved: in the first transfer it was 56.2%; and in the second it was 27.2%. Both figures gave a cumulative rate of 83.4% (Graph 2). However, in “Early receptive” a little more than 42.8% presented an alteration associated with RIF (Graph 1). This group shows how important it is to analyze the RE in patients who have a factor associated with RIF identified, because if they had not undergone ERA, the alteration of the WOI would not have been located nor would the application of endometrial stimulation have been corrected and, consequently, there would be a low prevalence of the implantation rate. On the contrary, the ERA in this group of patients, allowed in this study to identify the exact moment to apply endometrial stimulation, thus achieving an implantation rate in the first embryo transfer of 57.1%, in the second of 28.5%. With a cumulative rate of 85.6% (Graph 2). The “pre-receptive” showed at least 18.8% of some alteration associated with RIF and together with the patients who did not present alterations associated with implantation failure (Graph 1), when correcting the endometrial stimulation protocol as recommended by the ERA report, allowed an implantation rate in the first embryo transfer of 63.6%, in the second of 9.09, with a cumulative rate of 72.6% (Graph 2).
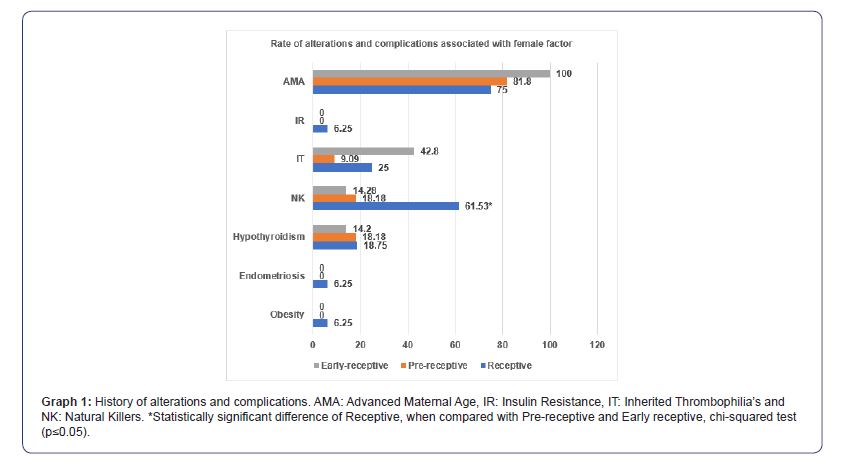
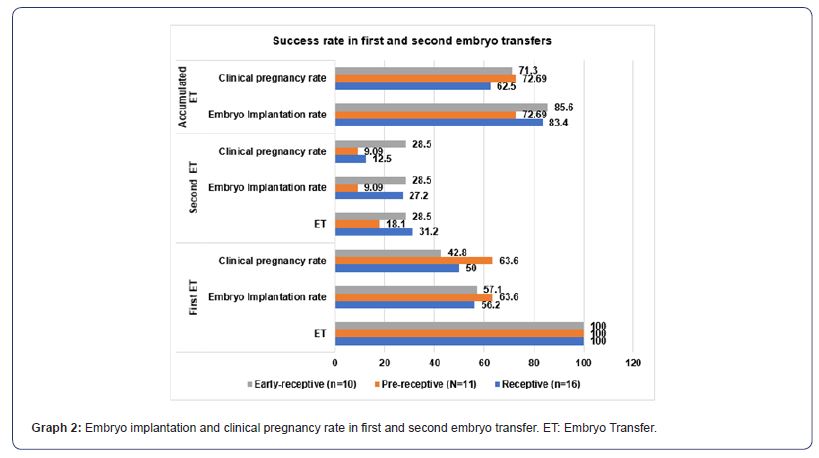
Our results coincide with other studies such as Tan J. et al., 2018, which included 88 patients and found that 44.3% of those who underwent ERA presented displaced WOI (Pre-receptive), achieving after following the recommendations of the ERA report, an implantation rate of up to 73.7% [29]. Similarly, Amin J, et al., 2022, in a group of 219 patients that included embryo transfer from own and donated oocytes, obtained an implantation rate of up to 78% in women with displaced WOI, after correcting endometrial stimulation as recommended in the ERA report [30]. In addition to this, Samadhiya R. et al., 2021 was a study that included 10 women who were non-receptive in ERA, they report an implantation rate of 45.5%, similar to that of the general population undergoing IVF techniques and with normal RE [31]. In addition, in the Pronatal Clinic a prevalence of women with displaced WOI of 56.72% (“pre-receptive” + “Early receptive”) was obtained, higher than that reported in different studies [10, 29]. The importance of this research lies in the fact that it is the first one carried out in a Mexican population. In addition, there is no study that refers to disorders such as obesity, endometriosis, hypothyroidism, pNK, IT and IR, which may be present in patients and which, in many cases, are a factor that influences not to perform ERA, since they are also associated with RIF.
Conclusions
Our experience shows that more than half of the patients with recurrent implantation failure have displaced implantation window and may benefit from a personalized adjustment of the endometrial stimulation protocol.
Patients with disorders such as obesity, endometriosis, hypothyroidism, natural killers ≥12%, inherited thrombophilia’s and insulin resistance, associated with recurrent implantation failure, may also have displaced implantation window, and will probably not achieve pregnancy if they do not undergo endometrial receptivity study. Transfer of euploid embryos to a uterus with a normal window of implantation may result in recurrent implantation failure due to altered natural killer levels in peripheral blood and the presence of inherited thrombophilia’s. We concluded that according to our increased prevalence of implantation failure in our private clinic in México city, we should implement endometrial receptivity assessment as a first line study in our Mexican population.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors declare they have no conflict of interest.
References
- E Margalioth, A Ben Chetrit, M Gal, T Eldar Geva (2006) Investigation and treatment of repeated implantation failure following IVF-ET. Human Reproduction 21(12): 3036-3043.
- M Borges, V Sarno, R Barini (2021) Lymphocyte immunotherapy in recurrent miscarriage and recurrent implantation failure. American Journal of Reproductive Immunology 85(4): e13408.
- H Achache, A Revel (2006) Endometrial receptivity markers, the journey to successful embryo implantation. Human Reproduction Update 12(6): 731-746.
- A Fukui, J Kwak, E Ntrivalas, A Gilman, S Lee et al. (2008) Intracellular cytokine expression of peripheral blood natural killer cell subsets in women with recurrent spontaneous abortions and implantation failures. Fertility and Sterilit 89(1): 157-165.
- E Grandone, D Colaizzo, A Lo Bue, M Checola, E Cittadini et al. (2001) Inherited thrombophilia and in vitro fertilization implantation failure. Fertility and Sterility 76(1): 201-202.
- Simon, N Laufer (2012) Assessment and treatment of repeated implantation failure (RIF). Journal of Assisted Reproduction and Genetics 29(11): 1227-1239.
- Y Shufaro, J Schenker (2011) Implantation Failure, Etiology, Diagnosis and Treatment. International Journal of Infertility and Fetal Medicine 2(1): 1-7.
- W Huang (2020) Chakra’s energy deficiency as the main cause of infertility in women. Obstetrics & Gynecology International Journal 11(2): 83-91.
- M Ruiz, D Blesa, C Simon (2012) The genomic of the human endometrium. Biochimica et Biophysica 1822(12): 1931-1942.
- N Mahajan (2015) Emdometrial receptivity array: clinical application. Journal of Human Reproductive Sciences 8(3): 121-129.
- G Adamson, J de Mouzon, G Cambers, F Zegers, R Mansour, et al. (2018) International committee for monitoring assisted reproductive technology: world report on assisted reproductive technology. Fertility and Sterility 110(6): 1067-1080.
- P Ferreira, P Jares, D Rico, G Gomez, A Martinez, et al. (2014) Transcriptome characterization by RNA sequencing identifies a major molecular and clinical subdivision in chronic lymphocytic leukemia. Genomic Research 24(2): 212-226.
- C Von Grothusen, P Lalitkumar, M Ruiz, N Boggavarapu, R Navarro, et al. (2018) Effect of mifepristone on the transcriptomic signature of endometrial receptivity. Human Reproduction 33(10): 1889-1897.
- C Von Grothusen, P Lalitkumar, M Ruiz, N Boggavarapu, R Navarro, et al. (2018) Effect of mifepristone on the transcriptomic signature of endometrial receptivity. Human Reproduction 33(10): 1889-1897.
- A Coates, A Kung, E Mounts, J Hesla, B Bankowski, et al. (2017) Optimal euploid embryo transfer strategy, fresh versus frozen, after preimplantation genetic screening with next generation sequencing: a randimized controlled trial. Fertility and Sterility 107(3): 723-730.e3.
- T Corona , J Halabe, G Vazquez, G Manjarrez, M Rodriguez (2019) Academia Nacional de Medicina de Mexico. septiembre 2019.
- J Lujan, J Guerrero, B Kava, F Avila, D Avila, et al. (2020) Autologous Mesenchymal Stem Cell Therapy in Patients with Unexplainable Low Ovarian Response: First Case in Mexico. Journal of Medical & Advanced Clinical Case Reports 2(1): 1-4.
- S Ozaltin, H Goksever, O Takmaz, E Yagmur, E Ozbasli, et al. (2019) Is Endometrial Receptivity Assay (ERA) Useful in Patients with Repeated Implantation Failure Undergoing Single, Autologous Euploid Embryo Transfer? Clinical and Experimental Obstetrics and Gynecology 49(9): 1-7.
- D Haouzi, H Dechaud, S Assou, J De Vos, S Hamamah, (2012) Insights into human endometrial receptivity from transcriptomic and proteomic data. Reproductive BioMedicine online 24(1): 23-34.
- H Achache, A Revel, (2006) Endometrial receptivity markers, the journey to successful embryo implantation. Human Reproduction Update 12(6): 731-746.
- S Messaoudi, I EL Kasmi, A Bourdiec, K Crespo, L Bissonnette, et al. (2019) 15 years of transcriptomic analysis on endometrial receptivity: what have we learnt? Fertility Research and Practice 5(9): 1-9.
- J Lujan, C Durand, R Hernandez, F Avila, D Avila, et al. (2022) Prevalence of peripheral blood natural killer’s cells ≥12% in women with recurrent pregnancy loss: study carried out in a private clinic of Mexico city. Obstetrics and Gynecology International Journal 13(2): 92-95.
- G Sack, Y Yang, E Gowen, S Smith, L Fay et al. (2012) Detailed Analysis of Peripheral Blood Natural Killer Cells in Women with Repeated IVF Failure. American Journal of Reproductive Immunology 67(5): 434-42.
- I Santillan, I Lozano, C Illan, V Verdu, S Coca, et al. (2015) Where and when should natural killer cells be tested in women with repeated implantation failure? Journal of Reproductive Immunology 108: 142-148.
- G Chang, J Han, H Seok, D Leen, T Yoon et al. (2013) Insulin resistance does not affect early embryo development but lowers implantation rate in in vitro maturation- in vitro fertilization-embryo transfer cycle. Clinical endocrinology 79(1): 93-99.
- S Moustafa, S Young, (2020) Diagnostic and therapeutic option in recurrent implantation failure. F1000 208: 1-9.
- C Tomassetti, C Meuleman, A Pexters, C Kyama, P Simsa et al. (2006) Endometriosis, recurrent miscarriage, and implantation failure: in there an immunological link? Reproductive BioMedicine Online 13(1): 58-64.
- S Morin, B Ata, E. Seli, (2017) Endocrine causes of imploantation failure. Recurrent Implantation Failure pp. 135-152.
- J Tan, A Kan, J Hitkari, B Taylor, N Tallon, et al. (2018) The role of the endometrial receptivity array (ERA) in patients who have failed euploid embryo transfer. Journal of Assisted Reproduction and Genetics 35(4): 683-692.
- J Amin, R Patel, G Jayes, J Gomedhikam, S Surakala et al. (2022) Perzonalized Embryo Transfer Outcomes in Recurrent Implantation Failure Parients Followings Endometrial Receptivity Array with Pre-Implantation Genetic Testing. Cures 14(6): e26248.
- R Samadhiya, G Prasad, A Singh, P Bhave, (2021) Role of endometrial Receptivity Array in Recurrent Implantation Failure. Fertillity Science and Research pp. 1-5.
-
Luján Irastorza Jesús Estuardo*, Durand Montaño Carlos, Pacheco Pineda Josué Giovani and Hernández Ramos Roberto etc al.. Is Endometrial Receptivity Assessment a First Line Study in Our Mexican Population? A Retrospective Study in Private Clinic. Arch of Repr Med.. Med. 1(3): 2023. ARM.MS.ID.000511.
-
Recurrent implantation failure, Natural killers, ERA, PGT-A, Thrombophilia’s, Endometrial receptivity assessment, Genetic abnormalities, Energy deficiency, Diagnosis, Endometrial receptivity, Obesity
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.