Case Report
Case Report
Prenatal Diagnosis of Congenital Leukemia with Pancreatic Enlargement: Case Report and Literature Review
Dottssa Marianna Silenzi1, Dott. Luca Bonadies2, Dott.ssa Marny Fedrigo3 and Dott.ssa Paola Veronese1*
1Maternal-Fetal Medicine Unit, Department of Women’s and Children’s Health, Padova, Italy
2Neonatal Intensive Care Unit, Department of Women’s and Children’s Health, Padova University Hospital, Padova, Italy
3Cardiovascular Pathology and Pathological Anatomy Unit, Department of Cardiac, Thoracic Vascular Sciences and Public Health, University of Padova, Padova, Italy
Dott.ssa Paola Veronese, Cardiovascular Pathology and Pathological Anatomy Unit, Department of Cardiac, Thoracic, Vascular Sciences and Public Health, University of Padova, Padova, Italy
Received Date: December 14, 2023; Published Date:December 19, 2023
Annotation
Background: Neonatal leukemias are rare, less than 1% of all childhood leukemias. Prenatal findings suggesting potential
abnormalities related to ALL include hepatomegaly, splenomegaly, fetal oedema, polyhydramnios and organ masses, however but
most prenatal tests show no abnormalities. However pancreatic ALL involvement is rare accounting for only few cases in adult and
children post-natally. We describe a extremely rare case report of prenatal leukemia pancreatic involvement
Case presentation: a 37 years-old pregnant woman was referred to our hospital at 35 4/7 ws. Our ultrasound showed
subcutaneous oedema mostly at the abdomen and prefrontal space, mild intraabdominal fluid and a tubular abdominal mass
moderatly vascularized with Color-Doppler. A C-section was performed due to reduced fetal movements. Palpation of the abdomen
revealed a soft, non-tender, middle-abdominal mass and splenomegaly. An urgent abdominal ultrasound showed a diffusely
hypoechoic voluminous mass in the pancreatic space with echoic areas in its context and marked vascularization by mesenteric
vessels, confirmed with a subsequent CT. WBC was 600.000/ mm3 with neutropenia, Hb 5.8 g/dl, PLT 16.000/mm3. Blood tests
showed Di coagulopathy with hypofibrinogenemia. Autoptic examination on the baby confirmed pancreatic leukemia infiltration.
Conclusion: This is the first described case of prenatal diagnosis of congenital leukemia with evidence of pancreatic enlargement
due to leukemic cells infiltration
Case Report
A 37 years-old pregnant woman, gravida 2 para 2, was referred to our hospital by her Gynaecologist for fetal abdominal and pericardial effusion at 35 4/7 weeks. Up to this point her pregnancy was eventful for a diagnosis of gestational diabetes with unremarkable ultrasounds up until the third trimester when the fetal stomach could not be visualized. Fetal growth was at the highest percentiles and amniotic fluid levels were normal. Our ultrasound showed subcutaneous oedema mostly at the abdomen (10 mm) and prefrontal space (8.5 mm), mild intra-abdominal fluid (5.4 mm) and a tubular abdominal mass moderatly vascularized with Color-Doppler with a disomogeneous echogenicity similar to the echogenicity of a clot with no peristaltic movements (Figure1,2). Fetal stomach cound not be visualized and amniotic fluid levels were normal. The ultrasound also showed reduced active fetal movements. Given the initial signs of fetal distress and the accomplished gestational age, it was decided to expedite the delivery via C- section. A female fetus was delivered, with meconium-stained amniotic fluid grade 1. In the delivery room she was ventilated due to bradycardia and apnea and subsequently intubated for the persistence of apnea. Apgar score was 3-6-7 respectively at 1’, 5’ and 10’ minute of life. Cord arterial blood gas showed anemia with 20% haematocrit and normal acid/base status. The birth weight was 2800 gr (84th centile). The baby persisted hypotonic, hyporeactive, pale, presented diffuse subcutaneous oedema and firm violaceous diffuse (face, thorax, limbs) cutaneous lesions (papules and nodules) suspicious for cutaneous metastasis (blueberry muffin baby). Palpation of the abdomen revealed a soft, non-tender, middle-abdominal mass and splenomegaly. She was then transferred to the NICU and underwent an urgent abdominal ultrasound showing: a diffusely hypoechoic voluminous mass (2,2 cm thickness) in the pancreatic space with echoic areas in its context and marked vascularization by mesenteric vessels without vessel compression. Splenomegaly with bipolar lenght of 7,4 cm with heterogeneous echotexture and hypoechoic triangularshaped areas in the subcapsular space. Transfontanellar Cerebral Ultrasound and Echocardiography Showed No Alterations.
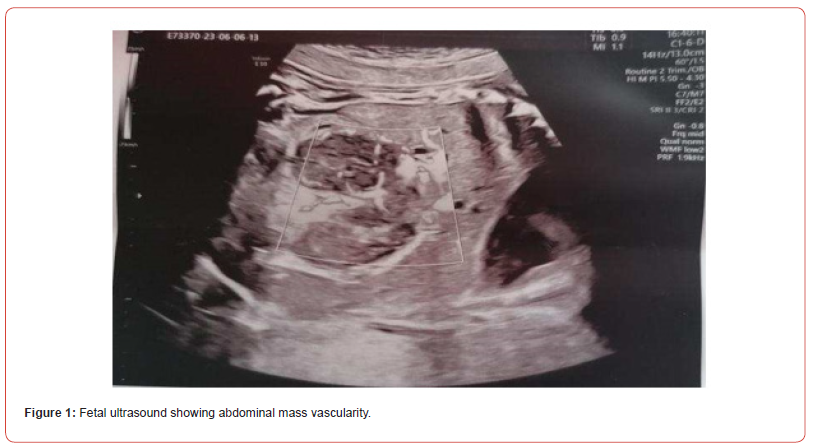
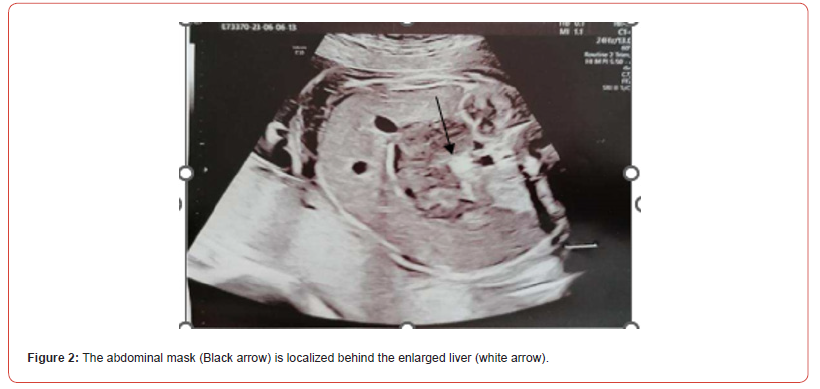
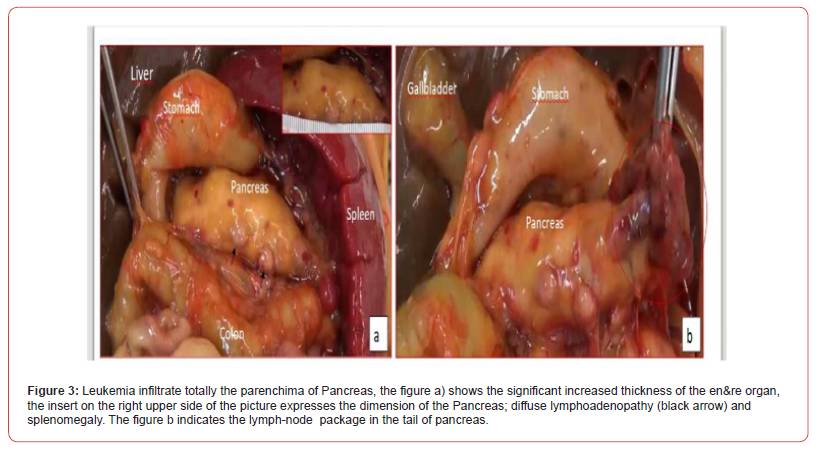
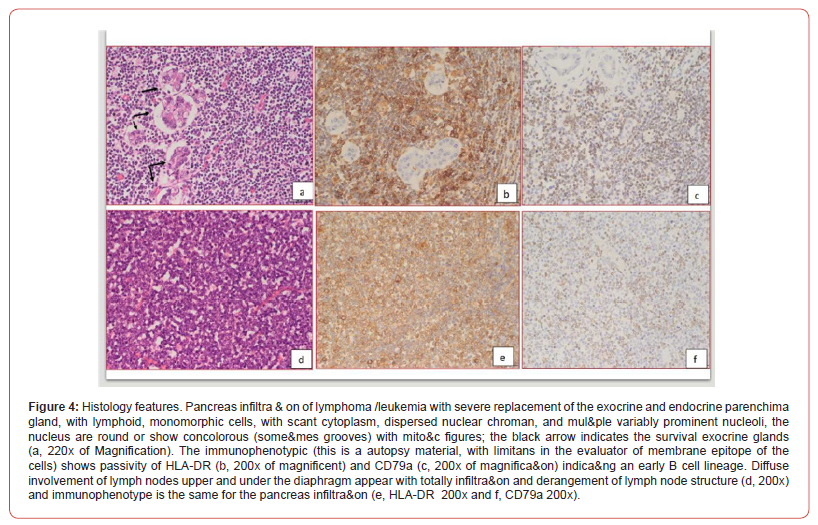
Surgical and hemato oncological consultations were requested for multidisciplinary evaluation and differential diagnosis (leukemia vs neuroblastoma vs vascular pancreatic mass with splenomegaly) and full hematological and biochemical analysis were performed following an umbilical central venous catheter placement. Their results showed WBC 600.000/mm3 with neutropenia, Hb 5.8 g/dl, PLT 16.000/mm3, discoagulopathy with hypofibrinogenemia, normal kidney function. The peripheral blood smear revealed > 90% small-medium blasts with lymphoid aspect; and peripheral blood cytometry showed a 96% of BCPALL blasts suggesting a possible 11q23RR immunophenotype of aprecursor b acute lymphoblastic leukemia. Given the high risk for hyperviscosity, an hyperhydratation regimen was started, and to correct laboratory and clinical parameters the patient received muliple platelets and plasma transfusions, prophylactic antibiotics, diuretic and inotropic therapy and anti-hyperuricemic drugs. A second transfontanellar ultrasound performed for anemization at about 12 hours of life revealed a midline left shift with a hyperechoic right temporo-occipital area and diffuse parenchimal hyperechogenicity. Subsequent cerebral CT and RM confirmed a wide right ischemic parietotemporo-occipital lesion and a subpial hemorrage with uncal herniation. EEG monitoring was started and Phenobarbital was initiated due to the evidence of epileptiform abnormalities. The abdominal CT confirmed the abdominal mass, measuring 40x70x41 mm, with, in its context, necrotic areas. The mass spread the hepatic and splenic arteries apart without compressing them, and was vascularized by the splenic and mesenteric arteries. Liver dimension and morphology were normal, with a mild ectasia of the hepatocholedochus. The spleen was enlarged with an 8.5 cm bipolar axis. Kidneys and adrenal glands showed no alterations. On day 9 of life the infant persisted in critical but stable conditions and given the improvement of the laboratory findings, with decreasing leukocyte count, it was decided to proceed with the hematooncologic treatments which initially consisted of exchange transfusion and steroid cytoreductive therapy. The following day the patient developed fever and rising of inflammatory markers which led to shifting the antibiotic and antifungal therapy. All cultures came back negative On day 16 of life the patient began chemotherapy (vincristine). However, blood tests showed progressive kidney failure and rising of white blood count; parallelly the increasing volume of abdominal mass lead to compartment syndrome and fluid overload. At the same time, abdominal ultrasound showed worsening of renal and bowel perfusion. Consequently, a multidisciplinary meeting involving neonatologists, oncohematologists, nephrologists, anestesiologists and pediatric surgeons with patient’s parents established that given the severity of the disease and its prognosis, summed to the lack of response to therapy, the ongoing treatment was disproportionate compared to its benefits. Palliative therapy was therefore initiated, and the patient died on day 18. The placental histological examination reported lymphoid cells infiltration of chorionic villi, villous stroma and umbelical cord vessels. Autoptic examination on the baby confirmed pancreatic leukemia infiltration (massive lymphoid infiltration with complete pancreatic glandular structure subversion) Figures 3 & 4 nodal and extra-nodal multiorgan leukemia localization (cutis, thyroid, thymus, lungs, heart, gastrointestinal tract mostly duodenum and colon, liver, spleen, kidneys, uterus), gastrointestinal and subarachnoid temporooccipital hemorrage.
Literature Review
Congenital or neonatal leukemia refers to leukemia diagnosed at birth or within the first 28 days of life. Neonatal leukemias are rare, accounting to less than 1% of all childhood leukemias according to retrospective studies and have a reported incidence that ranges from 1 to 5 per million live births. The majority of cases are represented by acute myeloid leukemias while most other cases, notably those of B lineage, are acute lymphoblastic leukemia. A few of them have cytogenetic or molecular anomalies, with t (4;11) (q21.3; q23.3)/KMT2A-AFF1 being the most prevalent, followed by t (1;22) (p13.3; q13.1)/RBM15-MKL1 and t (8;16) (p11.2; p13.3)/KAT6A-CREBBP and are frequently associated with Down Syndrome [1]. Hepatomegaly, splenomegaly and skin lesions (leukemia cutis) are the most common clinical signs of neonatal leukemia. Other possible manifestation are jaundice, ascites and pleural effusions while lymphadenopathy is less frequent [2].
Usually, leukemia cutis manifests as generalized firm violaceous or blue-gray nodules, a clinical feature described as a ‘blueberry muffin rash’. This sign however is not specific for neonatal leukaemia, but it also can be seen occasionally in other malignant conditions such as neuroblastoma, rhabdomyosarcoma or Langerhans cell histiocytosis as well as in congenital infections and in non-malignant conditions associated with extramedullary haemopoiesis (i.e haemolytic anaemias) [3-6]. Around half of cases present central nervous system infiltration with signs of increased intracranial pressure such as bulging fontanelle, papilloedema and retinal haemorrhages. The most frequent haematological feature of neonatal leukaemia is hyperleucocytosis usually associated with anemia and thrombocytopenia. Hyperleucocytosis can cause leucostasis which can lead to respiratory distress with hypoxia and acidosis, neurological symptoms and manifestations (reduced level of consciousness, stroke), cardiac failure and renal failure [7-10]. Prenatal findings suggesting potential abnormalities related to ALL include hepatomegaly, splenomegaly, fetal oedema, polyhydramnios and organ masses, however, most prenatal tests showed no abnormalities [11] and the majority of them were related to Trisomy 21 fetuses. Pancreatic ALL involvement, as in our case, is rare and has only been described in 6 adult [12-17] and 6 pediatric case reports [18-23] to date. In almost all of these cases patients had multiple extramedullary organ involvements and the prognoses were poor in most of them [24-27]. To our knowledge, this is the first described case of prenatal diagnosis of congenital leukemia with evidence of pancreatic enlargement due to leukemic cells infiltration, moreover in a non-Down syndrome fetus.
References
- Irene Roberts, Nicholas J (2018) Fordham, Anupama Rao 4 and Barbara J. Bain, Neonatal leukemia,2018 John Wiley & Sons Ltd British Journal of Hematology 182:170-184
- Roberts I, Fordham NJ, Rao A, Bain BJ (2018) Neonatal leukaemia. Br J Haematol 182(2): 170-184
- Bowden JB, Hebert AA, Rapini RP (1989) Dermal hematopoiesis in neonates: report of five cases. JAm Acad Dermatol 20(6): 1104-1110
- Shown TE, Durfee MF (1970) Blueberry muffin baby: Neonatal neuroblastoma with subcutaneous metastases. J Urol 104(1): 193-195
- Abdel-Latif ME, Sugo E (2010) Images in clinical medicine. Congenital cytomegalovirus infection. NEngl J Med 362(9): 833
- Schlegel (2020) Neonatal Acute Lymphoblastic Leukemia with Translocation Presenting as Blueberry Muffin Baby: Successful Treatment by ALL-BFM Induction Therapy, Allogeneic Stem Cell Transplantation from an Unrelated Donor, and PCR-MRD-Guided Post-Transplant Follow-Up, Am J Case Rep 21: e927153
- J Weis V, DeVito L, Allen D, Linder E (1085) Magenis Translocation X;10 in a case of congenital acute monocytic leukemia Cancer Genet Cytogenet 16(4): 357-364.
- Heikinheimo S, Pakkala E, Juvonen UM (1994) Saarinen Immuno- and cytochemical characterization of congenital leukemia: a case report Med Pediatr Oncol 22(4): 279-282.
- Elspeth C, Ferguson (2005) Polly Talley, Ajay Vora Translocation (6;17) (q23; q11.2): a novel cytogenetic abnormality in congenital acute myeloid leukemia? Cancer Genet Cytogenet 163(1): 71-73.
- Joyce C (2009) A van Dongen, Michiel Dalinghaus, Andre A Kroon, Andrica C H de Vries, Marry Mvan den Heuvel-Eibrink Successful treatment of congenital acute myeloid leukemia (AML-M6) in a premature infant J Pediatr Hematol Oncol 31(11): 853-854.
- Zhang Q, Ren Z, Yang J, Yin A (2019) Analysis of 59 cases of congenital leukemia reported between 2001 and 2016. J. Int. Med. Res 47: 4235-4625
- Daniel SV, Vani DH, Smith AM (2010) Obstructive jaundice due to a pancreatic mass: a rare presentation of acute lymphoblastic leukaemia in an adult. JOP 11: 72-74.
- Pamuk GE (2014) An adult patient with common B-cell acute lymphoblastic leukaemia who presented with pancreatic involvement, description of the second adult case and review of pediatric cases BMJ Case Rep.
- Choi EK, Byun JH, Lee SJ (2007) Imaging findings of leukemic involvement of the pancreaticobiliary system in adults. AJR Am J Roentgenol 188: 1589-1595.
- Yinghao Wang, Xuzhao Zhang, Linping Dong, Kezhong Tang (2019) Acute lymphoblastic leukemia with pancreas involvement in an adult patient mimicking pancreatic tumor: A case report Medicine (Baltimore) 98(23): e15685.
- Xing-Fang Jia, Liang Chen, Nana Wang, Xiao Liu, Hui Yan, et al. (2022) A case of acute lymphoblastic leukaemia disease with pancreatic mass as the first symptom confirmed by elastography combined with EUS-FNA Journal of International Medical Research 50(10): 1-9
- Zhang Cheng, Yunfeng Shan, Xiaoke Ji (2020) Acute Lymphocytic Leukemia with the Initial Presentation as a Pancreatic Mass Pancreas 49(6): e58-e59.
- Sato A, Imaizumi M, Chikaoka S (2005) Acute renal failure due to leukemic cell infiltration followed by relapse at multiple extramedullary sites in a child with acute lymphoblastic leukemia. Leuk Lymphoma 45: 825-828.
- Rausch DR, Norton KI, Glass RB (2002) Infantile leukemia presenting with cholestasis secondary to massive pancreatic infiltration. Pediatr Radiol 32: 360-361.
- Ikawa Y, Saikawa Y, Horisawa T (2007) Pancreatic and renal involvement in pediatric acutelymphoblastic leukemia/lymphoma. J Clin Oncol 25:451-453.
- Malbora B, Avci Z, Alioglu B (2008) A case with mature B-cell acute lymphoblastic leukemiaand pancreatic involvement at the time of diagnosis. J Pediatr Hematol Oncol 30: 87-89.
- Collado L, Dardanelli E, Sierre S (2011) Asymptomatic leukemic cell involvement of the pancreas: US findings. Pediatr Radiol 41: 779-780.
- Benjamin Wolf (2017) Astrid Monecke, Lars-Christian Horn, Ulrich Thome, Holger Stepan, SusanneSchrey-Petersen. Acute lymphoblastic congenital leukemia as a cause of perinatal death following massive cerebral hemorrhage; Case Reports in Perinatal Medicine 20160053
- Macones GA, Johnson A, Tilley D, Wade R, Wapner R (1995) Fetal hepatosplenomegaly associated with transient myeloproliferative disorder in trisomy 21. Fetal Diagn Ther 10: 131-133.
- Gaedicke G, Kleihauer E, Terinde R (1990) Acute non-lymphocytic versus transient leukaemoidreaction in fetuses with Down syndrome. Lancet 335-857
- Zerres K, Schwanitz G, Niesen M, Gembruch U, Hansmann M (1990). Prenatal diagnosisof acute non-lymphoblastic leukemia in Down syndrome. Lancet 335-117
- Robertson G, De Jong (2003) Prenatal diagnosis of congenital leukemia in a fetus at 25 weeks’ gestation with Down syndrome: case report and review of the literature; Ultrasound Obstet Gynecol 21: 486-489.
-
Dottssa Marianna Silenzi, Dott. Luca Bonadies, Dott.ssa Marny Fedrigo and Dott.ssa Paola Veronese*. Prenatal Diagnosis of Congenital Leukemia with Pancreatic Enlargement: Case Report and Literature Review. Arch of Repr Med.. Med. 1(3): 2024. ARM.MS.ID.000515.
-
Pregnant woman; C- section; Neonatal leukemias; leukemia; prophylactic antibiotics; plasma transfusions
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.