Review Article
Review Article
Exclusively Oral Treatment for Osteoarticular Infections in Children. Is It Time?
Rosa María Alcobendas1, Clara Udaondo1,2,4 and Cristina Calvo2,3,4*
1Pediatric Rheumatology Unit, Hospital Universitario La Paz, Madrid, Spain
2Pediatric Infectious Diseases Department, Hospital Universitario La Paz, Fundación IdiPaz, Madrid, Spain
3Translational Research Network in Pediatric Infectious Diseases (RITIP), Madrid, Spain
4CIBER Enfermedades Infecciosas (CIBERINFEC), Madrid, Spain
Cristina Calvo, Pediatric Infectious Diseases Department, University Hospital La Paz, Madrid, Spain.
Received Date: March 13, 2023; Published Date: March 31, 2023
Abstract
Osteoarticular infections (OAI) in children are bacterial infections that affect the bones and/or joints, such as osteomyelitis, septic arthritis, and spondylodiscitis. The conventional treatment approach for OAI consists of a prolonged course of intravenous antibiotics followed by oral therapy. However, there is an ongoing debate regarding the optimal duration of intravenous treatment and the efficacy of oral treatment for OAI.
When establishing the antibiotic strategies, the microbiological diagnosis is a key point. Novel molecular based techniques (multiplex polymerase chain reaction panels) BioFire® Joint Infection Panel can enable early diagnosis and adjusted treatment decisions. There is current evidence of the changing epidemiology of OAI, with Kingella kingae emerging as a common causative agent in young children, while Staphylococcus aureus remains prevalent in other age groups. Kingella kingae infections tend to be milder and have certain differential characteristics.
The minimally invasive approach to OAI consisting of performing arthrocentesis and joint lavage has been shown to be effective and with fewer complications than other approaches such as arthrotomy or arthroscopy.
All this gives rise to considering the possibility of carrying out an exclusively oral treatment and outpatient follow-up in selected cases of children without risk factors. This article reviews the data that support this new approach that is imposed in various infections with the maximum of: “Oral treatment is the new IV”.
Keywords: Osteomyelitis, Oral, Arthrocentesis, Children, Osteoarticular Infections, Treatment, Septic arthritis
Abbreviations:
BCI: Blood Culture Identification
JI: Joint Infection
MRSA: Methicillin-resistant S. aureus
OAI: Osteoarticular Infections
OM: Osteomyelitis
SA: Septic Arthritis
SD: Spondylodiscitis
PSI: Pyogenic sacroiliitis
Introduction
Osteoarticular infection (OAI) is an umbrella term for inflammation usually due to bacterial infection of bone and/or joints. The term OAI includes osteomyelitis (OM), septic arthritis (SA), spondylodiscitis (SD) and pyogenic sacroiliitis (PSI). Acute OAI are defined as the diagnosis within 2 weeks after the onset of clinical manifestations (symptoms or signs) in a previously uninfected location. This type of infection more frequently affects children, especially males (1.5-3:1) under 5 years of age. In developed countries, an annual incidence of 1-4 cases/100,000 children is estimated for septic arthritis and 2-13 cases/100,000 children for osteomyelitis [1,2]. Staphylococcus aureus has been the most prevalent microorganism associated with osteoarticular infections (OAI) in all age groups [3]. However, in children aged 6-48 months, Kingella kingae has emerged as the primary cause of OAI [4]. Compared to other bacteria, particularly S. aureus, K. kingae OAI generally exhibit milder symptoms, lower levels of inflammatory markers, and better outcomes [5]. Children with S. aureus OAI tend to be older, present with fever, and have a marked rise in acute phase reactants levels and white blood cell counts [5, 6]. Methicillin-resistant S. aureus (MRSA) infections are associated with more severe purulent complications, increased probability of secondary procedures, and a higher likelihood of admission to intensive care units [7, 8]. With the advent of modern molecular-based techniques like the BioFire® Joint Infection (JI) Panel, rapid identification of the causative agent of an infection is possible, enabling personalized approaches tailored to the specific microbiological agent involved [9, 10].
There is growing evidence that suggests favorable outcomes in primary hematogenous OAI when treated with a minimally invasive approach, involving stricter surgical indications and shorter courses or even no intravenous therapy. Due to the diverse clinical presentations and the advent of novel microbiological techniques, traditional recommendations must be re-evaluated, and a personalized approach to treatment should be adopted.
Discussion
For decades, children with acute osteoarticular infections (OAI) have been treated with intravenous antibiotics for several weeks before switching to oral therapy. However, the prolonged use of parenteral therapy has led to controversy due to extended hospitalization, higher costs, and potential complications related to central venous access [3]. To address these concerns, some experts propose reducing the duration of intravenous antibiotic therapy to a few days and then transitioning to oral therapy [11-13].
Studies have shown that a shorter duration of intravenous therapy followed by oral antibiotics can be effective in treating pediatric osteoarticular infections (OAI), including those caused by S. aureus. Peltola et al. conducted a prospective, randomized, and controlled study assessing 131 children aged 3 months to 15 years with culture positive OAI [13]. The patients were randomly assigned to receive clindamycin or a first-generation cephalosporin for 20 or 30 days, including an intravenous phase for the first 2 to 4 days. Their conclusion was that most cases of pediatric OAI could be effectively treated for only 20 days with a short initial period of intravenous therapy with large doses of a well-absorbed antimicrobial, including infections caused by S. aureus. Additionally, a recent systematic review and meta-analysis compared the effectiveness of short-course and long-course antibiotics for osteomyelitis in both children and adults, showing similar rates of treatment success. The study found that short-course antibiotics may be just as effective as long-course antibiotics for patients with osteomyelitis, although the results for vertebral osteomyelitis were inconsistent [14].
Beyond reducing intravenous treatment duration, there is growing evidence comparing oral and intravenous antibiotic therapy for blood and bone infections. In early 2022, Wald-Dickler et al. conducted a review of 20 randomized controlled trials that compared oral and intravenous therapy for blood and bone infections [15]. Among the trials, seven focused on osteomyelitis in 1,321 adult patients. The most common monomicrobial organisms found were Staphylococcus aureus and Pseudomonas aeruginosa. The researchers concluded that oral antibiotic therapy was at least as effective as intravenous therapy in treating these infections. Even in fact, they noted that the intravenous groups had higher rates of adverse events and lower patient satisfaction. They suggested considering oral therapy for patients who met specific criteria, such as being clinically and hemodynamically stable, having good oral tolerance, access to oral medication, and no psychosocial or logistical reasons for preferring intravenous therapy, and they coined the term “Oral is the new IV”. Interestingly, similar conclusions and selection criteria for exclusive oral therapy were described in Spanish children in 2018 based on a prospective study conducted exclusively in pediatric patients. The study compared 25 outpatients who received only oral antibiotics with 228 hospitalized children who initially received intravenous treatment. The patients who received oral antibiotics from the time of diagnosis had a good overall condition, adequate oral intake, close monitoring, and the approval of legal guardians. All the outpatients treated with oral antibiotics had a positive outcome without any long-term complications [6].
Recently, the Spanish Network of Osteoarticular Infections published the results of a nationwide multicenter registry. The study compared 893 children who initially received intravenous antibiotics (group 1) with 64 children who received exclusively oral therapy (group 2). Group 2 patients were younger, had a lower percentage of Staphylococcus aureus infections, and a higher proportion of Kingella kingae infections compared to group 1. No complications or long-term sequelae were observed in group 2. The researchers concluded that exclusive oral administration could be a safe option for selected patients with osteoarticular infections, particularly those clinically suggestive of K. kingae as the causative agent and without risk factors for complications. They proposed specific low-risk criteria for selecting patients for this treatment option [16] [Table 1].
Table 1:Proposed criteria for minimally invasive approach (all must be fulfilled)a (16).
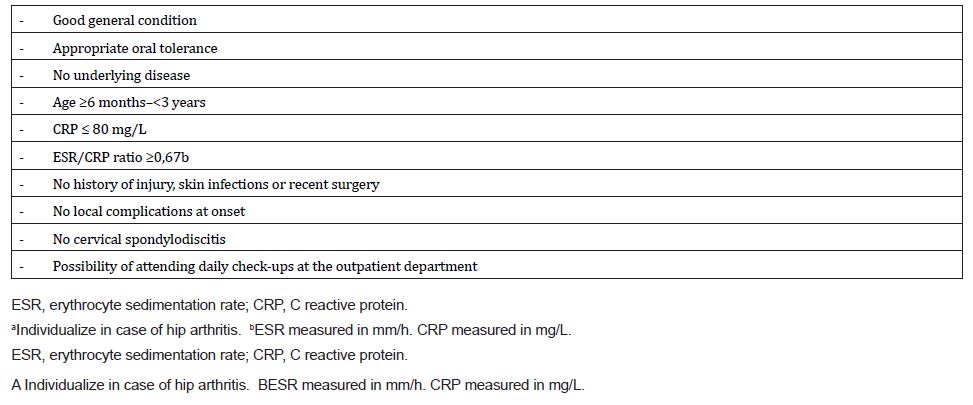
The oral treatment is generally well-tolerated, and instances of non-compliance are typically related to other factors such as poor oral tolerance or vomiting. In addition, oral therapy offers increased patient comfort and reduces the risk of nosocomial infections associated with prolonged intravenous treatment [17].
Molecular-based techniques have been shown to reduce the time required to diagnose infectious diseases, leading to improved patient outcomes. Although these methods have initially been studied in blood samples, Micó et al. evaluated the effectiveness of the BIOFIRE® Blood Culture a blood culture identification (BCID) panel bioMérieux (France) in analyzing clinical specimens other than blood, including cerebrospinal fluid, joint fluid, pleural fluid, ascitic fluid, bronchoscopy samples, and abscesses [9]. The overall sensitivity and specificity of the BIOFIRE® BCID panel were 71% and 97%, respectively. The sensitivity was lower in samples with a low bacterial load, such as ascitic and pleural fluids (25%), but higher in abscess samples (89%).
More recently, a specific panel for the microbiological diagnosis of OAI in the synovial fluid, the BIOFIRE® JI panel, was introduced in the market and there are already few studies showing its utility. For instance, in a multicentre retrospective evaluation of the BIOFIRE® JI panel, testing 399 synovial fluids from adults mostly and a few from children with acute OAI there was an increased diagnostic yield of the panel compared to routine culture [18]. Although they had only few cases of K. kingae and N. gonorrhoae, they mentioned that the ability to detect additional organisms might influence antibiotic choices. This adding to the fact of rapid detection of resistant strains such as methicillin-resistant S. aureus (MRSA), extended spectrum beta-lactamase producers (ESBL), and vancomycin-resistant enterococci (VRE). In this study, they also proposed an algorithm of use of the BIOFIRE® JI panel, targeting adults and children in general for the management of septic arthritis in routine clinical practice.
The results suggest that integrating BIOFIRE® JI panels with routine culture methods could improve our ability to diagnose the microorganisms causing OAIs in clinical practice, facilitating the selection of appropriate antimicrobial agents. However, before incorporating this method into microbiological diagnostic algorithms, it is important to conduct cost-benefit studies to assess its economic feasibility. Nevertheless, its use can enable the determination of the causative agent and identify children with K. kingae infection who can be exclusively treated orally, resulting in cost savings and a reduction in side effects associated with hospitalization.
Additionally, age and acute phase reactants have been studied as factors in septic arthritis. Tornero et al. conducted a retrospective study involving 74 children with septic arthritis in the knee who initially underwent needle joint aspiration. Their findings revealed that additional drainage was unnecessary for patients under one year old and for all patients between one and three years old with a C-reactive protein (CRP) level below 20 mg/L [19]. This observation could possibly be attributed to the presence of K. kingae, a pathogen associated with these age groups. Septic arthritis of small or hard-to-reach joints like the sacroiliac, sternoclavicular, or interphalangeal joints poses challenges when it comes to aspiration, particularly in children. Consequently, in such cases, it may be advisable to consider a trial of medical management using antibiotics as an alternative approach to surgery [16, 20, 21]. In patients from whom a sample from the site of infection cannot be obtained, the detection of K. kingae DNA in the oropharynx could point to the etiology by this agent [22].
In addition to antibiotics, surgery plays a crucial role in the treatment of acute osteomyelitis and septic arthritis in children [23] that we cannot forget. Surgery allows for the collection of biological samples to identify the causative agent and guide the selection and duration of antimicrobial therapy. For acute osteomyelitis, surgery can help remove necrotic bone tissue, clean the surrounding soft tissues, and reduce bacterial load [24]. While most cases of hematogenous osteomyelitis can be cured with antibiotic therapy alone, surgical intervention may be necessary in cases where patients do not respond to antibiotics or when complications are suspected. Surgery for spondylodiscitis is typically reserved for cases involving vertebral instability, neurological symptoms, or failure of conservative treatment [25-27].
In cases of acute septic arthritis, joint drainage is crucial to reduce the risk of complications such as bone avascular necrosis and permanent cartilage damage caused by elevated intra-articular pressure [28]. The European Society for Pediatric Infectious Diseases (ESPID) provides guidelines for the treatment of septic arthritis in children, which recommend joint drainage by aspiration (arthrocentesis), arthroscopy, or arthrotomy followed by intravenous administration of antibiotics [1]. However, there is limited literature available regarding the most effective drainage technique for children with septic arthritis.
Spans and Donders recently conducted three systematic reviews comprising retrospective and prospective studies, focusing on drainage techniques for septic arthritis in the hip, knee, and shoulder joints in children [29-31]. These joints are particularly significant in the pediatric population, as the hip and knee are commonly affected in cases of septic arthritis, and the hip and shoulder joints are of particular interest due to the potential risk of avascular necrosis. Overall, these systematic reviews indicate that both aspiration and arthrotomy can lead to positive clinical outcomes in the management of septic arthritis. It is worth mentioning that the time elapsed between the onset of symptoms and the initiation of treatment can potentially serve as a predictor of clinical and radiological outcomes. Specifically, studies have shown that delayed treatment is associated with poorer outcomes [32-35].
Therefore, and to be able to establish an exclusively oral treatment, we must ensure that we have the availability to perform an arthrocentesis before the patient is sent home, with joint lavage, if necessary, in the case of septic arthritis. And a capacity to monitor patients in 24-48 hours on an outpatient clinic, to confirm that they are evolving favorably and that they do not require a new evacuation and washing or surgery. Oral tolerance must be guaranteed. Parents must be reliable enough, and available to go to the hospital for review. A confirmed or highly suspected Kingella kingae infection is highly recommended. Ultimately, treatment must be individualized and closely controlled. But if all these requirements are met, we can offer a much less harmful treatment for our children, suitable for families and cost effective for the health system.
Despite all the above, further studies are needed to better understand this treatment approach. Currently, two multicenter clinical trials called CHILD@HOME_BJI (Oral only antibiotics for Bone and Joint Infections in Children) and BEST (Bone and Joint Infections—Simplifying Treatment in Children Trial) are underway, and their results may provide additional insights into this matter.
Conclusion
In summary, this paper provides a current perspective on the approach and treatment of pediatric patients with primary hematogenous OAI. Recent research suggests that, in selected patients, an individualized and minimally invasive approach and an exclusively oral treatment can be a safe and effective option. BCID panels can enable early and accurate diagnosis of the causative pathogens in OAIs using specimens such as joint fluid and bone tissue, facilitating a more individualized therapeutic approach. However, further research is needed to refine treatment strategies based on the specific causative agent.
Acknowledgement
To Isabel Mellado for defending this opinion in a debate at the ESPID (European Society of Pediatric Infectious Diseases) meeting (2023).
Conflict of Interest
The authors declare no conflict of interest.
References
- Saavedra-Lozano J, Falup-Pecurariu O, Faust SN, Girschick H, Hartwig N, et al. (2017) Bone and joint infections. Pediatr Infect Dis J 36: 788-799.
- Saavedra-Lozano J, Calvo C, Huguet Carol R, Rodrigo C, Núñez E, et al. (2015) SEIP-SERPE-SEOP consensus document on a etiopathogenesis and diagnosis of uncomplicated acute osteomyelitis and septic arthritis. An Pediatr (Barc) 82(4): 273.e1–273.e10.
- Castellazzi L, Mantero M, Esposito S (2016) Update on the management of pediatric acute osteomyelitis and septic arthritis. Int J Mol Sci 17(6): 855.
- Yagupsky P (2022) Kingella kingae reveals its secrets. Microorganisms 10(7): 1261.
- Gouveia C, Subtil A, Norte S, Arcangelo J, Santos MA, et al. (2022) Distinguishing Kingella kingae from pyogenic acute septic arthritis in young portuguese children. Microorganisms 10(6): 1233.
- Alcobendas R, Remesal A, Murias S, Nuñez E, Calvo C (2018) Outpatients with acute osteoarticular infections had favourable outcomes when they received just oral antibiotics without intravenous antibiotics. Acta Paediatr 107(10):1792-1797.
- Jain MJ, Bradko V, Zhu H, Inneh I, Shinava VR (2021) Pediatric osteoarticular infection: trend in surgically treated patients and association of methicillinresistant Staphylococcus aureus with requirement of secondary procedures. J Pediatr Orthop B 30(6): 579-584.
- Kaushik A, Kest H (2018) Pediatric methicillin-resistant Staphylococcus aureus osteoarticular infections. Microorganisms 6(2): 40.
- Micó M, Navarro F, De Miniac D, Yésica González, Albert Brell, et al. (2015) Efficacy of the FilmArray blood culture identification panel for direct molecular diagnosis of infectious diseases from samples other than blood. J Med Microbiol 64(12): 1481-1488.
- Hirai J, Mori N, Sakanashi D, Yusuke Morishita, Yuji Kuge, et al. (2023) Usefulness of the Film Array blood culture identification panel for identifying causative pathogens of bone and joint infections. J Infect Chemother 29(7): 722-725.
- Jagodzinski NA, Kanwar R, Graham K, Bache CE (2009) Prospective evaluation of a shortened regimen of treatment for acute osteomyelitis and septic arthritis in children. Pediatr Orthop 29(5): 518-525.
- Jaberi FM, Shahcheraghi GH, Ahadzadeh M (2002) Short-term intravenous antibiotic treatment of acute hematogenous bone and joint infection in children: a prospective randomized trial. J Pediatr Orthop) 22(3): 317-320.
- Peltola H, Pääkkönen M, Kallio P, Kallio MJ (2010) Osteomyelitis-septic arthritis study group. Short- versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture-positive cases. Pediatr Infect Dis J 29(12): 1123-1128.
- Huang CY, Hsieh RW, Yen HT, Hsu TC, Chen CY, et al. (2019) Short versus long-course antibiotics in osteomyelitis: a systematic review and meta-analysis. Int J Antimicrob Agents 53(3): 246-260.
- Wald-Dickler N, Holtom P, Phillips MC, Centor RM, Lee RA, et al. (2022) Oral is the new IV-challenging decades of blood and bone infection dogma: a systematic review. Am J Med 135(3):369-379.e1.
- Alcobendas Rueda RM, Núñez E, Martín L, Hernández MB, Saavedra-Lozano J, et al. (2022) Oral versus intravenous antibiotics for pediatric osteoarticular infection: when and to whom? Pediatr Infect Dis J. (2022) 41(9): e351-e357.
- Tetzlaff TR, Mc Cracken GH, Nelson JD (1978) Oral antibiotic therapy for skeletal infections of children. J Pediatr 92: 485-490.
- Tornero E, Josep Maria De Bergua-Domingo, Pedro Domenech, Francisco Soldado, Ferran Torner, et al. (2019) Knee arthritis in children: when can it be safely treated with needle joint aspiration? A large children’s tertiary hospital study. J Pediatr Orthop 39(3): 130-135.
- Alhariri S, Kalas MA, Hassan M, Carter JT, Ghafouri SR, et al. (2022) Medical management of septic arthritis of the sternoclavicular joint with extended spectrum beta-lactamase-producing Escherichia coli: a case report. Cereus 14(4): e23969.
- Kwon HY, Cha B, Im JH, Baek JH, Lee JS (2020) Medical management of septic arthritis of sternoclavicular joint: a case report. Medicine (Baltimore) 99(44): e22938.
- Ceroni D, Belaieff W, Kanavaki A, Della Llana RA, Lascombes P, et al. (2013) Possible association of Kingella kingae with infantile spondylodiscitis. Pediatr Infect Dis J 32(11):1296-1298.
- Pääkkönen M, Peltola H (2011) Simplifying the treatment of acute bacterial bone and joint infections in children. Expert Rev Anti Infect Ther 9: 1125-1131.
- Woods CR, Bradley JS, Chatterje A, Coppley LA, Robinson J, et al. (2021) Clinical practice guideline by the pediatric infectious diseases society and the infectious diseases society of America: guideline on diagnosis and management of acute hematogenous osteomyelitis in pediatrics. J Pediatric Infect Dis Soc 10(8): 801-844.
- Roversi M, Mirra G, Musolino A, Barbuti D, Lancella L, et al. (2021) Spondylodiscitis in children: a retrospective study and comparison with non-vertebral osteomyelitis. Front Pediatr 9:727031.
- Skaf GS, Domloj NT, Fehlings MG, Bouclaous CH, Sabbagh AS, et al. (2010) Pyogenic spondylodiscitis: an overview. J Infect Public Health 3(1): 5-16.
- De Moraes Barros Fucs PM, Robert M, Yamada HH (2012) Spinal infections in children: a review. Int Orthop 36(2): 387-95.
- Pääkkönen M, Peltola H (2012) Management of a child with suspected acute septic arthritis. Arch Dis Child 97(3): 287-292.
- Spaans AJ, Donders CM, Bessems JHJM, van Bergen CJA (2021) Aspiration or arthrotomy for pediatric septic arthritis of the shoulder and elbow: a systematic review. EFORT Open Rev 6(8):651-657.
- Donders CM, Spaans AJ, Bessems JHJM, van Bergen CJA (2021) Arthrocentesis, arthroscopy or arthrotomy for septic knee arthritis in children: a systematic review. J Child Orthop 15(1): 48-54.
- Donders CM, Spaans AJ, Bessems JHJM (2022) A systematic review of the optimal drainage technique for septic hip arthritis in children. Hip Int 32(5): 685-693.
- Umer M, Hashmi P, Ahmad T, Ahmed M, Umer M (2003) Septic arthritis of the hip in children–aga khan university hospital experience in Pakistan. J Pak Med Assoc 53(10): 472-478.
- Rutz E, Spoerri M (2013) Septic arthritis of the pediatric hip-a review of current diagnostic approaches and therapeutic concepts. Acta Orthop Belg 79(2): 123-134.
- Bos CF, Mol LJ, Obermann WR, Tjin a Ton ER (1998) Late sequelae of neonatal septic arthritis of the shoulder. J Bone Joint Surg Br 80: 645-650.
- Ernat J, Riccio AI, Fitzpatrick K, Jo C, Wimberly RL (2017) Osteomyelitis is commonly associated with septic arthritis of the shoulder in children. J Pediatr Orthop 37(8): 547-552.
-
Rosa María Alcobendas, Clara Udaondo and Cristina Calvo*. Exclusively Oral Treatment for Osteoarticular Infections in Children. Is It Time?. Arch Rheum & Arthritis Res. 2(5): 2023. ARAR.MS.ID.000548.
-
Osteoarticular infections, Oral treatment, Septic arthritis, Antibiotic strategies, Microbiological diagnosis, Blood Culture Identification (BCI), Staphylococcus aureus, Pyogenic Sacroiliitis (PSI), Pediatrics
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.