Case Report
Case Report
Atypical Griscelli Syndrome Presenting with Immune Dysregulation, Systemic Granulomatosis and Normal Pigment Secondary to A Structural Variant in RAB27A
Gunderman L1*, Wlodaver A1, Ochfeld E2, Klein Gitelman M1, Klein Gitelman M1, Yap K1 and Khojah A1
1Department of Allergy and Immunology, Ann and Robert H Lurie Children’s Hospital of Chicago, USA
2Department of Allergy and Immunology, Children’s Hospital of Philadelphia, USA/p>
Gunderman Lauren M, Department of Allergy and Immunology, Ann and Robert H Lurie Children’s Hospital of Chicago, USA.
Received Date: January 27, 2022; Published Date: February 15, 2022
Introduction
Griscelli Syndrome Type 2 (GS2) is a rare autosomal recessive disease characterized by organ granulomas, central nervous system inflammation, hemophagocytic lymphohistiocytosis (HLH) and partial albinism. Patients have immune dysregulation secondary to dysfunctions in natural killer (NK) cell and T cell cytotoxicity as a result of poor vesicular transport [1-2]. Typically, patients also have abnormal skin and hair pigmentation from accumulation of melanosome clumps in hair shafts and melanocytes. Homozygous or compound heterozygous pathogenic variants in RAB27A are associated with GS2. However, patients with atypical GS2 harbor a structural rearrangement in RAB27A not detected by typical genetic sequencing [3]. We present two cases of atypical GS2 (below and in supplement) characterized by systemic granulomatosis, neuroinflammation and normal skin and hair pigment, secondary to a structural variant (SV) in RAB27A and a second pathogenic sequence variant on the other allele of the gene.
Patient Case
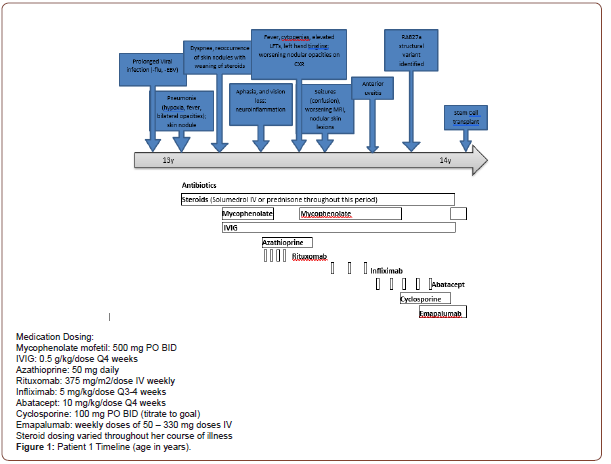
Our patient (patient 1) is a previously healthy Caucasian female of distant Lithuanian descent who presented at age 13 with pneumonia requiring IV antibiotics and supplemental oxygen. Despite antibiotics, her respiratory status worsened. She was unable to wean from oxygen until the addition of high-dose corticosteroids. Extensive infectious workup was negative. Immunology workup revealed mild hypogammaglobulinemia, persistent lymphopenia and mild neutropenia. Biopsy of a skin nodule showed non-specific mild perivascular and interstitial dermatitis. Bone Marrow biopsy showed a hypo-cellular marrow with progressive multi-lineage hematopoiesis, but no evidence of leukemia. Nodular opacities were visualized on Chest CT. Of interest, the lung biopsy portrayed intra-alveolar macrophages and extensive non-necrotizing intraalveolar granulomas, concerning for interstitial lung disease (ILD).
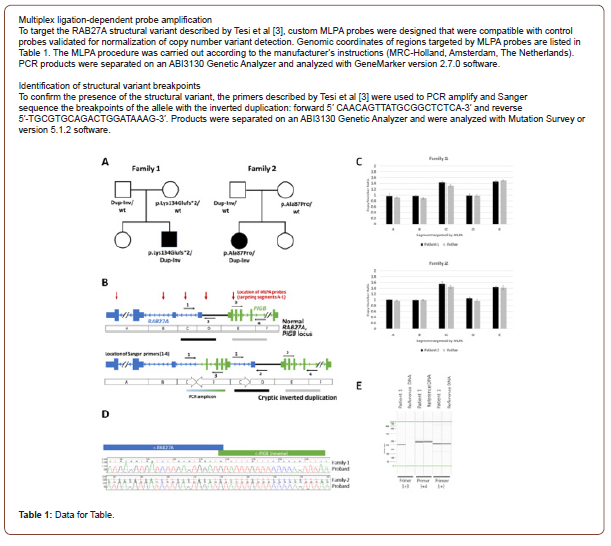
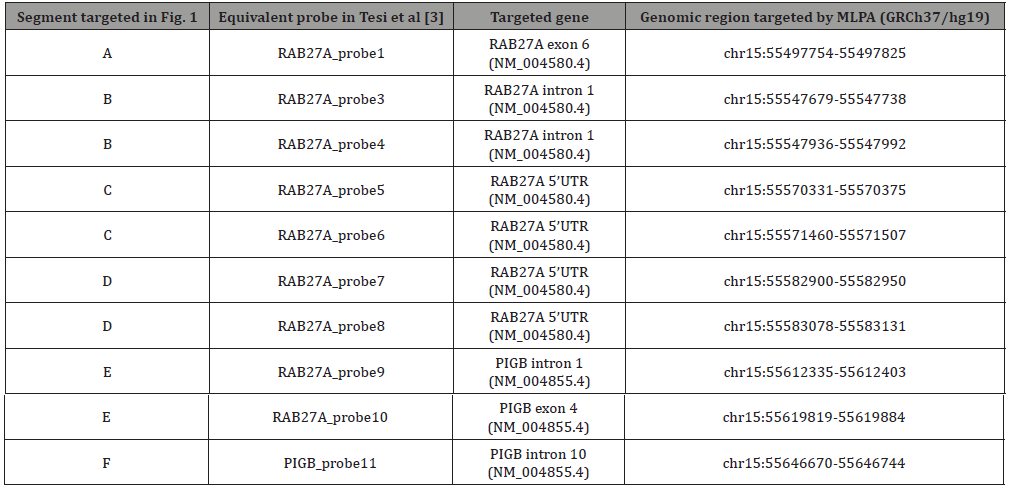
After discharge, she re-presented with dyspnea despite continued steroids. Intravenous Immunoglobulin (IVIG) and mycophenolate (a steroid-sparing agent) were started for suspected GLILD (granulomatous lymphocytic interstitial lung disease) and steroids were decreased. However, attempts to further wean steroids led to reoccurrence of hypoxia and skin nodules. The disease course was further complicated by an episode of aphasia, unilateral loss of peripheral vision and severe headache. Brain MRI revealed numerous subcortical, cerebral white matter and basal ganglia lesions concerning for an inflammatory or demyelinating process. Due to concerns for relapse, Mycophenolate was switched to Azathioprine and Rituximab, standard treatment for GLILD. Around this time, singleton WES resulted in multiple variants of uncertain significance (VUS), including a heterozygous likely pathogenic variant in RAB27A (c.259G>C, p. Ala87Pro). She was not diagnosed with Griscelli Syndrome because of the normal skin pigmentation and lack of a second pathogenic variant in the RAB27A gene. and Mycophenolate was restarted for possible better disease control. Yet after 4 weeks on Mycophenolate, episodes of confusion developed and nodular skin lesions worsened. The confusion was attributed to seizures based on Brain MRI results showing acute diffusion restriction in the left temporal lobe and insula, and she began antiepileptic therapy. In the following 6 months, the patient developed asymptomatic anterior uveitis of the eye, retinal granulomas and vasculitis. Prolonged steroid use caused significant side effects including insulin resistance, short stature, central obesity and mild compression fractures. She switched from Rituximab to Infliximab to Abatacept for immune suppression; all failed to gain disease control. Seizures worsened with attempts to wean steroids. Labs became significant for low CD107a (NK cell degranulation) and elevated serum neopterin (62 nmol/L, Reference range: <10.0 nmol/L), despite the normal soluble IL-2 receptor. Therefore, evaluation for a RAB27A SV was conducted via a multiplex ligation-dependent probe amplification (MLPA) assay using 10 probes in coding and non-coding regions of RAB27A and the nearby PIGB gene (Table 1). A complex duplicationinversion SV was found in the 5’ untranslated region (5’UTR) of RAB27A and confirmed by Sanger sequencing. The SV was classified as pathogenic. This, in conjunction with the likely pathogenic RAB27A variant found on WES, resulted in the diagnosis of atypical GS2. Parental genetic testing revealed the variants were inherited in trans. After diagnosis, she was switched from Mycophenolate to Cyclosporine for immune modulation, and Abatacept was discontinued after a 3-month trial. Despite changes in therapy, the neuroinflammation worsened and neopterin levels (76.3 nmol/L) were found elevated in the CSF. Given this progression, she started anti-interferon gamma therapy (Emapalumab; a cytokine therapy) as a bridge to stem cell transplant to reduce inflammation and control disease progression. While on this medication, the CSF neopterin levels declined and repeat MRI was stable. Stem cell transplant was completed. Unfortunately, the patient (Patient 2) did not survive complications from the post-transplant period (Figure 1).
Discussion
The RAB27A gene encodes the Rab27a protein and is important for vesicle transport and docking in a variety of cell types (platelets, leukocytes and melanocytes). Variants in RAB27A lead to GS2, resulting in an immune dysregulatory phenotype with increased susceptibility to infection, cytopenias, HLH, neurologic involvement and complete to partial albinism [2]. Lack of pigment and immunodeficiency in GS2 are linked as both result from a dysfunction in secretory vesicles, a component essential for the proper functioning of cytotoxic T lymphocytes, natural killer cells and melanocytes.
Unlike the variants in RAB27A associated with typical GS2, RAB27A structural variants associated with atypical GS2 are not easily detected. The SV found in the 5’ untranslated region (5’UTR) of RAB27A in our patients was previously reported by Tesi et al., in 5 children from 5 unrelated families of the Baltic Sea population who had characteristic findings of GS2 (immune dysregulation, including neuroinflammation, skin granulomas, and late-onset HLH) but the atypical finding of normal skin and hair pigment [3]. In these families, heterozygous carriers of the SV were not affected by disease, though those homozygous or compound heterozygous for the SV and a separate pathogenic RAB27A variant were affected by atypical GS2 [4]. Expression studies in a homozygous carrier of the SV, using RNA from peripheral blood mononuclear cells, provided evidence that the inverted duplication interrupts the transcription start site of the longest RAB27A transcript (NM_183235.2) [5]. This transcript is the predominant RAB27A isoform in lymphocytes but is expressed to a lesser extent in melanocytes, according to FANTOM CAGE data and expression studies in melanocytes from control individuals [6].
The custom designed targeted MLPA assay used to detect the SV in RAB27A was key to the diagnosis in these patients (Table 1). Typical next-generation sequencing (NGS) based assays are unable to detect the SV as non-coding regions of the genes are not routinely sequenced in panel testing or WES studies. Without the MLPA analysis, it would have been challenging to recognize that these patients had atypical GS2, due to lack of pigment abnormalities and also differences in age of presentation and variability in HLH as a predominant feature. Patients who present with skin granulomas, neurologic involvement, concern for HLH and ancestry in the Baltic Sea region should be evaluated for structural RAB27A variants, even with the absence of pigment abnormalities. If a RAB27A variant is found on NGS and phenotypic features of GS2 are present, a MLPA assay is necessary to evaluate for this SV. This case outlines the importance of early diagnosis in atypical GS2, reiterates the usefulness of targeted genetic testing for early diagnosis and offers a playbook of therapeutic options, including both the success and failures of targeted therapies.
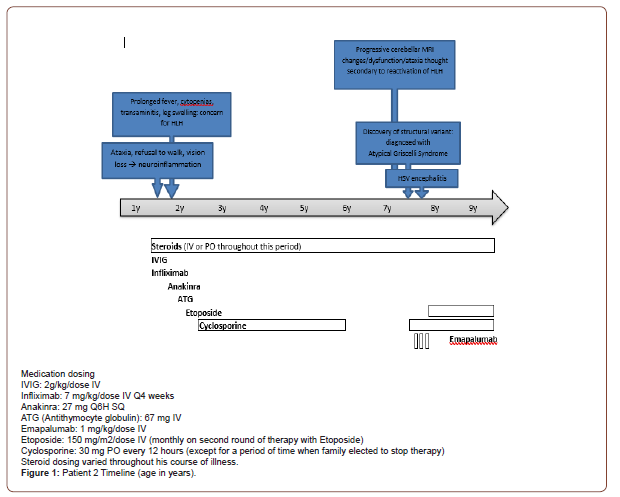
Table 1:MLPA probes used in this study.
Conflict of Interest
None.
Acknowledgements
References
- Ménasché G, Pastural E, Feldmann J, Certain S, Ersoy F, et al. (2000) Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet 25(2): 173-176.
- Zamani R, Shahkarami S, Rezaei N (2021) Primary immunodeficiency associated with hypopigmentation: A differential diagnosis approach. Allergologia et immunopathologia 49(2): 178-190.
- Tesi B, Rascon J, Chiang S, Burnyte B, Löfstedt A, et al. (2018) A RAB27A 5' untranslated region structural variant associated with late onset hemophagocytic lymphohistiocytosis and normal pigmentation. The Journal of allergy and clinical immunology 142(1): 317-321.e8.
- Bahadoran P, Aberdam E, Mantoux F, Buscà R, Bille K, et al. (2001) Rab27a: A key to melanosome transport in human melanocytes. The Journal of cell biology 152(4): 843-850.
- Valentina Cetica, Yvonne Hackmann, Samantha Grieve, Elena Sieni, Benedetta Ciambotti, et al. (2015) Patients with Griscelli syndrome and normal pigmentation identify RAB27A mutations that selectively disrupt MUNC13-4 binding. The Journal of allergy and clinical immunology 135(5): 1310-1318.e1.
- Ohishi Y, Ammann S, Ziaee V, Strege K, Groß, et al. (2020) Griscelli Syndrome Type 2 Sine Albinism: Unraveling Differential RAB27A Effector Engagement. Frontiers in immunology 11: 612977.
-
Gunderman L, Wlodaver A, Ochfeld E, Klein Gitelman M, Klein Gitelman M, Yap K, Khojah A. Atypical Griscelli Syndrome Presenting with Immune Dysregulation, Systemic Granulomatosis and Normal Pigment Secondary to A Structural Variant in RAB27A. Arch Rheum & Arthritis Res. 2(1): 2021. ARAR.MS.ID.000530.
-
Griscelli Syndrome Type 2 (GS2), Hemophagocytic Lymphohistiocytosis (HLH), Intravenous Immunoglobulin (IVIG), RAB27A gene, Cytopenia’s, interstitial lung disease (ILD).
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.