Research Article
Research Article
Firmicutes: Bacteroidetes Ratio Correlate Negatively with Treg: Th1 Ratio in Rheumatoid Arthritis Patients
Perdana Aditya Rahman1*, Handono Kalim1, C Singgih Wahono1, Bagus Putu Putra Suryana1, Bogi Pratomo2, Arief Nurudhin3, Indra Kasman4, Duma Aulia Sianturi4, Fiqih Faruz Ramadhan4, Tri Wahyudi Iman Dantara4 and Muhammad Fahmi Rizqi Syaban5
1Rheumatology-Immunology Division, Department of Internal Medicine, Faculty of Medicine, Universitas Brawijaya, Indonesia
2Gastroenterohepatology Division, Department of Internal Medicine, Faculty of Medicine, Universitas Brawijaya, Indonesia
3Rheumatology Division, Department of Internal Medicine, Faculty of Medicine, Universitas Sebelas Maret, Solo, Central Java, Indonesia
4Department of Internal Medicine, Faculty of Medicine, Universitas Brawijaya – RSUD dr. Saiful Anwar Malang, Indonesia
5Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia
Perdana Aditya Rahman, Rheumatology Division Department of Internal Medicine, Faculty of Medicine Universitas Brawijaya, Malang, Indonesia
Received Date:November 11, 2023; Published Date:February 08, 2024
Abstract
Backgrounds: In the initiation of rheumatoid arthritis [RA], Prevotella and Firmicutes were increased and Bacteroidetes was decreased, yet
Prevotella also correlate with CRP. However, studies showing inconsistency regarding microbiome composition. Currently, study regarding dysbiosis
and RA disease activity are lacking.
Aims: To assess difference of Firmicutes/Bacteroidetes ratio and Prevotella count in RA patients between remission-low and moderate-high
disease activity, and the correlation with DAS-28 ESR.
Subjects and Methods: Sampling was performed consecutively including 6 subjects for each disease activity group. Study performed using
case-control design. Microbiome examination performed using 16S rRNA sequencing targeting V3-V4 region. Flowcytometry performed to identify
Th1 and Treg cells. Statistical analysis for independent t-test and correlation performed using SPSS.
Results and Discussions: There are no significant difference on Firmicutes:Bacteroidetes ratio, Prevotella count, Th1 and Treg counts between
remission-low disease activity and moderate-high disease activity, with p value 0.663, 0.379, 0.744, 0.976 respectively. There is no correlation
between Firmicutes: Bacteroidetes and Prevotella counts with DAS-28 ESR, with r=0.263 [p=0.409] and 0.291 [p=0.358]. Weak correlation between
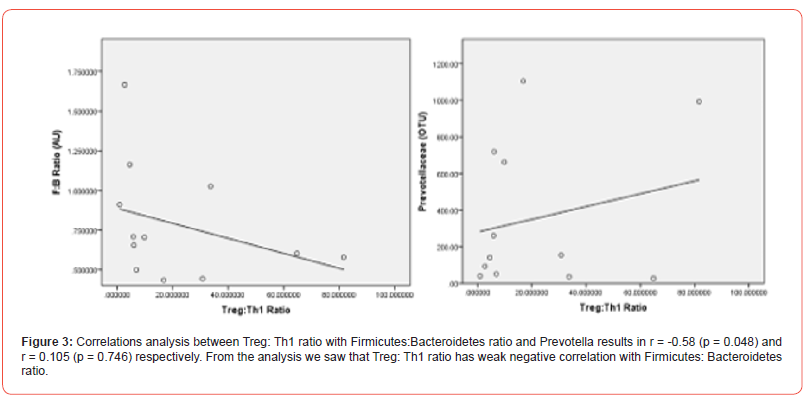
Treg/Th1 ratio and Firmicutes/Bacteroidetes ratio [r=-0.58, p=0.048] obtained, there is no correlation between Treg/Th1 ratio with Prevotella
counts [r=0.105, p=0.746]. There is propensity that Bacteroidetes and Prevotella are more abundant in remission-low disease group. Bacteroidetes
suggested producing propionate and butyrate that suppress inflammation. This preliminary study should be followed by more subjects and including
cellular function and cytokine assays.
Conclusion: This study does not result in significant difference, but there is tendency that Bacteroidetes and Prevotella are more abundant in
remission-low disease group.
Keywords:Firmicutes/Bacteroidetes ratio; Prevotella; Treg/Th1 ratio; DAS-28 ESR
Introduction
Interaction between gut microbiome has been a recent interest in the pathogenesis study of human diseases. Rheumatoid arthritis is one of the diseases that exhibit the interaction with microbes, Porphyromonas gingivalis in oral cavity, produce peptidylarginine deiminase-4 [PADI-4] that able to citrullinate arginine and results in autoantigen formation. Gut microbiome also altered inflammatory response through other mechanism; [a] modulation of immune response through PRR; [b] alteration of cytokine productions and functions; [c] molecular mimicry; and [d] bystander activation [1- 6].
Presence of gut microbiota also results in production of shortchain fatty acid [SCFA] such as propionate, butryate, and acetate, thus altered microbiota composition will results in alteration of SCFA composition. Other microbial products, such as toxin, could also altered mucosal immune response [3,4,7]. Several studies have shown some pattern of microbiome alteration in the predevelopmental stage and clinical course of rheumatoid arthritis, also in relation to DMARD treatment responses [6]. The interaction between microbiome composition and clinical aspects of rheumatoid arthritis remains unsolved, studies mostly performed in animal models. Animal studies mostly performed at pre-clinical stage, where dysbiosis become a risk factor for development of clinical manifestation after arthritogenic substance given to animal models. While human studies on microbiome is another challenge, many factors affecting gut microbiome composition, age, dietary composition, environmental, intestinal pathology, and drugs.
Analysis of microbiome, currently not used in clinical settings, despite the high cost, currently no clinical significance observed. However, several commercial examinations to identify SCFA composition are available. SCFA probably recognized as the most important pathway in dysbiosis mechanism, yet there is another pathway for dysbiosis to alter inflammation process. Identification of microbiome composition, in most of research settings are using 16s rRNA Next Generation Sequencing, however this is expensive. PCR targeting several microbes can also be performed for identification. Bacteroides and Firmicutes considered representatives, since these two phyla dominate the intestinal microbial composition.
However, there are lacking of study regarding microbiome composition, dysbiosis, short chain fatty acid, and toxins in the relation to immune response and disease activity of rheumatoid arthritis in human subjects. This preliminary study tries to identify the microbiome composition in relation with disease activity of rheumatoid arthritis patient.
Material and Methods
Study Design, Sample and Subjects
A cross-sectional study and correlations analysis performed to identify the relationship between microbiome compositions, number of Th1 cells, number of Treg cells, and disease activity in RA subjects. Ethical approval obtained from Dr. Saiful Anwar General Hospital Ethical Commission, number 400/213/K.3/302/2020. Twelve rheumatoid arthritis patients, consecutively recruited to this study, divided into two categories based on disease activity, which are remission-low disease activity and moderate-high disease activity. Stools were collected from subjects for microbiome examination. Subjects were also examined for disease activity, consist of tender joint counts, swollen joint counts, pain scale, global health scale and ESR. Blood samples were also taken for flowcytometry to identify Treg and Th1 cells.
Disease Activity Measurement
Disease activity score measured using DAS-28 ESR using the
formula.
0.56*v[TJC28] +0.28*v[SJC28] +0.014*VAS+0.70*ln [ESR]
Number of Firmicutes, Bacteroidetes and Prevotella
Stool samples were collected, 180 – 200 mg stool sample were prepared for DNA isolation [TianAmp Stool DNA kit, Cat. no. 4992205]. Genome extraction from DNA isolate perform using CTAB/SDS method. Amplicon were made targeting V3-V4 region using Phusion® HighFidelity PCR Master Mix [New England Biolabs], Mixture and purification of PCR product were performed using Qiagen Gel Extraction Kit [Qiagen, Germany]. Library generation conducted using NEBNext® UltraTM DNA Library Prep Kit for Illumina and quantified by Qubit and Q-PCR. Sequencing and processing of raw tags performed using Qiime [V1.7.0] and compared to database, using UCHIME algorithm to eliminate chimeric sequence. Number of Firmicutes, Bacteroidetes and Prevotella were count from KRONA diagram from the NGS Analysis Report.
Identification of Th1 and Treg Cells
Blood samples using EDTA anticoagulant collected for flowcytometry analysis. Identification of Th1 cells using FITC-CD4 [Biolegend 317408] and PE-IFNγ [Biolegend 502509], while Treg cells identify using FITC-CD4, PE/CyCD25 [Biolegend 302608], and PE-FoxP3 [Biolegend 320108]. Flowcytometry performed using BD FACS Melody. Intracellular marker [FoxP3 and IFNγ] staining procedure performed after extracellular marker [CD4 and CD25] staining done.
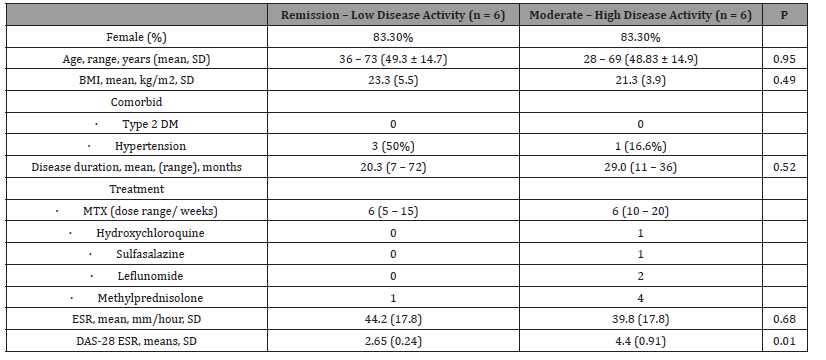
Twelve subjects included in the study, from Table 1 no significant difference between two groups in regards of age, BMI, and disease duration. In the moderate-high disease activity group, MTX dose were higher and combination with other DMARDS and glucocorticoids more frequent, marking that this group of patients seems have an intractable inflammation. Interestingly, mean ESR were not significantly different between two groups, this may suggest another inflammatory markers or clinical/imaging findings reflecting arthritis are necessary.
Number of Firmicutes, Bacteroidetes and Prevotella
Number of Firmicutes and Bacteroidetes phyla and number of Prevotella genus determined by counting the composition show in KRONA display. The difference between two groups do not show significant difference, despite there is trends that in remissionlow disease activity group the composition is higher, especially in Bacteroidetes and Prevotella composition (Figure 1).
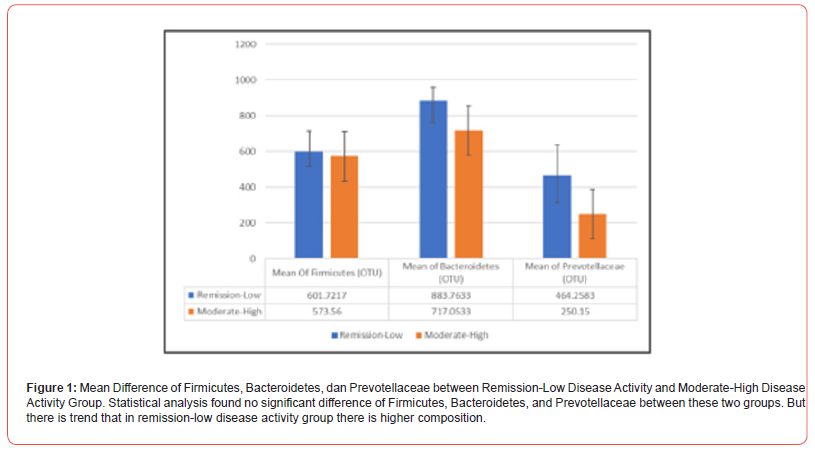
Table 1:Baseline Characteristic of Subjects.
Discussions
Most of the subjects in this study are women [83.3%], with age ranging from 28 – 73 years old. Mean BMI is 22.3 kg/m2 and hypertension found in on-third of subjects. Subjects included in this study had ESR elevation despite their disease activity are remission low. Global data showing increase age-standardized prevalence and incidence of RA by 7.4% and 8.2% respectively, distribution may vary between region.25 Female gender dominates the case, ranging from 67 – 70%. Obesity [IMT ≥ 30 kg/m2] also found more prevalent in RA subjects, while in our study mean of BMI are less than 25 kg/m2 [8,9]. In our subjects most of subjects [n = 5] in remission-low disease activity are treated with MTX alone, while one subjects receiving MTX and HCQ combination. In the moderatehigh disease activity group, most of subjects receiving combination DMARD and glucocorticoids. No subjects receiving biologic DMARD, regarding limitation of coverage in national health insurance. The global trends show that some treating physician or rheumatologist initiate biologic DMARD in several patients [10].
Microbiome Composition and Disease Activity
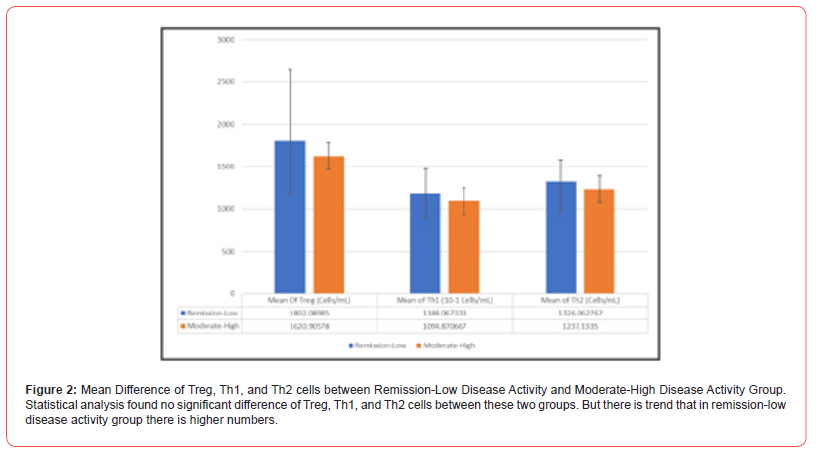
Despite not statistically significant, this study showing trends that Firmicutes, Bacteroidetes and Prevotella are higher in remission-low disease activity. Study on dysbiosis results in variable distribution of microbes and many factors affecting microbial composition. In CIA mice added with P. gingivalis, Bacteroides decreased and two study found different pattern of Firmicutes. In pre-clinical RA, Prevotella found increased. The study on microbiome pattern in RA mostly not controlled all the factors influencing microbial composition [11-13]. In clinical phase of RA, Bacteroidetes:Firmicutes ratio lower compare to osteoarthritis subjects and Prevotella was higher [14]. Other studies show different results for Prevotella in clinical RA [15-17]. In this initial study, subjects not divided into treatment group, while in several studies Enterobacteriales composition may affected by MTX treatment [18] (Figure 2).
Inconsistency that found in microbiome study might result because of that most study do not control the factors affecting intestinal microbial composition. Several factor affecting microbial composition including, age, races, comorbid, dietary pattern, drugs, and in RA, clinical phase of the disease and DMARD may affect the composition. Intestinal microbes’ “ecosystem” itself shared a mechanism that known as quorum sensing that provide the balancing between microbes and their products such as toxins, metabolites, and biofilms [19].
Number of Th1 and Treg and Disease Activity
In this study, correlation between Th1 and Treg percentage with DAS-28 ESR were not established, with r = 0.032 [p = 0.922] and r = 0.095 [p = 0.770] respectively. Unfortunately, our study did not analyse cytokine and other factors such as Bcl-2, Bim, IL-2, IL-4, IL- 7, IL-15, and IL-21 which may be affected by treatment [20]. In the other side, T cell subsets in RA are dynamics, in the different stage of disease development and progressions, T cell subsets may vary. Treatment may also affect T cell subsets, as most of DMARDs action are targeting lymphocyte. In anti-CCP positive subjects, number of Treg and naïve cells are predictive for clinical RA development [21]. Treg may also exhibit plasticity, where Treg produce IL-17 and act as proinflammatory cells like Th17. Different locations, blood and synovial tissue may share different populations of T cells subset, for example IL-17 produce by synovial Treg [22].
Number of Th1 and Treg and Microbiome Composition
Several pathways has been identified how microbiome composition affecting inflammation, one is through alteration in T cells number and function. Several bacteria with invading properties can be phagocytized by dendritic cells and results in Th1 polarization. Microbial products can also bind to immunoregulatory cells surface receptors and results in anti-inflammatory states.
Bacteroides species can induce Foxp3 expression and RORγt+ pTreg differentiation, mediated by goblet cells and CD103+ DC antigen presentation and secretion of high levels of TGF-β and IL- 10. Bacteroides’ sphingolipids [GSL-Bf717] may bind to CD1 on DCs and iNKT cells and results in tolerogenic responses [23-24]. In our study, we found negative correlations between Treg: Th1 ratio and Firmicutes:Bacteroidetes ratio, which means Treg and Bacteroidetes were in line. Prevotella show non-significant correlation with Treg: Th1 ratio in our study. Prevotella thought to be “pro-inflammatory bacteria” as it may induce Th17 differentiation,23 which is not measured in this study.
In (Figure 3) we describe the interactions between microbes and balance between cells with ratio, regarding the possibility of “quorum sensing” in maintaining the balance of microbe numbers and Firmicutes and Bacteroidetes are dominating the microbial composition in intestinal tract.
Conclusions
This study does not found difference of Firmicutes: Bacteroidetes ratio, Prevotella, Th1 and Treg counts between remission-low disease activity group and moderate-high disease activity group. We find no correlations between microbial composition and DAS- 28 ESR score, no correlations between Prevotella to Treg: Th1 ratio, and inversely weak correlation between Firmicutes: Bacteroidetes ratio to Treg:Th1 ratio. These findings may suggest other pathways beside T cell-mediated inflammation that involved in dysbiosisrelated inflammatory state. Further research that involving more subjects, more controlled factors such as diet, medications, comorbid, and studying more T-cell subsets should be performed for better understanding.
Acknowledgement
None.
Conflict of Interest
No conflict of Interest.
References
- De Luca F, Shoenfeld Y (2019) The microbiome in autoimmune diseases. Clin Exp Immunol 195(1): 74-85.
- Leech MT, Bartold PM (2015) The association between rheumatoid arthritis and periodontitis. Best Pract Res Clin Rheumatol 29(2): 189-201.
- Forbes JD, Chen CY, Knox NC, Marrie RA, El-Gabalawy H, De Kievit T, et al; (2018) A comparative study of the gut microbiota in immune-mediated inflammatory diseases - Does a common dysbiosis exist? Microbiome 6(1): 1-15.
- Kang Y, Cai Y, Zhang X, Kong X, Su J (2017) Darmmikrobiota und rheumatoide Arthritis: von der Pathogenese zu neuen therapeutischen Strategien. Z Rheumatol 76(5): 451-457.
- Sandhya P, Danda D, Sharma D, Scaria V (2016) Does the buck stop with the bugs?: An overview of microbial dysbiosis in rheumatoid arthritis. Int J Rheum Dis 19(1): 8-20.
- Zhang L, Song P, Zhang X, Metea C, Schleisman M, Karstens L, et al; (2020) Alpha-glucosidase inhibitors alter gut microbiota and ameliorate collagen-induced arthritis. Front Pharmacol pp. 1-13.
- Horta Baas G, Romero Figueroa MDS, Montiel Jarquín AJ, Pizano Zárate ML, García Mena J, et al. (2017) Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. J Immunol Res 2017: 4835189.
- Almutairi KB, Nossent JC, Preen DB, Keen HI, Inderjeeth CA (2021) The prevalence of rheumatoid arthritis: A systematic review of population-based studies. J Rheumatol 48(5): 669-676.
- Myasoedova E, Davis J, Matteson EL, Crowson CS (2020) Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985-2014. Ann Rheum Dis 79(4): 440-444.
- Mease PJ, Stryker S, Liu M, Salim B, Rebello S, Gharaibeh M, et al; (2021) Treatment patterns in rheumatoid arthritis patients newly initiated on biologic and conventional synthetic disease-modifying antirheumatic drug therapy and enrolled in a North American clinical registry. Arthritis Res Ther 23(1): 1-13.
- Sato K, Takahashi N, Kato T, Matsuda Y, Yokoji M, Yamada M, et al (2017) Aggravation of collagen-induced arthritis by orally administered Porphyromonas gingivalis through modulation of the gut microbiota and gut immune system. Sci Rep 7(1): 1-13.
- Nemoto N, Takeda Y, Nara H, Araki A, Gazi MY, Takakubo Y, et al; (2020) Analysis of intestinal immunity and flora in a collagen-induced mouse arthritis model: Differences during arthritis progression. Int Immunol 32(1): 49-56.
- Alpizar-Rodriguez D, Lesker TR, Gronow A, Gilbert B, Raemy E, Lamacchia C, et al; (2019) Prevotella copri in individuals at risk for rheumatoid arthritis. Ann Rheum Dis 78(5): 590-593.
- Sun Y, Chen Q, Lin P, Xu R, He D, Ji W, et al; (2019) Characteristics of Gut Microbiota in Patients With Rheumatoid Arthritis in Shanghai, China. Front Cell Infect Microbiol 9: 369.
- Maeda Y, Kurakawa T, Umemoto E, Ito Y, Gotoh K, Hirota K, et al; (2016) Full Length Arthritis & Rheumatology This article has been accepted for publication and undergone full peer review but has not been through the copy editing, typesetting, pagination and proofreading process which may lead to differences between this vers pp. 2-36.
- Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, et al; (2013) Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2: 1-20.
- Kishikawa T, Maeda Y, Nii T, Okada Y (2022) Response to: ’Comment on “Metagenome-wide association study of gut microbiome revealed novel aetiology of rheumatoid arthritis in the Japanese population” by Kishikawa et al.’ by Kitamura et al. Ann Rheum Dis 81(5): 72.
- Diamanti AP, Panebianco C, Salerno G, Di Rosa R, Salemi S, Sorgi ML, et al; (2020) Impact of mediterranean diet on disease activity and gut microbiota composition of rheumatoid arthritis patients. Microorganisms 8(12): 1-14.
- Jimenez AG, Sperandio V (2019) Quorum Sensing and the Gut Microbiome [Internet]. Quorum Sensing: Molecular Mechanism and Biotechnological Application. Elsevier Inc pp. 151-169.
- Pandiyan P, Lenardo MJ (2008) The control of CD4+CD25+Foxp3+ regulatory T cell survival. Biol Direct 3: 1-12.
- Ponchel F, Burska AN, Hunt L, Gul H, Rabin T, Parmar R, et al; (2020) T-cell subset abnormalities predict progression along the Inflammatory Arthritis disease continuum: implications for management. Sci Rep 10(1): 1-10.
- Wang T, Sun X, Zhao J, Zhang J, Zhu H, Li C, et al; (2015) Regulatory T cells in rheumatoid arthritis showed increased plasticity toward Th17 but retained suppressive function in peripheral blood. Ann Rheum Dis 74(6): 1293-301.
- Brown EM, Kenny DJ, Xavier RJ (2019) Gut Microbiota Regulation of T Cells During Inflammation and Autoimmunity. Annu Rev Immunol 37: 599-624.
- Ivanov I, Tuganbaev T, Skelly A, Honda K (2022) T Cell Responses to the Microbiota. Annual Review of Immunology 40(1): 559-587.
- Finckh A, Gilbert B, Hodkinson B, Bae S, Thomas R, Deane K et al; (2022) Global epidemiology of rheumatoid arthritis. Nature Reviews Rheumatology 18(10): 591-602.
-
Perdana Aditya Rahman*, Handono Kalim, C Singgih Wahono and Bagus Putu Putra Suryana etc al.. Firmicutes: Bacteroidetes Ratio Correlate Negatively with Treg: Th1 Ratio in Rheumatoid Arthritis Patients. Arch Rheum & Arthritis Res. 3(1): 2024. ARAR.MS.ID.000553.
-
Postural orthostatic tachycardia syndrome, Neuropathy, Autoimmune diseases, Inflammation, Rheumatoid arthritis, chronic fatigue syndrome, Immunizations.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.