Case Report
Case Report
A Rare Case of Methotrexate Induced Pancytopenia with Neutropenic Sepsis Unveiling Rhupus
Richmond R Gomes, Ad-Din Women’s Medical College Hospital, Dhaka, Bangladesh
Received Date:December 11, 2023; Published Date:January 09, 2024
Abstract
Rhupus syndrome is a rare entity, is the co-existence of rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) and is characterized by the presence of erosive arthritis together with symptoms and signs of systemic lupus erythematosus. It manifests more RA and less SLE related damage. The incidence of rhupus in patients with arthritis is 0.01%-0.2% and <2% in patients with connective tissue diseases. However, we report a rare case of rhupus in a 35-year-old lady who was known case of seropositive RA (both RA factor and anti CCP positive), presenting with MTX induced pancytopenia with neutropenic sepsis. Later on further work up she was found to be positive for ANA, Anti dS DNA, anti neucleosome, anti Ku and Anti Smith antibody. She was treated conservatively with whole blood transfusion, G-CSF(Granulocyte colony stimulating factor), broad spectrum antibiotic and anti-fungal agent.
Keywords:Rheumatoid arthritis; Systemic lupus erythematosus; Rhupus syndrome; Methotrexate; Neutropenic Sepsis; Pancytopenia
Introduction
Autoimmune diseases Rheumatoid arthritis (0.5-1.0% of adult population)1 and SLE (10-400/100,000)2 are not uncommon and are considered to be separate entity. The coexistence of two or more connective tissue diseases in the same patient is a rare phenomenon, particularly for the coexistence of SLE and RA, which has been estimated between 0.01% and 2%.3,4,5 Joint compromise in systemic lupus erythematosus (SLE) is one of the common manifestations of this disease, with only a small fraction of patients (∼5%) developing deformity in the form of Jaccoud’s arthropathy. Less than one percent of patients with SLE develop erosive disease which is indistinguishable from rheumatoid arthritis (RA), an entity known as rhupus.6The term “rhupus” is traditionally used to describe patients with coexistence of rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE). Toone et al. Performed the first clinical observations that helped to identify this disease,7 who found the presence of LE cell phenomenon in patients with RA, which was considered an exclusive feature seen in SLE patients. Later the term rhupus was proposed by Schur in 1971.8 The exact etiology and triggers of rhupus remain unknown till date with limited studies suggesting the combined role of genetic, immunological, hormonal, and environmental factors in the progression of the disease9 The prognosis in rhupus syndrome depends on internal organ damage, but it is better than SLE and worse than RA. RA is responsible for Th1-mediated inflammation, whereas SLE is associated with Th2-mediated pathologies 10.It is important to categorically differentiate the patients with rhupus because their therapy and outcome differ from those having RA or SLE alone.5 Here in, we report one such rare case.
Case report
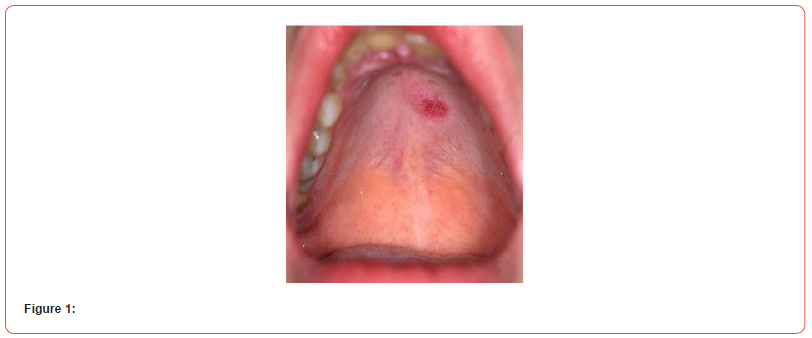
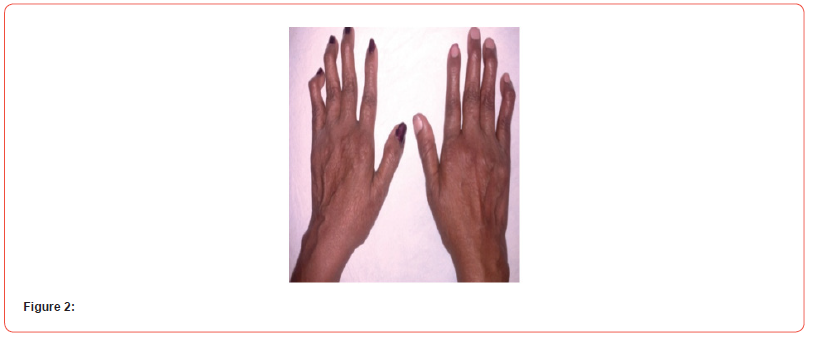
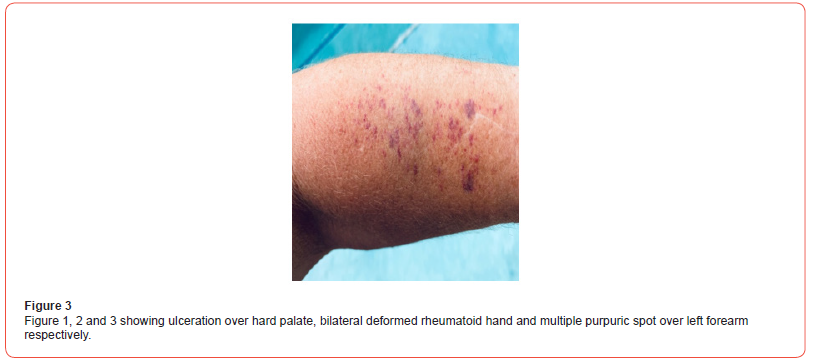
A 35 years non diabetic, normotensive lady from rural Bangladesh, known to have seropositive RA (positive for both RA factor and Anti CCP) for last 6 years (on oral prednisolone 7.5 mg daily and Methotrexate 20 mg once weekly) presented to us with high grade, intermittent fever(highest being recorded as 1040F) for 6 days, blood mixed urine for 3 days and generalized purpuric spot for 2 days. She denied any dysuria, cough, abdominal pain, seizure, abnormal behavior, bleeding from any other site, vomiting or headache. There was no history raynaud’s phenomenon, dysphagia or muscle weakness. On general examination, patient was febrile with temp 1020F, moderate pallor, blood pressure 110/70 mmHg, pulse 100/min, regular, respiratory rate 16/ min, alopecia, ulcers over hard palate and buccal mucosa (figure 1), multiple bilateral symmetrical small & large joint swelling, tenderness and hand deformities (figure 2), generalized purpuric rash (figure 3) There was no skin nodules, lymphadenopathy, hepato-splenomegaly, sclerodactyly. Other systemic examination revealed no abnormalities.
Complete blood count showed normocytic normochromic anemia with hemoglobin 7.3 gm/dl(normal 12-16 gm/dl), MCV 84.4 fL (normal 78-98 fL), MCH 28.5 pg (normal 26-32 pg), total white cell count 1.6×103/uL (N-12%,ANC 192, L-82.9%), total platelet count 15×103/ uL( 150-450×103/uL). Peripheral blood film showed pancytopenia with negative direct coomb’s test, Serum reticulocyte count was reduced 0.50% (normal 1.5%-2.5%). LDH was raised to 1860U/L (normal 160-450 U/L), CRP- 329 mg/ dl (normal <5 mg/dl) procalcitonin 11.6ng/dl(normal <0.5 ng/ ml). Serum bilirubin 2.1 mg/dl (normal 0.5-1.8mg/dl) SGPT- 24 U/L(normal up to 31U/L), serum creatinine 0.68 mg/dl(normal 0.5-1.3 mg/dl). Serum electrolyte showed sodium 139 mmol/L (normal 135-145 mmol/L), potassium 3.8 mmol/L (normal 3.5- 5.5 mmol/L). TSH was mildly raised 8.42 mIU/mL (normal 0.35- 5.5mIU/mL). Urine routine examination showed plenty RBC without significant pyuria, proteinuria or any casts. ANA and d’s DNA was positive with values (87.51 U/ml, normal < 20 U/ml) and 123 IU/ml (normal 1-10 IU/ml). PT and aPTT were normal. ENA (extractable nuclear antigen) was sent which showed positivity for anti-smith antibody, anti-Ku and anti neucleosome antibody. She was started inj. Meropenem and inj. Voriconazole after sending blood culture which later showed growth of Elizabethkingia meningoseptica. She was also given G-CSF subcutaneously for 3 days. She was also transfused 2 units of whole blood. Methotrexate was discontinued.
With treatment she made significant clinical recovery. Meropenem lasted for 10 days and voriconazole for 14 days. She was discharged on 16th day of admission. During discharge her hemoglobin was 10.7 gm/dl (normal 12-16 gm/dl), total white cell counts 10.4×103/uL (N-72%, ANC 7488, L-22.7%), total platelet count 385×103/ uL (150-450×103/uL). CRP- 41.5 mg/dl (normal <5 mg/dl) procalcitonin 0.3ng/dl (normal <0.5 ng/ml). She was discharged with oral prednisolone 30 mg daily, hydroxycloroquine 200 mg twice daily and azathioprine 100 mg once daily. On follow up after two weeks she was doing well.
Discussion
Overlap syndromes have been defined as entities satisfying the classification criteria of at least two CTDs occurring at the same or at different times in the same patient. Rhupus is one such condition with the co-existence of SLE and rheumatoid arthritis with a prevalence varying from 0.01% to 2%. Among genetic factors, HLA-DR alleles have been strongly associated with rhupus, being present in nearly 67% of the reported cases 5,9.
In general, the syndrome is described with higher incidence in women, although it may occur in male patients,11 appearing first the manifestations of RA and then those of SLE, in most cases, another important group of patients begins with manifestations of the 2 entities simultaneously, and patients who begin with manifestations of SLE and later of RA are reported rarely [12].
With regard to the time of appearance of the manifestations of one or another disease, it is reported that in the patients with RA the time for the onset of the clinical manifestations of SLE ranges between 4 and 7 years [12, 13]. in the case of the patients with initial SLE, is reported a period of approximately 4 years for the onset of the articular manifestations of RA [11-14].
RA is a progressive inflammatory autoimmune disease with articular and systemic effects. Its exact cause is unknown, but genetic and environmental factors are contributory. T-cells, B-cells, and the orchestrated interaction of pro-inflammatory cytokines play key roles in the pathophysiology of RA. Predominant role of Th 1 pathway is there with release of cytokines such as IL-17, TNF-α, IL-6, and IL-1, which cause synovial inflammation. However, the exact patho-aetiology of SLE remains elusive. An extremely complicated and multifactorial interaction among various genetic and environmental factors is probably involved. The loss of immune tolerance increased antigenic load, defective B-cell suppression, and the shifting of Th1 to Th2 immune responses lead to B-cell hyperactivity and the production of pathogenic autoantibodies. Hence, the coexistence of both these entities with different pathogenesis is unique [15].
SLE is a connective tissue disease that is characterized by the involvement of multiple systems [16]. It generally starts with joint involvement, and its most frequently observed symptoms are arthritis that does not lead to deformity and myalgia. Proximal interphalangeal and metacarpophalangeal joints are primarily involved [17,18].
Rhupus patients have been found to have a lower incidence of malar rash, hemolytic anemia, and renal and neurological involvement compared with the SLE group. In addition, rhupus patients rarely have severe renal disorders such as nephrotic syndrome and renal insufficiency. The SLEDAI score that is an indicator of disease activity in SLE, initial corticosteroid dosages, and the requirement of methylprednisolone pulse therapy have been found to be lower in the rhupus patients [19]. Previous studies have also shown that rhupus patients have a lower incidence of visceral organ involvement compared with SLE patients without RA.4 Many authors have classified Rhupus syndrome as a subset of SLE with severe arthritis. SLE shows 3 types of articular involvement: intermittent non-erosive polyarthritis usually found in the hands, wrists, and knees; non-erosive deforming arthritis referred to as Jaccoud’s joint; and arthritis with joint deformities and specific erosion, i.e., Rhupus syndrome [20, 21]. Most patients with SLE have transient, migratory, and reversible arthritis without erosion. Some rheumatologists suggest that the presence of rheumatoid nodules in SLE patients could be a risk factor for Rhupus syndrome [22] Tani et al. also found an association of rheumatoid factor positivity and joint erosion in SLE patients [23].
The results of the diagnostic methods played a key role in the definitive diagnosis of the disease. Laboratory findings such as anti- CCP Ab, anti-dsDNA, or anti Smith Ab positivity are the diagnostic criteria for rhupus syndrome24. A high titration of anti-RNP Ab (+positive) is very important in the overlap syndrome diagnosis 17,24. Hypocomplementemia is not characteristic in rhupus syndrome [24]. Anti-dsDNA antibodies are specific for SLE [24]. Rheumatoid factor with high sensitivity and low specificity is the diagnostic criterion for RA. On the other hand, positive antiCCP antibody has 98% specificity for diagnosis of the disease. Anti- CCP antibodies for RA are known as a more specific parameter compared to rheumatoid factor [25, 26].
Imaging studies show an erosive joint pattern with the presence of radiological signs, such as juxta-articular bone demineralization and the presence of erosions which are part of the diagnostic criteria for RA [27].
With regard to the therapeutic approach, the incorporation of methotrexate is justified by the presence of the polyarticular, erosive and seropositive inflammatory picture; with the addition of folic acid supplements and the increase in the dose of steroids in order to control, in the short term, the articular inflammatory process and to achieve an improvement in constitutional symptoms. Otherdrugs such as mycophenolate mofetil, cyclosporin A, and tumor necrosis factor inhibitors have shown little effect on rhupus syndrome [28] but have role especially if there is renal involvement [29].
RhS is an infrequent condition, but when occurs it can cause complications that determine different degrees of disability in the patients who suffer from it, the constant monitoring of the clinical manifestations of this entity becomes the most secure way to prevent the complications derived from it.
Conclusion
Rhupus syndrome is a special overlap syndrome of RA and SLE that is manifested characteristically by more RA and less SLE-associated damage. The treatment and prognosis of Rhupus syndrome have been found to be different from that of RA or SLE. Despite being a rare entity, it is important to know the clinical and humoral elements that allow its early diagnosis, making it easier to start treatment in a timely manner and reduce its possible complications and improving patient prognosis.
Conflict of interest
None.
Acknowledgements
No Conflict of interest.
References
- Shah A, E William St. Clair. Chapter 351, Rheumatoid Arthritis, epidemiology, harrison principle of internal medicine 18 Edn. pp. 2741.
- Hahn B H. chapter 319prevalence, SLE, harrison principle of internal medicine 18edn, pp: 2724.
- Simon JA, Alcocer Varela J (2001) Cuál es la definición de rhupus? Rev Mex Reum 16(2): 111-119.
- Simon JA, Granados J, Cabiedes J, Ruiz Morales J, Alcocer Varela J (2002) Clinical and inmunogenetic characterization of Mexican patients with ‘Rhupus’ Lupus 11(5): 287-292.
- Panush R, Edwards NL, Longley S, Webster E (1988) ‘Rhupus’ syndrome. Arch Intern Med 148(7): 1633-1636.
- Alarcón Segovia D, Abud Mendoza C, Diaz Jouanen E, Iglesias A, De los Reyes V, Hernandez Ortiz J (1998) Deforming arthropathy of the hands in systemic lupus erythematosus. J Rheumatol 15(1): 65-69.
- Toone E, Irby R, Pierce EL (1960) The cell LE in rheumatoid arthritis. Am J Med Sci 240: 599608.
- Schur PH (1971) Systemic lupus erythematosus in Cecil-Loeb. In: Beeson P B, McDermott W (Eds.), Textbook of Medicine. Philadelphia, PA: Saunders pp: 821.
- Al Fadhli S, Nizam R (2014) Rhupus: A crosswalk between lupus and rheumatoid arthritis. OA Arthritis 2(3).
- Arbuckle MR, Mc Clain MT, Rubertone MV, R Hal Scofield, Gregory J Dennis, et al. (2003) Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 349(16):1526-1533.
- Carrillo Nánez L, Huaringa Marcelo J, Carrillo García P (2012) Rhupus en un paciente varó Rev Soc Peru Med Interna 25: 1315
- Ignacio Benavente EP, Oscar Paira S (2011) Rhupus: report of 4 cases. Reumatol Clin 7(5):333-335.
- Abarca Acuña B, Atamari Anahui N, Contreras Sotomayor S, Sucasaca-Rodríguez C, Nieto-Portocarrero R (2015) Rhupus, un síndrome poco frecuente: Reporte de un caso. Rev Med Hered 26: 51-4.
- Talarico R, Caramella D, Bombardieri S, Mosca M, Tani C, et al. (2013) Rhupus syndrome: assessment of its prevalence and its clinical and instrumental characteristics in a prospective cohort of 103 SLE patients. Autoimmun Rev 12(4): 537-541.
- Mok CC, Lau CS (2003) Pathogenesis of systemic lupus erythematosus. J Clin Pathol 56(7): 481-490.
- Sarkar S, Saha K (2012) Bilateral acute lupus pneumonitis in a case of rhupus syndrome. Lung India 29(3):280-282.
- Baykal C (2012) Dermatoloji atlası. 3rd ed. Istanbul: Nobel Tıp Kitabevi.
- Ziaee V, Moradinejad MH, Boyat R (2013) Rhupus syndrome in children: a case serieand literature review. Case Rep Rheumatol 819629.
- Li J, Wu H, Huang X, Xu D, Zheng W, et al. (2014) Clinical analysis of 56 patients with rhupus syndrome: Manifestations and comparisons with systemic lupus erythematosus: A retrospective case-control study. Medicine (Baltimore) 93(10): e49.
- Pipili C, Sfritzeri A, Cholongitas E (2008) Deforming arthropathy in systemic lupus erythematosus. Eur J Intern Med 19(7):482-487.
- Santiago MB (2011) Miscellaneous non-inflammatory musculoskeletal conditions. Jaccoud's arthropathy. Best Pract Res Clin Rheumatol 25(5): 715-725.
- Richter Cohen M, Steiner G, Smolen JS, Isenberg DA (1998) Erosive arthritis in systemic lupus erythematosus: Analysis of a distinct clinical and serological subset. Br J Rheumatol 37(4): 421-424.
- Tani C, Daniello D, Delle Sedie A, Carli L, Cagnoni M, et al. (2013) Rhupus syndrome: Assessment of its prevalence and its clinical and instrumental characteristics in a prospective cohort of 103 SLE patients. Autoimmun Rev 12: 537-541.
- Verdoorn, BP, McDonald FS (2011) 62-year-old woman with fever, dyspnea, pleuriticchest pain and weight loss. Mayo Clin Proc 86(2): 152-155.
- Klareskog L, Padyukov L, Rönnelid J, Alfredsson L (2006) Genes, environment and immunity in the development of rheumatoid arthritis. Curr Opin Immunol 18(6): 867-875.
- Visser H, le Cessie S, Vos K, Breedveld FC, Hazes JM (2006) How to diagnose rheumatoid arthritis early: a prediction model for persistent (erosive) arthritis. Arthritis Rheum 46(2): 357-365.
- Gómez A (2011) Nuevos criterios de clasificación de artritis reumatoide. Reumatol Clín 6: 33-37.
- Showman O, Langevitz P, Shoenfeld Y (2015) Rhupus: unusual presentations. Clin Rheumatol 34(12): 2041-2046.
- Andrade-Ortega L, Irazoque-Palazuelos F, Muñoz-López S, Rosales-Don Pablo VM (2013) Eficacia y tolerabilidad de rituximab en el tratamiento de pacientes con Rhupus. Reumatol Clin 9(4): 201-205.
-
Richmond Ronald Gomes, Habiba Akhter. A Rare Case of Recurrent Hypokalemic Paralysis Associated with Distal (Type1) Renal Tubular Acidosis Due to Primary Sjogren’s Syndrome with Type 3 Cryoglobulinemic Vasculitis with Autoimmune Hypothyroidism. Arch Rheum & Arthritis Res. 2(2): 2022. ARAR.MS.ID.000551.
-
Postural orthostatic tachycardia syndrome, Neuropathy, Autoimmune diseases, Inflammation, Rheumatoid arthritis, chronic fatigue syndrome, Immunizations.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.