 Research Article
Research Article
Therapeutic Potency of the Polar and Non-Polar Extracts of Andrographis Paniculata Leaf Against some Pathogenic Bacterial Isolates
Ayanwale O Abraham1*, Adabara U Nasiru1, Adeniyi K Abdulazeez2, Oyewole O Seun3 and David W Ogonna4
1Department of Microbiology, Federal University of Technology, Nigeria
2Department of Animal Biology, Federal University of Technology, Nigeria
3Department of Microbiology, Obafemi Awolowo University, Nigeria
4Department of Biochemistry and molecular biology, Obafemi Awolowo University, Nigeria
Ayanwale O Abraham, Department of Microbiology, Federal University of Technology, Minna, Nigeria.
Received Date:November 04, 2019; Published Date: November 18, 2019
Abstract
The increasing resistance among virulent pathogens and the toxicity of synthetic antibiotic has led to the quest for bioactive natural products which are safe, potent, and affordable for therapeutic purpose. This study evaluated the antibacterial activities of polar and non-polar extracts from Andrographis paniculata leaf (80, 120, 160 and 200 mg/mL) against Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, and Salmonella typhi. Phytochemicals screening were conducted using standard procedures while the antibacterial activity was evaluated using agar well diffusion technique. Results revealed the presence of alkaloid, phenol, flavonoids, steroids, terpenoids, and saponins in methanol extract while the N-Hexane extract contains alkaloids, phenols, and steroids. Cardiac glycosides and tannins were absent in both extracts. Both extracts exhibited increase growth inhibition of S. aureus, E. coli and S. typhi with increase extract concentration. Methanol extract exhibited higher activities (3.00±0.01 and 25.00±0.01 mm) than the hexane extract (3.00±0.01 and 20.00±0.01 mm). The MIC of methanol extract were 1.60, 0.32 and 0.32 mg/mL while hexane extract recorded MIC of 8.0, 2.60 and 2.60 mg/mL against S. aureus, E coli and S. typhi respectively. Thus, Andrographis paniculata leaf might be a useful tool in the future for pharmaceuticals antibiotics.
Keywords: Andrographis paniculate; Antibacterial activities; Phytochemicals; Polar; Non-polar solvents
Introduction
Infectious diseases remain the primary cause of mortality and morbidity in developing countries, resulting in substantial economic loss and threaten the attainment of the Sustainable Development Goals (SDGs), which highlighted healthcare as one of the topmost challenges for 2030 [1,2]. The re-current emergence of drug-resistant pathogens and the toxicity of conventional synthetic antibiotics [3], further necessitate the need for the discovery of less toxic and yet potent natural alternatives, hence the continuous screening of plants and other natural products for therapeutic efficacy [4,5]. Medicinal plants have grown enormously from the use of herbal products as natural cosmetics and as self-medication by the general public scientific for their beneficial effects [6]. Medicinal plants still play important role in human and animal health care and about 60% of the world’s population (and 80% of Africa’s population) depend on herbal medicine for their primary health care [7]. Many of these plants have been screened for their phytochemical constituents and antimicrobial properties with the view to authenticate their folkloric uses and safety [8]. Plants have various phytochemical compounds and metabolites occurring in the stem, roots, bark, leaves and have been implicated to have therapeutic efficacy against man’s pathogens such as fungi and bacteria [9]. Andrographis paniculata, commonly known as “king of bitters,” is a shrub found in many countries of Africa, Asia, and America. It has been well documented for treating diabetes, high blood pressure, skin diseases, influenza, dysentery, bronchitis, leprosy, ulcer, flatulence [10]. Extract from the plant has been reported to contain xanthones, flavonoids, diterpenes, noriridoides, which have demonstrated antiprotozoal, anti-inflammatory, antioxidants, immunostimulants, anti-diabetics, anti-angiogenic, cytotoxicity, sex hormones functions [10,11]. Evidence from published literature recently concentrated on the aqueous and methanol extracts of Andrographis paniculata. The lack of current information informed this study to elucidate the effects of polar and non-polar bioactive extracts from A. paniculata against some clinical bacteria isolates.
Materials and Methods
Collection and identification of the plant materials
Leaves of Andrographis paniculata were collected from the Botanical garden of Federal University of Technology, Minna, Latitude N9039’17.32608” and Longitude E6031’39.07164.” The collected sample was identified at the Department of Plant Biology of the Federal University of Technology, Minna.
Bacteria strains
The bacteria pathogens used in the antimicrobial activity screening of the methanol and n-hexane extracts of Andrographis paniculata were E. coli, Staphylococcus aureus, Streptococcus pyogenes and Salmonella typhi. The test organisms were obtained from Niger State General Hospital, Minna.
Processing and extraction of plant material
Freshly collected leaves of the A. paniculata were thoroughly rinsed with distilled water and dried at room temperature for one week; the dried leaves were then processed into powder form using a clean electric blender. A 40g of the powder was extracted using the Soxhlet apparatus with solvents methanol (160 ml) and N-hexane (150 ml). The concentration of the extracts was done using the reflux method, as described by [12]. The crude extracts were weighed and preserved in a sterile air-tight universal bottle and stored at 4o C [13].
Phytochemical screening
The crude methanol and hexane extracts of were analyzed for qualitative phytochemical composition including; Alkaloid, phenol, flavonoids, steroids, terpenoids, saponins, cardiac glycosides and tannins using standard procedures Poongothai et al. Hosamani et al. [14].
Culture and standardization of the bacteria strain
The clinical isolates of the test organism were plated out on nutrient agar by streaking method, a loopful of the test microorganisms were then transferred into 5ml of nutrient broth; this was later incubated for 24 hours at 37oC. After incubation, 0.2 ml of the culture was transferred into 20ml of nutrient brought and incubated for 3-5 hours to standardize the culture to 106 cfu/ml Babayi et al.
Antibacterial assay
The following bacteria: S. pyogenes, Salmonella typhi, Staphylococcus aureus and Escherichia coli were the species used for the experiments. Organisms were isolated by standard methods, maintained on agar plates and refrigerated until further use. The antibacterial activity of the methanol and hexane extract of Andrographis paniculata at various concentrations (80, 120, 160 and 200 mg/ml) was carried out using agar-well diffusion method according to the method of [15] as described by [16] For comparison, Ampicillin and tween 80 oil were used as positive and negative control respectively. Zones of inhibition obtained were measured with meter rule in millimeter, 5 mm which is the diameter of the used cork borer was subtracted from each measured inhibition zones, the final result is taken as the zones of inhibition [17]. A broth micro-dilution method [18] was used to determine the minimum inhibitory concentration (MIC) of the extracts.
Data analysis
Data generated were analyzed using statistical package for social science (SPSS) version 21. Differences between groups were compared by analysis of variance (ANOVA) followed by Duncan’s Multiple Range Test. The significance level was considered P<0.05.
Result
Phytochemical composition andrographis paniculata
The phytochemical constituents of the methanol and N-Hexane extract of A. panicaulata is presented in Table 1 Methanol extract of Andrographis paniculata was found to contain alkaloid, phenol, flavonoids, steroids, terpenoids, and saponins while the N-Hexane extract contains alkaloids, phenols, and steroids. Cardiac glycosides and tannins were absent in both extracts (Table 1).
Table 1: Phytochemical composition of the methanol and n-hexane extract of Andrographis panculata.
Antibacterial activity
The zone of inhibition of m ethanol and N-hexane extracts of A. paniculata on the tested organism is shown in Table 2,3 respectively. Methanol extract of Andrographis paniculate inhibited the growth of S. aureus, E. coli and S. typhi with inhibition zone range of 3.00±0.01 and 25.00±0.01 mm. However, the extract was completely in active against S. pyogenes. The inhibition zone of the extract against the organism increase with increase extract concentration from 80 to 200 mg/mL. the extract was more active against E. coli (6.00±0.01 mm to 25.00±0.01 mm), followed by S. typhi (10.00±0.00 mm and 22.00±0.03 mm) while the least activity was recorded against S. aureus (3.00±0.01 mm and 18.00±0.02 mm) Table 2. Similarly, hexane extract was in active against S. pyogenes but the inhibition zones (3.00±0.01 and 20.00±0.01 mm) caused by the extract against S. aureus, E. coli and S. typhi increase with increase extract concentration from 80 to 200 mg/mL. The extract was more active against E. coli and less active against S. aureus (Table 2,3).
Table 2: Zone of inhibition (mm) of the Methanol extract of Andrographis paniculata against the organism.
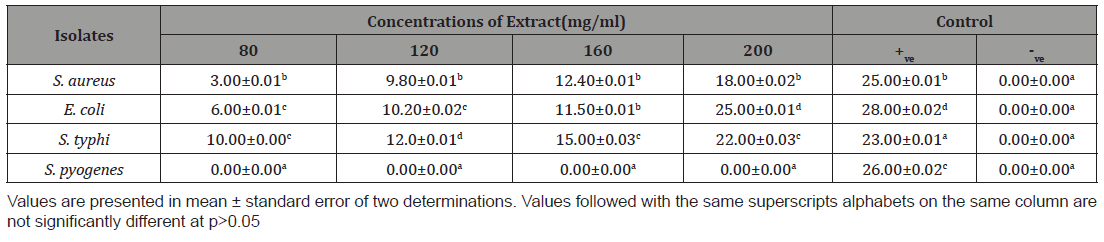
Table 3: Zone of inhibition (mm) of the n-Hexane extract of Andrographis paniculata against the organism.
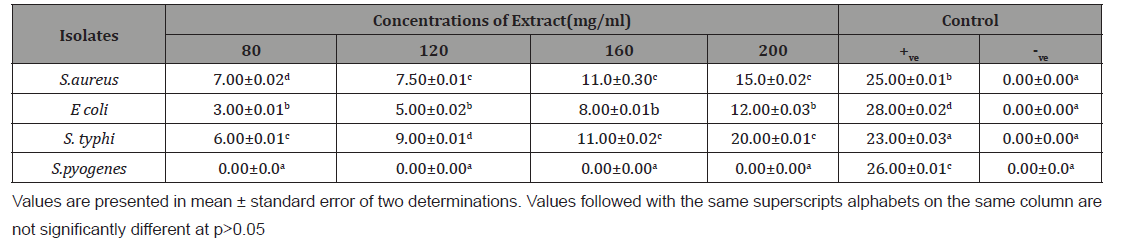
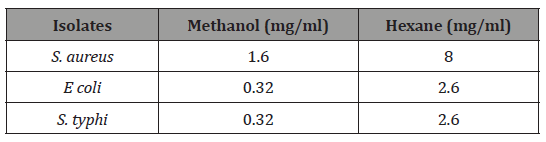
Table 4: Minimum Inhibitory Concentration of Methanol and hexane Extract of Andrographis paniculate.
Minimum Inhibitory concentration (MIC)
The minimum inhibitory concentration of methanol and hexane extracts of Andrographis paniculate is shown in Table 4. The MIC of methanol extract of Andrographis paniculate were 1.60, 0.32 and 0.32 mg/mL against S. aureus, E coli and S. typhi respectively. Hexane extract however, recorded MIC of 8.0, 2.60 and 2.60 mg/mL against S. aureus, E coli and S. typhi respectively (Table 4).
Discussion
Medicinal plants have gained high attention as alternative to conventional therapy. Studies on the medicinal potentials of plants as reported by [19,20] revealed that extraction solvents such as n-hexane, ethyl acetate, methanol and water have different affinities for phytochemical compounds. In the present study, methanol extract of Andrographis paniculata was found to contain alkaloid, phenol, flavonoids, steroids, terpenoids, and saponins while the N-Hexane extract contains alkaloids, phenols, and steroids. In agreement with the present study [21], demonstrated the presence of Alkaloids, Phenols, and Terpenoids and Saponin in the methanol extracts of A. paniculata. While the study of [22] revealed the presence of Alkaloids and Terpenoids in the N-Hexane extract of A. paniculata. The presence of these bio-active metabolites such as Alkaloids, phenols, and steroids is an indication that the plant will possess therapeutic activities [5] Alkaloids, phenols and flavonoids, for instance, have been previously identified as antimicrobial compounds [23,24]. They have been extensively used in disinfection and remained the standard with which other bactericides are compared [25]. Also, alkaloids and flavonoids are used as basic medicinal agents for their bactericidal, analgesic and antispasmodic effects [26,24]. These phytochemicals may be acting singly or synergistically with each other to bring about a cidal or static effect on the microorganisms [27]. The discrepancy in the phytochemical compositions of the two extract and also the absence of Cardiac glycosides and tannins in both extracts could be attributed to the differences in the polarity of the solvent used. Previous study has reported that not all phytochemicals are present in all plants and also that phytochemical composition of medicinal plants can be rationalized in terms of the organ of the plant used as well as the solvent used in the extraction process [28].
In the present study, the plant extract showed dose-dependent antibacterial activities against E. coli, S. typhi, and S. aureus. These could be as a result of an increase in extract concentration which gives a resultant increase in bioactive compound constituent and efficacy. According to [29], dose-dependent potency is common to bioactive compounds from plants. The methanol extract showed higher antibacterial potency than the N-Hexane extract. This could be attributed to the higher qualitative phytochemical composition of the methanol extract than the hexane extract. The observation may be correlated to the nature of bioactive compounds whose activity can be increased in the presence of more polar solvents. According to [30], polar solvents have a higher ability to extract more bioactive compounds than non-polar solvents. The tested microorganisms were inhibited at a very low concentration of the Methanol and N-hexane extract of Andrographis paniculata. E. coli and Salmonella were the most susceptible to having a zone of inhibition of 25 mm and 20 mm, respectively. The MIC values 0.32 mg/ml, obtained for the tested pathogens for the methanol extract in both the E. coli and S. typhi, is an indication of a strong therapeutic potency at low concentrations. This result is similar to that obtained by [31], who discovered that the methanol extract of A. paniculata has high antibacterial activity against Salmonella and E. coli than on Gram-positive bacteria. However [32,33], showed contradicting results to the present study [32]. reported that the methanol extracts of A. paniculata have stronger antibacterial activity against gram positive bacteria than on Gram Negative bacteria. From his study, Staphylococcus aureus has an inhibitory zone of (21mm) at 25μl of methanol extract of the plant, at this same concentration, E. coli was not inhibited. [33] also reported that the methanol and the hexane extract of A. paniculata have no inhibitory effect on E. coli even at a high concentration of 200 μg/ ml. The variation in results may be due to the differences in the nature of the phytochemical constituents available in the extracts, which are primarily determined by the method of extractions [14]. Also, difference in geographical location of the plant material could also cause variation in the quality as well as the quantity of bioactive metabolite in the plant, thus contributing to the variation in biological activitie [34].
Conclusion
The current study revealed the presence of bioactive metabolites in both methanol and N-Hexane extracts of A. paniculata. The methanol and the N-Hexane extracts from the leaf of A. paniculata showed significant antibacterial activity against Salmonella typhi, E. coli, S. aureus. Methanol extract however, demonstrated higher activities than the Hexane extract. Thus, it might be a useful tool in the future for pharmaceuticals antibiotics. It will be of utmost benefit to study the antiviral potency of this leaf against debilitating viral infections such as Rabies, Dengue, Hepatitis, etc.
Acknowledgement
None
Conflict of interest
The authors declare that no conflict of interest.
References
- Asokan GV, Kasimanickam RK (2013) Emerging Infectious Diseases, Antimicrobial Resistance and Millennium Development Goals: Resolving the Challenges through One Health. Central Asian journal of global health 2(2): 76.
- Holmes KK, Bertozzi S, Bloom BR, Jha P, Gelband H, et al. (2017) Major Infectious Diseases: Key Messages from Disease Control Priorities, Third Edition. Holmes KK, Bertozzi S, Bloom BR (Eds) Major Infectious Diseases. 3rd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank, Chapter 1.
- Nurgali K, Jagoe RT, Abalo R (2018) Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Frontiers in pharmacology 9: 245.
- Cheesman MJ, Ilanko A, Blonk B, Cock IE (2017) Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacognosy reviews 11(22): 57-72.
- Ibrahim AM, Lawal B, Abubakar AN, Tsado NA, Kontagora GN, et al. (2017) Antimicrobial and Free Radical Scavenging Potentials of N-Hexane and Ethyl Acetate Fractions of Phyllanthus Fraternus. Nigerian Journal of Basic and Applied Science 25(2): 06-11.
- Sharma M, Joshi S (2011) Comparison of anti-oxidant of Andrographis paniculata and Tinospora cordifolia leaves. J Curr Chem Pharm Sc 1(1): 1-8.
- WHO (2016) General Guidelines for Methodologies on Research & Evaluation of Traditional Medicine? World Health Organization 2000, Geneva.
- S Tahir Ali, A Ayub, SN Ali, S Begum, BS Siddiqui, et al. (2017) Antibacterial activity of methanolic extracts from some selected medicinal plants. Fuuast Journal of Biological sciences 7(1): 123-125
- Ngulde SI, Sandabe UK, Tijjani MB, Barkindo AA, Hussaini IM (2013) Phytochemical constituents, antimicrobial screening & acute toxicity studies of the ethanol extract of Carissa edulis Vahl. root bark in rats & mice. American Journal of Research Communication 11(9): 91-110.
- Akhbar S (2011) Andrographis paniculata: A review of pharmacological activities and clinical effects. Alternative Medicine Effect 16(1): 66-77.
- Aniel KO, Mutyala NL, Rao KGR (2010) In vitro antibacterial activity in the extracts of Andrographis paniculata Burm. F. International Journal of Pharmaceutical Research 2(2): 1383-1385.
- Zhang QW, Lin L G, Ye WC (2018) Techniques for extraction and isolation of natural products: a comprehensive review. Chinese medicine 13: 20.
- Gurupriya S, Cathrine L (2016) Antimicrobial activity of Andrographis paniculata stem extracts. International Journal of Scientific & Engineering Research 7(8): 2229-5518.
- Ramos JU, David SE, Waing GD (2016) Phytochemical screening and antibacterial testing of different varieties of Morus spp. (Mulberry). Journal of Biological Engineering Research and Review 3(1): 44-48.
- CLSI (2012) Method for antimicrobial susceptibility testing of Anaerobic Bacteria; Approved standard, eight edition. CLSI document M11-A8. Wayne, PA. Clinical and Laboratory Standard Institute.
- Tsado NA, Lawal B, Ossa PC, Jagaba A, Kontagora GN, et al. (2016a) Antioxidants and Antimicrobial Activities of Methanol Extract of Newbouldia laevis and Crateva adansonii. Journal of Pharmacy and Allied Health Sciences 6(1-2): 14-19.
- Morsi N (2000) Antimicrobial effect of crude extracts of Andrographis paniculata on multiple resistants bacteria. Journal of Microbiology 49: 63-74.
- Eloff JNA (1998) Sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica 64(8): 711-713.
- Ibrahim AM, Lawal B, Tsado NA, Yusuf AA, Jimoh AM (2015) Phytochemical Screening and GC-MS Determination of Bioactive Constituents from Methanol Leaf Extract of Senna occidentalis. Journal of Coastal Life Medicine 3(12): 992-995.
- Tsado NA, Lawal B, Kontagora GN, Muhammad BM, Yahaya MA, et al. (2016b) Antioxidants and Antimicrobial- Activities of Methanol Leaf Extract of Senna occidentalis. Journal of Advances in Medical and Pharmaceutical Sciences 8(2): 1-7.
- Das P, Srivastav AK (2014) Phytochemical Extraction and Characterization of the Leaves of Andrographis Paniculata for Its Anti-Bacterial, Anti-Oxidant, Anti-Pyretic and Anti Diabetic Activity. International Journal of Innovative Research in Science, Engineering and Technology 3(8): 15176-15184.
- Chandra P, Sudhakran R, Krishna A, Arya B, Priya S, et al. (2017) Analysis of Phytochemical Constituents, Anthelmintic and Insecticidal Properties of Leaf Extracts of Andrographis paniculata. The Pharmaceutical and Chemical Journal 4(5): 98-106.
- Othman L, Sleiman A, Abdel Massih RM (2019) Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Frontiers in microbiology 10: 911.
- Umar SI, Lawal B, Mohammed BA, Obiekezie CI, Adewuyi AH, et al. (2019) Antioxidant and Antimicrobial Activities of Naturally Occurring Flavonoids from M. heterophylla and the Safety Evaluation in Wistar Rats. Iran J Toxicol 13(4): 39-44.
- Ogbunugafor HA, Ugochukwu CG, Kyrian Ogbonna AE (2017) The role of spices in nutrition & health: a review of three popular spices used in Southern Nigeria. Food Quality & Safety 1(3): 171-185.
- Jigam AA, Mahmood F, Lawal B (2017) Protective effects of crude and alkaloidal extracts of Tamarindus indica against acute inflammation andnociception in rats. Journal of Acute Disease 6(2): 78-81.
- Yusuf AA, Lawal B, Yusuf MA, Omonije YO, Adejoke AA, et al. (2018) Free Radical Scavenging, Antimicrobial Activities and Effect of Sub-Acute Exposure to Nigerian Xylopia Aethiopica Seed Extract on Liver and Kidney Functional Indices of Albino Rat. Iranian journal of toxicology 12(3): 51-58.
- Lawal B, Ossai PC, Shittu OK, Abubakar AN (2014) Evaluation of Phytochemicals, Proximate, Minerals and Anti-Nutritional Compositions of Yam Peel, Maize Chaff and Bean Coat. International Journal of Applied Biological Research 6(2): 21-37.
- Zhao Y, Wu Y, Wang M (2015) Bioactive Substances of Plant Origin. Cheung P, Mehta B (Eds.), Handbook of Food Chemistry, Heidelberg, Germany.
- Dhawan D, Gupta J (2016) Comparison of different solvents for phytochemical extraction potential from Datura metel plant leaves. International Journal of Biological Chemistry 11(1): 17-22.
- Shalini VB, Narayanan JS (2015) Antibacterial activity of Andrographis paniculata Nees against selective human pathogens. African Journal of Microbiology Research 9(16): 1122-1127.
- Nanthini R, Athinarayanan G, Ranjitsingh Aja, Mariselvam R, Chairman K, et al. (2013) antimicrobial activity of leaf extracts of the medicinal plants andrographis paniculata and Melia Azadirach L. International Journal of Current Research 5(11): 3563-3566.
- Deepak S, Pawar A, Shinde P (2014) Study of antioxidant and antimicrobial activities of Andrographis paniculate. Pelagia Research Library Asian Journal of Plant Science and Research 4(2): 31-41.
- Lawal B, Shittu OK, Oibiokpa FI, Berinyuy EB, Muhammed H (2016) African natural products with potential antioxidants and hepatoprotectives properties: a review. Clinical Phytoscience 2(23): 1-66.
-
RAyanwale O Abraham, Adabara U Nasiru, Adeniyi K Abdulazeez, Oyewole O Seun, David W Ogonna. Therapeutic Potency of the Polar and Non-Polar Extracts of Andrographis Paniculata Leaf Against some Pathogenic Bacterial Isolates. Arch Phar & Pharmacol Res. 2(3): 2019. APPR.MS.ID.000539.
-
Andrographis paniculata, Antibacterial activities, Phytochemicals, Polar, Non-polar solvents, pathogens, toxicity, antibiotics, enormously, E coli, Staphylococcus aureus, Streptococcus pyogenes, Salmonella typhi, Alkaloid, Phenol, Flavonoids, Steroids, Terpenoids, Saponins
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






