 Review Article
Review Article
Solutions of Formaldehyde in Water and Alcohols
Michael M Silaev*
Department of Chemistry, Lomonosov Moscow State University, Russia
Michael M Silaev, Department of Chemistry, Lomonosov Moscow State University, Vorobievy Gory, Moscow 119991, Russia.
Received Date:September 05, 2018; Published Date: September 26, 2018
Abstract
A mechanism of the initiated nonbranched-chain process of forming 1,2-alkanediols and carbonyl compounds in alcohol– formaldehyde systems is suggested. The quasi-steady-state treatment is used to obtain kinetic equations that can describe the nonmonotonic (with a maximum) dependences of the formation rates of the products on the concentration of free unsolvated formaldehyde. The experimental concentrations of the free unsolvated form of formaldehyde are given at the different temperatures, solvent permittivity, and total concentrations of formaldehyde in water and alcohols. An empirical equation for calculating the free formaldehyde concentration in alcohol–formaldehyde (including water/ethanediol–formaldehyde) systems at various temperatures and total formaldehyde concentrations and an equation for evaluating solvent concentrations in these systems were derived.
Keywords: Nonbranched-chain process; Free formaldehyde; 1-Hydroxyalkyl radical; Formyl radical; Competing reaction; Equation
Introduction
Free radicals add to the carbon atom at the double bond of the carbonyl group of dissolved free (unsolvated, monomer) formaldehyde. The concentration of free formaldehyde in the solution at room temperature is a fraction of a percent of the total formaldehyde concentration, which includes formaldehyde chemically bound to the solvent [1]. The concentration of free formaldehyde exponentially increases with increasing temperature [2]. The energy released as a result of this addition, when the C=O bond is converted into an ordinary bond, is 30 to 60kJ mol–1 (according to the data on the addition of С1–С4 alkyl radicals in the gas phase under standard conditions [3–6]). The resulting free 1:1 adduct radicals can both abstract hydrogen atoms from the nearestneighbor molecules of the solvent or unsolvated formaldehyde and, due to its structure, decompose by a monomolecular mechanism including isomerization [7,8].
The analysis of stable liquid-phase products was carried out by the gas chromatographic method. The quasi-steady-state treatment is used to obtain kinetic equations.
Addition of 1-Hydroxyalklyl Free Radicals with Two or More Carbon Atoms
Free 1-hydroxyalkyl radicals (which result from the abstraction of a hydrogen atom from the carbon atom bonded to the hydroxyl group in molecules of saturated aliphatic alcohols but methanol under the action of chemical initiators [9,10], light [11,12], or ionizing radiation [13,14]) add at the double bond of free formaldehyde dissolved in the alcohol, forming 1,2-alkanediols [7–10,12–18], carbonyl compounds, and methanol [14,15] via the chaining mechanism. (The yields of the latter two products in the temperature range of 303 to 448K are one order of magnitude lower). In these processes, the determining role in the reactivity of the alcohols can be played by the desolvation of formaldehyde in alcohol–formaldehyde solutions, which depends both on the temperature and on the polarity of the solvent [2,14]. For the γ -radiolysis of 1(or 2)-propanol–formaldehyde system at a constant temperature, the dependences of the radiation-chemical yields of 1,2-alkanediols and carbonyl compounds as a function of the formaldehyde concentration show maxima and are symbatic [13,15]. For a constant total formaldehyde concentration of 1 mol dm–3, the dependence of the 1,2-alkanediol yields as a function of temperature for 303–473 K shows a maximum, whereas the yields of carbonyl compounds and methanol increase monotonically [14] (along with the concentration of free formaldehyde [2]). In addition to the above products, the nonchain mechanism in the γ-radiolysis of the solutions of formaldehyde in ethanol and 1- and 2-propanol gives ethanediol, carbon monoxide, and hydrogen in low radiationchemical yields (which, however, exceed the yields of the same products in the γ-radiolysis of individual alcohols) [7,14,15]. The available experimental data can be described in terms of the following scheme of reactions.
Scheme
Chain initiation
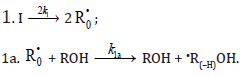
Chain propagation
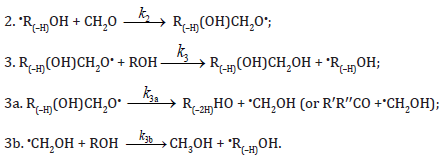
Inhibition

Chain termination
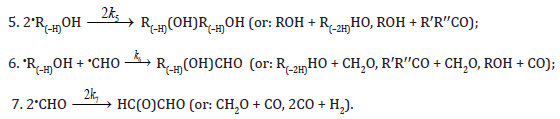
In these reactions, I is an initiator, e.g., a peroxide [9,10]; •R0/ , some reactive radical (initiator radical); R, an alkyl; ROH, a saturated aliphatic alcohol, either primary or secondary, beginning from ethanol; CH2O, the unsaturated molecule – free formaldehyde; •СН2ОН, the 1-hydroxymetyl fragment radical; •R(–H)OH, the reactive 1-hydroxyalkyl addend radical, beginning from 1-hydroxyethyl; R(–H)(ОH)СН2О•, the reactive hydroxyalkoxyl 1:1 adduct radical; •СНО, the low-reactive formyl radical (inhibitor radical); R0H, the molecular product; R(–H)(OH)СН2ОН, 1,2-alkanediol; R(–2H)HO, an aldehyde in the case of a primary alcohol and an R’R”CO ketone in the case of a secondary alcohol; R(–H)(ОH)R(–H)ОH, a vicinal alkanediol; R(–H)(OH)CHO, a hydroxyaldehyde. The chain evolution stage of Scheme includes consecutive reaction pairs 2–3, 2–3a, and 3a–3b; parallel (competing) reaction pairs 3–3a, 3–3b, 3–4, and 3a–4; and consecutive–parallel reactions 2 and 4.
In addition, it seems unlikely that free adduct radicals will add to formaldehyde at higher temperatures the reaction of adding is unlikely because this would result in an ether bond. The addition of hydroxymethyl radicals to formaldehyde, which is in competition with reaction 3b, is not included as well, because there is no chain formation of ethanediol at 303–448 K [14]. At the same time, small amounts of ethanediol can form via the dimerization of a small fraction of hydroxymethyl radicals, but this cannot have any appreciable effect on the overall process kinetics. The addition of free formyl radicals to formaldehyde cannot proceed at a significant rate, as is indicated by the fact that there is no chain formation of glycol aldehyde in the systems examined [14]. The mechanism of the decomposition of the free adduct radical via reaction 3a, which includes the formation of an intramolecular Н⋅⋅⋅О bond and isomerization, can be represented as follows [7,8,15]:

The probability of the occurrence of reaction 3a should increase
with increasing temperature. This is indicated by experimental
data presented above [7,8,15]. The decomposition of the hydroxy
alkoxyl radical. R(–H)(ОH)СН2О• (reaction 3a) is likely endothermic.
The of reaction 3a is indirectly indicated by the fact that the
decomposition of simple C2−C4 alkoxyl radicals RО• in the gas phase
is accompanied by heat absorption: (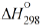 = 30−90 kJ mol–1 [4−6]).
Reaction 3b, subsequent to reaction 3a, is exothermic, and its heat
for C2−C3 alcohols in the gas phase is =
= 30−90 kJ mol–1 [4−6]).
Reaction 3b, subsequent to reaction 3a, is exothermic, and its heat
for C2−C3 alcohols in the gas phase is = 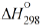 −40 to −60 kJ mol–1
[4–6]. As follows from the above scheme of the process, reactions
3a and 3b, in which the formation and consumption of the highly
reactive free radical hydroxymethyl take place (at equal rates under
steady-state conditions), can be represented as a single bimolecular
reaction 3a,b occurring in a “cage” of solvent molecules. The free
formyl radical resulting from reaction 4, which is in competition
with reactions 3 and 3a, is comparatively low-reactive because its
spin density can be partially delocalized from the carbon atom via
the double bond toward the oxygen atom, which possesses a higher
electron affinity [3]. For example, in contrast to the methyl and
alkoxyl π-radicals, the formyl σ-radical can be stabilized in glassy
alcohols at 77 K [19]. In the gas phase, the dissociation energy of
the C–H bond in formyl radicals is half that for acetyl radicals and is
about 5 times lower than the dissociation energy of the Сα–Н bond
in saturated C1–C3 alcohols [3].
−40 to −60 kJ mol–1
[4–6]. As follows from the above scheme of the process, reactions
3a and 3b, in which the formation and consumption of the highly
reactive free radical hydroxymethyl take place (at equal rates under
steady-state conditions), can be represented as a single bimolecular
reaction 3a,b occurring in a “cage” of solvent molecules. The free
formyl radical resulting from reaction 4, which is in competition
with reactions 3 and 3a, is comparatively low-reactive because its
spin density can be partially delocalized from the carbon atom via
the double bond toward the oxygen atom, which possesses a higher
electron affinity [3]. For example, in contrast to the methyl and
alkoxyl π-radicals, the formyl σ-radical can be stabilized in glassy
alcohols at 77 K [19]. In the gas phase, the dissociation energy of
the C–H bond in formyl radicals is half that for acetyl radicals and is
about 5 times lower than the dissociation energy of the Сα–Н bond
in saturated C1–C3 alcohols [3].
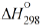 = −135 to −385 kJ mol–1 [3−6].
= −135 to −385 kJ mol–1 [3−6].
The rates of the chain formation of 1,2-alkanediols in reaction 3 (and their nonchain formation in reaction 4), carbonyl compounds in reaction 3a, and methanol in reaction 3b are given by the equations1 :
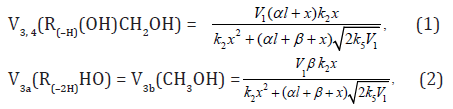
where V1 is the initiation rate, l is the molar concentration of
the saturated alcohol at the given total concentration c0 of
formaldehyde2 dissolved in it, x is the molar concentration of free formaldehyde (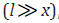 ), k2 is the rate constant of reaction 2
(addition of 1-hydroxyalkyl free radical to free formaldehyde), and
α = k3/k4 and β = k3а/k4 (mol dm–3) are the ratios of the rate constants
of competing (parallel) reactions. Estimates of 2k5 were reported by
Silaev et al. [22 24]. From the extremum condition for the reaction
3a rate function, , we derived the following analytical expression:
), k2 is the rate constant of reaction 2
(addition of 1-hydroxyalkyl free radical to free formaldehyde), and
α = k3/k4 and β = k3а/k4 (mol dm–3) are the ratios of the rate constants
of competing (parallel) reactions. Estimates of 2k5 were reported by
Silaev et al. [22 24]. From the extremum condition for the reaction
3a rate function, , we derived the following analytical expression:
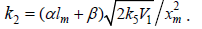
footnote
1The rate equations were derived by quasi-steady-state treatment, which is most suitable for describing the processes including at least eight to ten
reactions with four to six different free radicals and at most three to seven experimental points in their functional curves, using the condition for the first
steps of the process that makes it possible to reduce the exponent of term 2k5[•R(−H)OH]2 to 1 in equation d[R•]/dt = 0 [7, 15]: k6 = 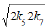 [20] and V1 = V5
+ 2V6 + V7 = (
[20] and V1 = V5
+ 2V6 + V7 = ( 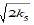 [•R(–H)OH] +
[•R(–H)OH] + 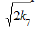 [•CHO])2. The relations between the reaction rates and radiation-chemical yields for radiation-chemical processes are
reported by Silaev [21].
[•CHO])2. The relations between the reaction rates and radiation-chemical yields for radiation-chemical processes are
reported by Silaev [21].
2 The alcohol concentration in alcohol–formaldehyde solutions at any temperature can be estimated by the method suggested in [22, 23]. The data necessary for estimating the concentration of free formaldehyde using the total formaldehyde concentration in the solution are reported by Silaev et al. [2, 22].
Equations (1) and (2) taken on the condition that 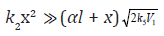 (the descending branch of the curve having a maximum)
can be transformed to equations that can be used for a preliminary
estimation of parameters α and β:
(the descending branch of the curve having a maximum)
can be transformed to equations that can be used for a preliminary
estimation of parameters α and β:
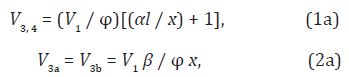
where φ = 2 for the maximum (when 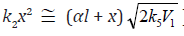 ) and φ
= 1 for the descending portion of the curve.
) and φ
= 1 for the descending portion of the curve.
The overall process rate is a complicated function of the formation and disappearance rates of the •R(–H)OH and •СНО free radicals:
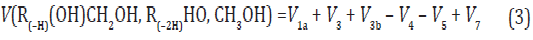
The ratios of the rates of the competing reactions are V3/V4 = αl/x and V3a/V4 = β/x, and the chain length is ν = (V3 + V3a)/V1. The ratio of the rates of formation of 1,2-alkanediol and the carbonyl compound is a simple linear function of x:
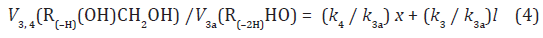
The equations for the rates of chain-termination reactions 5–7 are given by the following equations:
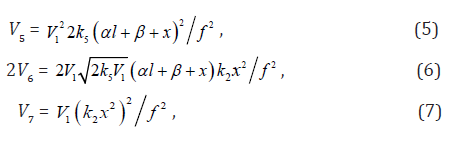
where 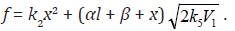
Neutral formaldehyde solutions in alcohols at room temperature primarily consist of a mixture of formaldehyde polymer solvates reversibly bound to alcohols; these polymer solvates differ in molecular mass and have the general formula RO(CH2O)nH, where n = 1–4 [1]. The concentration of formaldehyde that occurs in solution as a free, unsolvated active species chemically unbound to the solvent (this species is capable of scavenging free radicals) at room temperature is lower than a percent of the total formaldehyde concentration [1]. The concentration x of the free formaldehyde species in solutions was determined by high-temperature UV spectrophotometry in the range 335–438 K at the total formaldehyde concentration c0 (free and bound species including the concentration of polymer solvates) of 1.0–8.4 mol dm–3 in water, ethanediol, methanol, ethanol, 1-propanol, 2-propanol, and 2-methyl-2-propanol [2] (Table 1). This concentration increases with temperature according to an exponential law, and it can be as high as a few percent of the total concentration in solution under the test conditions, up to 19.3% in the case of 2-methyl-2-propanol at a total concentration of 1.0 mol dm–3 and a temperature of 398 K. The following empirical equation relating the concentration x (mol dm–3) of free formaldehyde to temperature T (K) and the total concentration c0 in the solution (measured at room temperature), was developed by the treatment of 101 data points [2,22]:
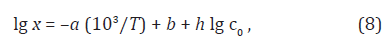
where the coefficients a and b were calculated as the parameters of a straight-line equation by the least-squares technique from the dependence of lg x on 1/T at c0 = 1.0 mol dm–3 for various solvents, and the coefficient h was obtained as the average value of the slopes of lg x as linear functions of lg c0 at various series of fixed temperatures. The Table 2 summarizes these coefficients for each solvent. As regards the experimental data, the error in the calculations of the concentration x of free formaldehyde made by Eq. (8) in the specified temperature range was no higher than 25% .
Table 1: The experimental concentrations x (mol dm–3) of free formaldehyde at different temperatures T (K) and total formaldehyde concentrations c0 (mol dm–3) in various solvents.
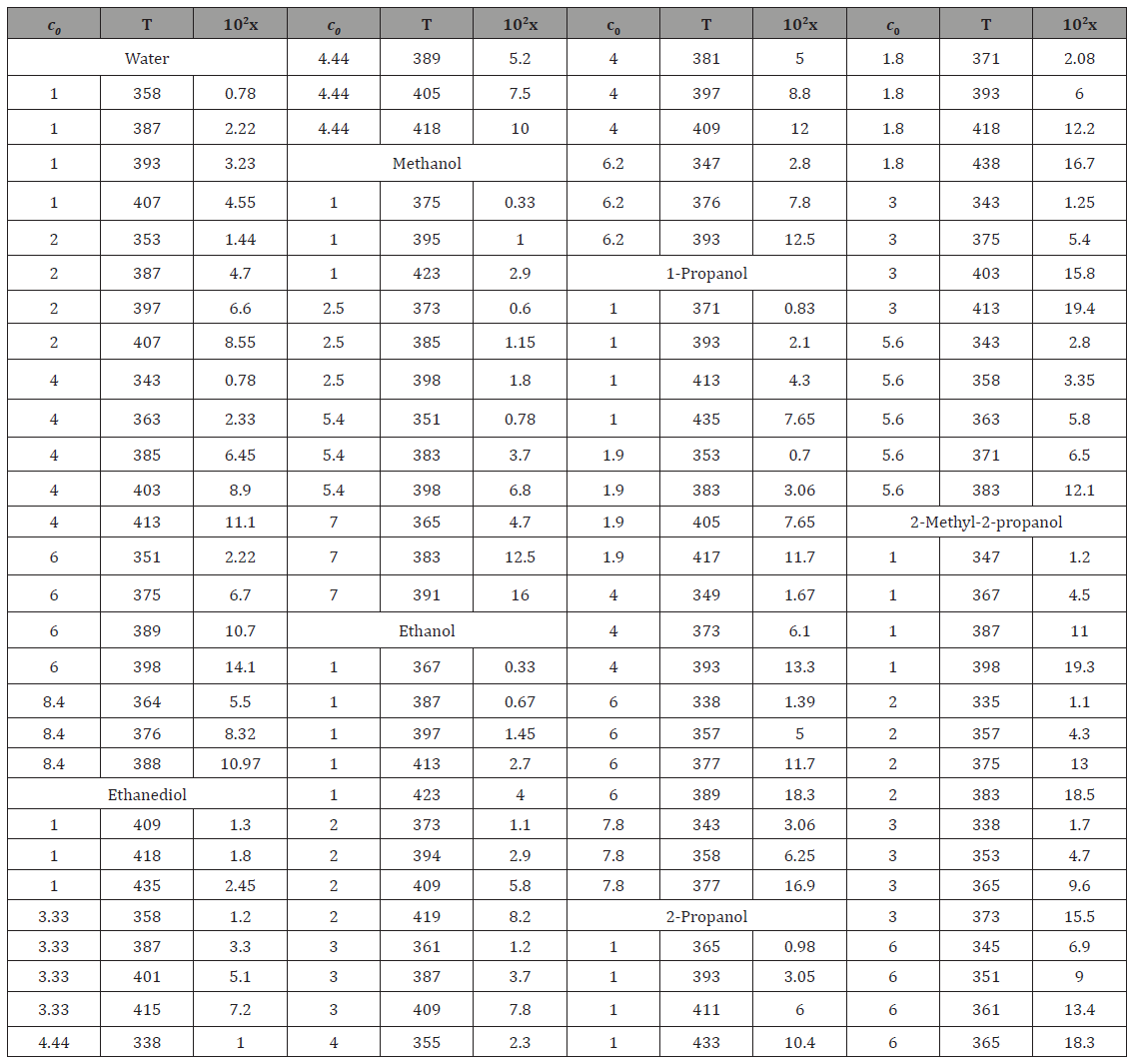
Table 2: Coefficients of the empirical Eq. (8) for the estimation of the concentration x of free formaldehyde in polar solvent–formaldehyde systems.
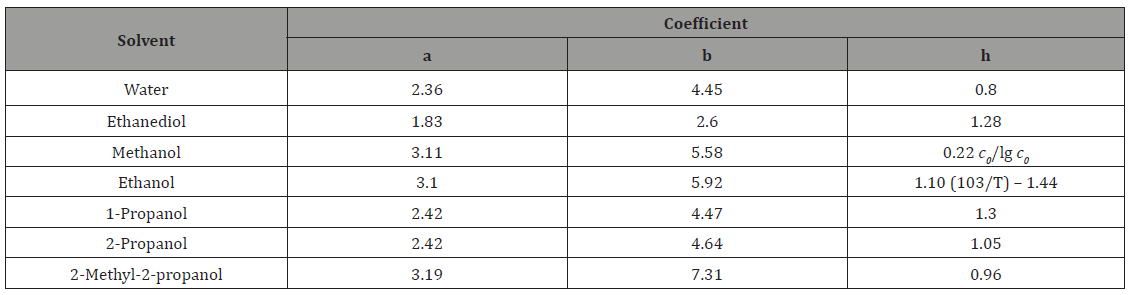
On the assumption that the dependence of the density of a given solution on the concentration of formaldehyde is similar to the analogous linear dependence found for aqueous formaldehyde solutions (0–14 mol dm–3; 291 K) [25], the concentrations lT (mol dm–3) of alcohols in alcohol–formaldehyde solutions at a certain temperature can be estimated by the equation
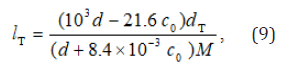
where c0 is the total formaldehyde concentration (mol dm-3); M is the molecular mass (g mol–1) of the solvent; d and dT are the solvent densities (g cm–3) at room and given temperatures, respectively; the coefficients 8.4 × 10–3 and 21.6 have the units of 103g mol–1 and g mol–1, respectively.
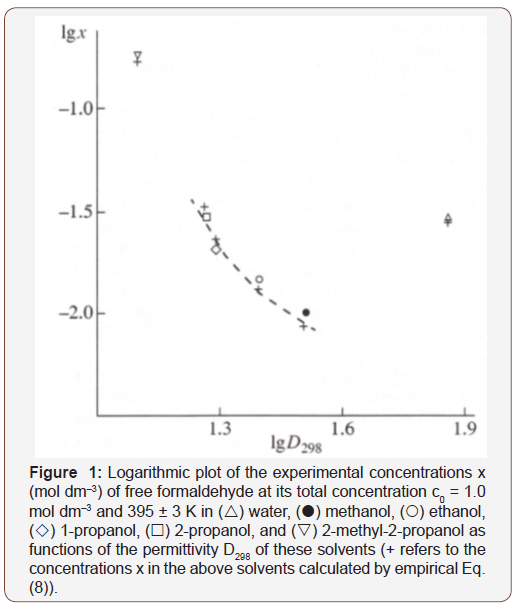
Earlier [2], it was found that the concentration x of the free formaldehyde species decreased with the solvent permittivity D298 at a constant temperature. Water is an exception. Although water is more polar than alcohols, the concentration x of free formaldehyde in an aqueous solution is anomalously high and reaches the level of its concentration in 2-propanol, all other factors being the same (see Figure. 1) [2, 22]. This can be due to the specific instability of hydrated formaldehyde species and the ease of their conversion into free formaldehyde with increasing temperature (Figure 1).
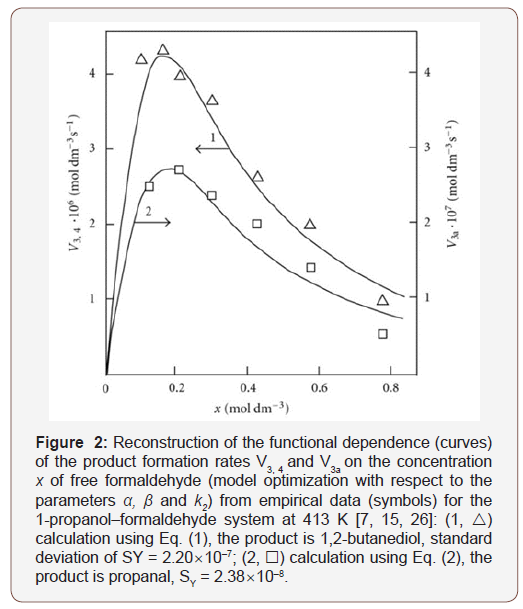
As an example, the Figure 2 illustrates the use of Eqs. (1) and (2) for describing the experimental dependences of the formation rates of 1,2-butanediol (curve 1) in reactions 3 and 4 and propanal (curve 2) in reaction 3a on the concentration x of free formaldehyde in the 1-propanol–formaldehyde reacting system at the total formaldehyde concentration c0 of 2.0 to 9.5 mol dm–3 and temperature of 413 K [7,15,26]. The concentration dependence of the propanal formation rate was described using the estimates of kinetic parameters obtained for the same dependence of the 1,2-butanediol formation rate. We considered these data more reliable for the reason that the carbonyl compounds forming in the alcohol–formaldehyde systems can react with the alcohol and this reaction depends considerably on the temperature and acidity of the medium [1]. The mathematical modeling of the process was carried out using a 137Cs γ-radiation dose rate of P = 0.8 Gy s–1 [13,26], a total initiation yield of G(CH3СН2ĊНОН) = 9.0 particles per 100 eV [7,15] (V1 = 4.07 × 10–7 mol dm–3 s–1), and 2k5 = 4.7 × 109 dm3 mol–1 s–1. The following values of the parameters were obtained: α = 0.36 ± 0.07, β = 0.25 ± 0.05 mol dm–3, and k2 = (6.0 ± 1.4) × 103 dm3 mol–1 s–1 (Figure 2).
Note that, as compared to the yields of 1,2-propanediol in the γ-radiolysis of the ethanol–formaldehyde system, the yields of 2,3-butanediol in the γ-radiolysis ossssssf the ethanol–acetaldehyde system are one order of magnitude lower [26]. Using data from [7,15], it can be demonstrated that, at 433 K, the double bond of 2-propen-1-ol accepts the 1-hydroxyethyl radical 3.4 times more efficiently than the double bond of formaldehyde [27].
Addition of Hydroxymethyl Free Radicals
The addition of hydroxymethyl radicals to the carbon atom at the double bond of free formaldehyde molecules in methanol, initiated by the free-radical mechanism, results in the chain formation of ethanediol [16]. In this case, reaction 3a in Scheme is the reverse of reaction 2, the 1-hydroxyalkyl radical •R(–H)OH is the hydroxymethyl radical •СН2ОН, so reaction 3b is eliminated (k3b = 0), and reaction 5 yields an additional amount of ethanediol via the dimerization of chain-carrier hydroxymethyl radicals (their disproportionation can practically be ignored [28]). The scheme of these reactions is presented in [17].
The rate equation for ethanediol formation by the chain mechanism in reaction 3 and by the nonchain mechanism in reactions 4 and 5 in the methanol–formaldehyde system has a complicated form3 as compared to Eq. (1) for the formation rate of the other 1,2-alkanediols [8]:
Foot note 3
3 an earlier publication [15], this equation does not take into account reaction 3a.
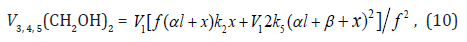
where 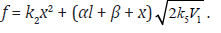
Equation (10) when 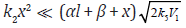 (ascending branch
of the curve having a maximum) and
(ascending branch
of the curve having a maximum) and 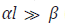 (practically without
reaction 3a) is transformed to a simple directly-proportional
dependence on the concentration x of free formaldehyde, which can
be used to pre-estimate the parameter k2:
(practically without
reaction 3a) is transformed to a simple directly-proportional
dependence on the concentration x of free formaldehyde, which can
be used to pre-estimate the parameter k2:
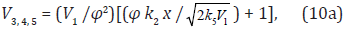
where φ = 1 for the ascending portion of the curve and φ = 2 for the maximum, when
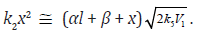
If the rate of ethanediol formation by the dimerization mechanism in reaction 5 is ignored for the reason that it is small as compared to the total rate of ethanediol formation in reactions 3 and 4, Eq. (10) will be identical to Eq. (1). After the numerator and denominator on the right-hand side of Eq. (1) are divided by k–2 ≡ k3a, one can replace k2 in this equation with K2 = k2/k–2, which is the equilibrium constant for the reverse of reaction 2. Ignoring the reverse step of reaction 2 (k3a = 0, β = 0) can further simplify Eq. (1). In this case, the rate constant k2 is effective.
Conclusion
In summary, the material on the kinetics of nonbranchedchain addition of free saturated 1-hydroxyalkyl radicals to the double bonds of free formaldehyde molecules makes it possible to describe, using Eq. (1) obtained by quasi-steady-state treatment, experimental dependences with a maximum of the formation rates of molecular 1:1 adducts (1,2-alkanediols) on the concentration of an unsaturated compound over the entire region of its change in binary reaction systems consisting of saturated (alcohol) and unsaturated (free formaldehyde) components (Figure 2).
The proposed addition mechanism involves the reaction of a free hydroxyalkoxyl 1:1 adduct radical with an unsaturated free formaldehyde molecule yielding a low-reactive free formyl radical (the reaction 4 competing with the chain propagation reactions in the Scheme). In such binary reaction systems, the unsaturated compound is both a reactant and an autoinhibitor, specifically, a source of low-reactive free radicals shortening kinetic chains.
Acknowledgement
None
Competing Interests
No Conflict of Interest.
References
- Ogorodnikov SK (1984) Formal’degid (Formaldehyde), Khimiya, Leningrad.
- Silaev MM, AV Rudnev AV, Kalyazin EP (1979) Formaldehyde III Concentration of Free Formaldehyde as a Function of Temperature, Polarity of Solvents, and Total Concentration of Formaldehyde in Solution. Zhurnal Fizicheskoi Khimii 53(7): 1647-1651.
- Gurvich LV, Karachevtsev GV, Kondrat’ev VN, Lebedev YA, Medvedev VA, et al. (1974) Energii razryva khimicheskikh svyazei. Potentsialy ionizatsii i srodstvo k elektronu. Kondrat’ev VN (Eds) Bond Dissociation Energies, Ionization Potentials, and Electron Affinity, Nauka, Moscow, Capital of Russia.
- Benson SW (1976) Thermochemical Kinetics: Methods for the Estimation of Thermochemical Data and Rate Parameters. (2nd edn), Wiley, New York, USA.
- Pedley JB, Naylor RD, Kirby SP (1986) Thermochemical Data of Organic Compounds. (2n2ndedn), Chapman & Hall, London.
- Orlov YD, Lebedev YA, Saifullin IS (2001) Termokhimiya organicheskikh svobodnykh radikalov. AM Kutepov (Eds) Thermochemistry of Organic Free Radicals, Nauka, Moscow, Capital of Russia.
- Silaev MM, Bugaenko LT (1994) Kinetics of the Addition of α-Hydroxyalkyl Radicals to 2-Propen-1-ol and Formaldehyde. Kinetics and Katalysis 35(4): 463-467.
- Silaev MM (2007) Simulation of Nonbranched Chain Processes for Producing 1,2-Alkanediols in Alcohol–Formaldehyde Systems. Thoretical Foundations Chemical Engineering 41(4): 357–361.
- Oyama M (1965) A Free-Radical Reaction of Primary and Secondary Alcohols with Formaldehyde. The Journal of Organic Chemistry 30(7): 2429–2432.
- Nikishin GI, Lefor D, Vorob’ev ED (1966) Free Radical Reaction of Primary Alcohols with Formaldehyde. Izvestiya Akademii Nauk SSSR, Ser. Khimiya: 1271–1272.
- Urry WH, Stacey FW, Huyser ES, Juveland OO (1954) The Peroxide- and Light-Induced Additions of Alcohols to Olefins. Journal of the American Chemical Society 76(2): 450–455.
- Dzhurinskaya MB, Rudnev AV, Kalyazin EP (1984) High Temperature UV Photolysis of Formaldehyde in Liquid Methanol. Vestnik Moskovskogo Universiteta, Ser. 2: Khimiya, 25(2): 173–176.
- Kalyazin EP, Petryaev EP, Shadyro OI (1977) Reaction between Oxyalkyl Radicals and Aldehydes. Zhurnal Organicheskoi Khimii 13(2): 293–295.
- Novoselov AI, Silaev MM, Bugaenko LT (2004) Effect of Temperature on the Yields of Final Products in the γ-Radiolysis of Formaldehyde Solutions in C1–C3 Alkanols. High Energy Chemistry 38(4) 236–238.
- Silaev MM, Bugaenko LT (1992) Mathematical Simulation of the Kinetics of Radiation Induced Hydroxy alkylation of Aliphatic Saturated Alcohols. Radiation Physics and Chemistry 40(1): 1–10.
- Novoselov AI, Silaev MM, Bugaenko LT (2008) Dependence of Ethanediol Yield on Formaldehyde Concentration in γ-Radiolysis of Methanol–Formaldehyde System at 373–473 K. High Energy Chemistry 42(1): 69–70.
- Novoselov AI, Silaev MM, Bugaenko LT (2010) γ-Induced Single-Step Synthesis of Ethylene Glycol from Methanol–Formaldehyde Solution. Theoretical Foundation of Chemical Engineering 44(4): 432–435.
- Novoselov AI, Silaev MM, Bugaenko LT (2007) Dependence of 1,2-Propanediol Yield on Formaldehyde Concentration in γ-Radiolysis of Ethanol–Formaldehyde System at 373–473 K. High Energy Chemistry 41(1): 53-54.
- Pshezhetskii SY, Kotov AG, Milinchuk VK, Roginskii VA, Tupikov VI (1972) EPR svobodnykh radikalov v radiatsionnoi khimii ESR of Free Radicals in Radiation Chemistry, Khimiya, Moscow, Belarus.
- L Bateman (1954) “Olefin Oxidation”. Quarterly Reviews 8(2): 147–167.
- Silaev MM (2007) Simulation of the Nonbranched-Chain Addition of Saturated Free Radicals to Alkenes and Their Derivatives Yielding 1:1 Adducts. Theoretical Foundations of Chemical Engineering 41(3): 454– 454.
- Silaev MM (2002) Applied Aspects of the γ-Radiolysis of C1–C4 Alcohols and Binary Mixtures on Their Basis. High Energy Chemistry 36(2): 70–75.
- Silaev MM (1993) Estimating the Solvent Concentration in Formaldehyde Solutions at Various Temperatures. Zhurnal Fizicheskoy Khimii 67(9): 1944.
- Silaev MM, Bugaenko LT, Kalyazin EP (1986) On the Possibility of Adequately Estimating of the Rate Constants for the Reaction of Hydroxyalkyl Radicals with Each Other Using the Self-Diffusion Coefficients or Viscosities of the Corresponding Alcohols. Vestnik Moskovskogo. Univiversiteta, Ser. 2: Khimiya, 27(4), pp. 386–389.
- Walker JF (1957) Formaldehyde, Reinhold, New York, 1953, English Translation under the title Formal’degid, Goskhimizdat, Moscow, Belarus, p. 106.
- Shadyro OI (1975) Radiation-chemical Conversions of Aldehydes in Various Systems (1st edn), Belarusian State University, Minsk, Belarus.
- Silaev MM (1993) Relative Reactivity of α-Hydroxyethyl Radicals for 2-Propene-1-ol and Formaldehyde Double-Bond Addition. Vestnik Moskovskogo Universiteta 34(3): 311.
- Seki H, Nagai R, Imamura M (1968) γ-Radiolysis of a Binary Mixture of Methanol and Water. The Formation of Formaldehyde in the Radiolysis of Liquid Methanol. Bulletin of the Chemical Society of Japan 41(12): 2877–2881.
-
Michael M Silaev. Solutions of Formaldehyde in Water and Alcohols. Arch Phar & Pharmacol Res. 1(1): 2018. APPR. MS.ID.000504.
-
Nonbranched-chain process, Free formaldehyde, 1-Hydroxyalkyl radical, Formyl radical, Competing reaction, Equation, Formaldehyde, Water, Alcohols, Chemistry, Alcohol-formaldehyde, Ethanediol–formaldehyde, Carbon monoxide, Hydrogen, Hydroxymethyl free radicals, Alkanediols, Neutral formaldehyde solutions
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






