 Research Article
Research Article
Evaluation of Novel Aspirin Suppository Formulation in HT-29 Human Adenocarcinoma Cells
Miriam A Ansong*, Rocco J Rotello, Danielle Eaton, Tiffany Hong, Mallory Thompson, Sarah Meyers6, Joseph Newman, Nathanael Smith, Nicole Stute and Yang Wameng*
1-9Department of Pharmacy Practice, Cedarville University School of Pharmacy, USA
1010Department of Clinical and Administrative Sciences, California Health Sciences University, USA
Miriam A Ansong, E.MBA, Pharm.D. Assistant Dean for Assessment and Program Excellence, Professor of Pharmacy, Clinical and Administrative Sciences. California Health Sciences University, 120 N Clovis Ave, Clovis CA, 93612, USA.
Received Date:January 02, 2020; Published Date: January 14, 2020
Abstract
Objective: The objective of this study was to evaluate a newly developed novel aspirin suppository that may have potential properties that enhance its absorption in the rectum.
Methods: This preclinical in vitro study utilized a human cell line, HT-29, to approximate the environment of the human rectum/colon. Six different suppository formulations, including zinc carnosine and acetyl salicylic acid, were used as new excipients aimed at increasing absorption of aspirin into these cells. The amount of aspirin absorbed was indirectly determined by the quantitating levels of 12(S)-HETE in pg/ml. A 12(S)-HETE competitive ELISA assay served as the primary outcome measure. Each suppository formulation was tested for its effect on cultured HT-29 cells.
Key findings: The activity measure was accomplished using light absorbance spectroscopy recorded in units of optical density (Tables 2-5). Despite some variations in the standard curve measures as described in the 12(S)-HETE kit, the authors observed absorbance of aspirin in the HT-29 cells. The results provide a platform for further preclinical studies to analyze suppository formulations in challenging in vitro models for advancing into clinical trials research.
Conclusion: Further studies utilizing a different endpoint with more specific COX-1 and possibly COX-2 assay measures and with more uniquely defined suppository mixtures may yield precise data.
Keywords: Aspirin; NPO; Suppository; Alternative
Introduction
As the baby-boomer population reaches an older age, there is an expected continual increase in hospitalization and the number of patients who are unable to take medications orally (NPOnothing through the mouth). An alternative drug delivery system for NPO patients is thus essential in providing optimal medication therapy. Specifically, the administration of a rectal suppository dosage form allows NPO patients an alternative route of drug administration. This could play an essential role in patients who suffer from gastrointestinal diseases such as peptic ulcer disease (PUD) or gastroesophageal reflux disease (GERD). Acetylsalicylic acid (Aspirin®), is among the many drugs used by the aging population. Avoiding aspirin contact with the stomach lining is therefore paramount [1]. As a result, special consideration must be given to suppository formulation of medications that are used in this population. The literature shows 50 percent usage of Aspirin in patients age 65 years or older compared to 27 percent in patients age 45 to 64 years [1].
Currently, two aspirin suppositories are marketed in the United States. One by Paddock Laboratories based out of Minneapolis, MN, and the other by Perrigo Company based out of Dublin, Ireland. The current Aspirin suppositories are available in 300 mg and 600 mg strengths, packaged in twelve-unit doses per box. A review of the literature indicated that very little research regarding absorption and retention of aspirin suppositories has been published since the mid 1970’s. The research that was done from 1974-1979 gave varying and conflicting results [2-4]. A dated clinical study done by Nowak [2] concluded that absorption of Aspirin suppositories when given to children after 10 hours is slow [2]. Another clinical study by Connolly [4] found aspirin rectal administration effective in reducing temperature in children after open heart surgery [4]. A more recent preclinical study by Broome [5] concluded that, in horses, aspirin serum levels peaked at 0.33 hours and becomes undetectable after 4 hours in plasma serum [5]. Despite exhaustive consensus, most researchers agree that aspirin suppositories are less efficacious than orally dosed aspirin [5]. Many of these studies examined the extent to which aspirin is absorbed rectally from a suppository by taking blood serum samples or urine samples [2,4].
Given the pro-drug nature of aspirin, its cleavage into salicylic acid makes it more absorbable through the upper GI tract. Due to the difference in chemical composition of the colorectal junction as compared to the stomach, the major difference is the pH [6]. The pH of the stomach ranges from 1.2 to 2 depending on the presence of food. In contrast, the rectum has a pH of 7 in the absence of fecal matter [6]. In the rectum, the salicylic acid is completely ionized and unable to pass through the cells, making the stomach an ideal environment for hydrolysis [7]. Even though hydrolysis could still take place in the rectum, the absorption and rate of onset of the drug would be reduced [8,9]. With this in mind, coupled with the results from the literature, the purpose of this study was to develop a novel aspirin suppository to enhance absorption in the rectum using a unique excipient zinc-carnosine. The goal is to improve the absorption of aspirin suppositories in the rectal medium. The excipient that became the focus of this study was zinc-carnosine. zinc-carnosine acts as an antioxidant, with anti-inflammatory properties that are prostaglandin-independent. This product has been studied as a supplement for stomach ulcers and ulcerative colitis [10]. Since the target patient population consists of elderly patients, who have compromised cell integrity in the rectum, the stabilizing effects of zinc carnosine on the cells are speculated to enhance absorption. There is an apparent lack of literature exploring how different excipients and bases might affect the absorption of aspirin from a suppository dosage form.
With limited current literature on the topic, the investigators decided to explore the use of additives to aspirin suppositories to evaluate its impact on enhancing absorption through the rectal medium first in a preclinical setting to foster advancement in clinical trials [2,4]. The major gap in the literature regarding aspirin suppositories is one of the major motivations for researching this topic as evidenced though the report by the United Health Foundation [11,12].
The objective of this study is to assess the absorption of an aspirin suppository formulated with zinc-carnosine excipients to improve therapy for NPO patients. Our hypothesis is that the addition of zinc-carnosine will aid in the absorption into the lower rectum.
Methods
This preclinical in-vitro method development study investigated the effects of a novel suppository formulation on cells cultured in vitro. Initially two human colonic cell lines were obtained from ATCC, CaCo2 colonic and HT-29. These cells were cultured over and assessed for viability using trypan blue exclusion (Figure 1).
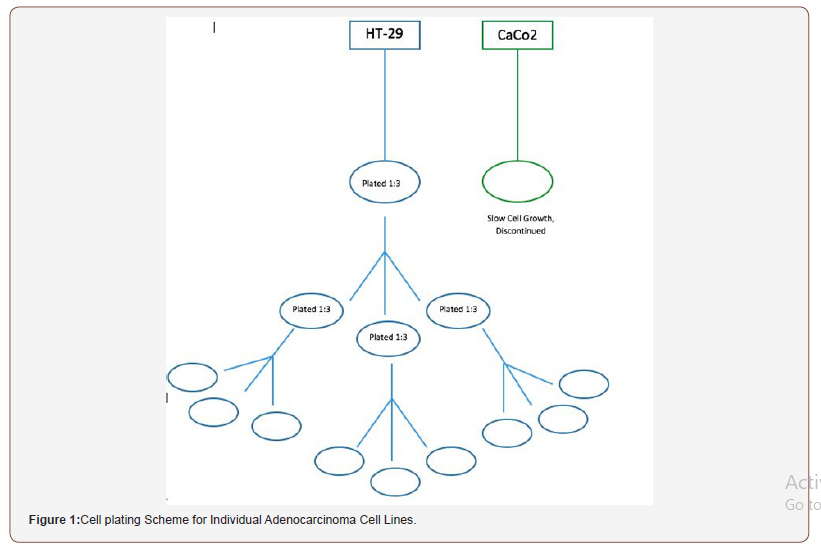
Due to limitations in numbers and variables with suppositories and complexity of assay, the HT-29 cell line was pursued for this research. Five different suppository formulations were compounded using: zinc-carnosine 150 mg, aspirin 600 mg with zinc-carnosine 75 mg, aspirin 600 mg with zinc-carnosine 150 mg, aspirin 600 mg, and a placebo fatty base suppository.
The suppository formulations were administered to the HT- 29 cells. The appropriate amount of the suppository to be added to the cells was determined to avoid toxicity. The rationale for testing effects is based on inhibiting the Cox-1 pathway which the HT-29 cell line has been reported to be present [13]. If the Cox-1 pathway is suppressed the 12-S- HETE pathway to be maintained and possibly increased as molecules are forced into lipogenesis route. If this pathway is dominant in the presence of aspirin then concentration of 12-S-HETE enzymes should be increased, implying a delivery and effect on colonic epithelium.
Given the fact that there is no approved industry standard to determine the in vitro/in vivo correlation for absorption, a trial and error process was followed for the protocol. Initially, an aspirin suppository was placed on the cell plate and incubated for 2 hours. This resulted in cell death due to toxicity (Figure 2, Figure 3).
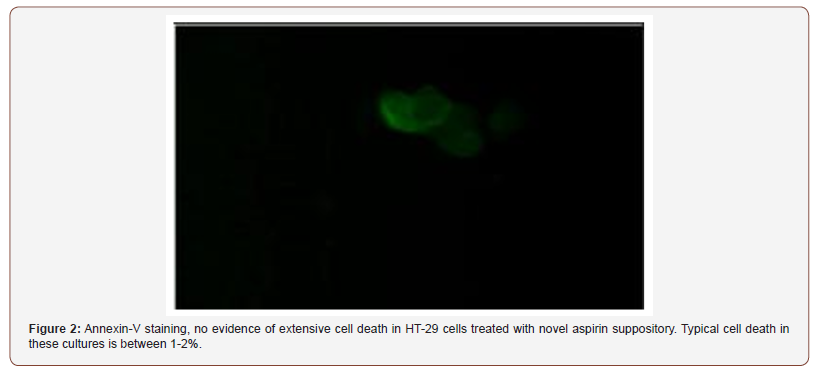
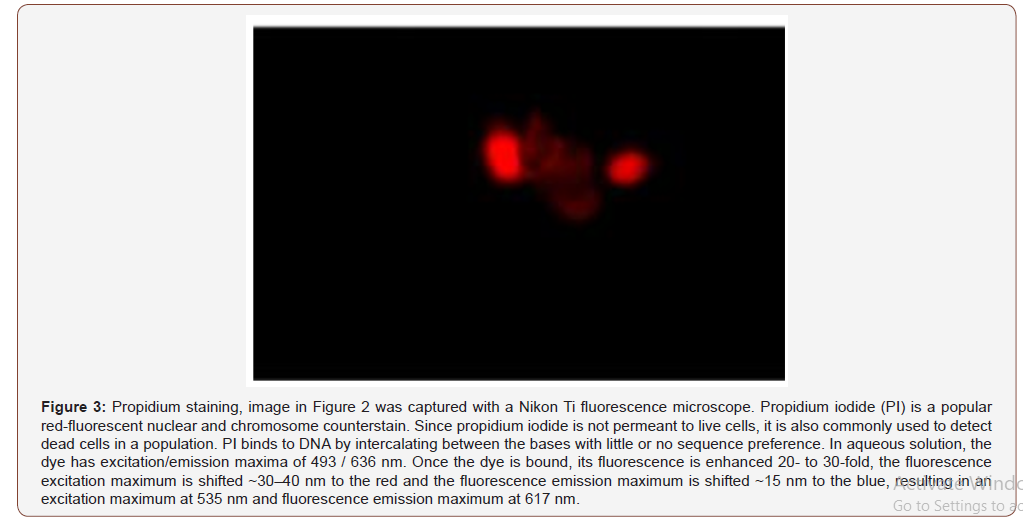
The protocol was adjusted by applying thin shavings (equal mass, 400mg) of the suppositories to the cells to better approximate the ratio of suppository to rectal cell exposure. This also resulted in some cell death but not to the extent as experienced in the first protocol. In the third trial, the suppositories were melted at 37 C and 2.8 mL of each formulation was applied to the cells with DMEM media containing serum. These plates were incubated for 24 hours and few surviving cells were detected (Figure 4).
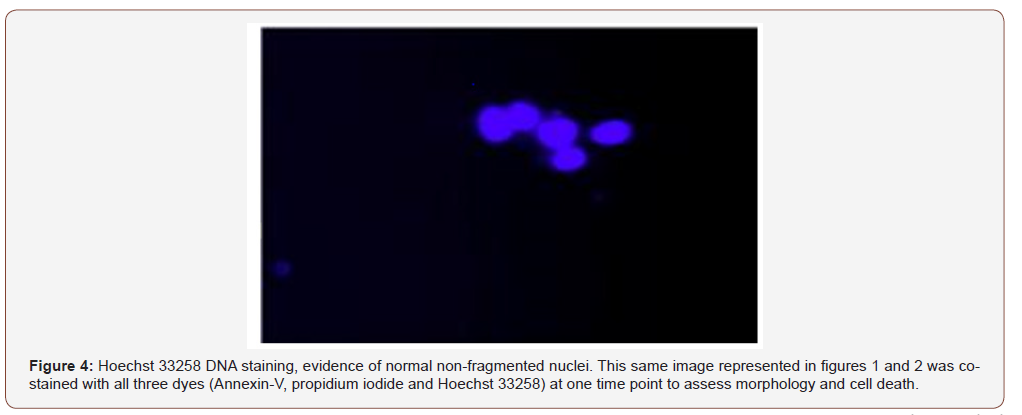
Once the appropriate amount of suppository that did not overtly affect cell survival was established, each sample was prepared for the assay. HT-29 cells were cultured as six 100cm2 plates (approximately 80-90% confluent). The six different suppository formulations prepared and stored at -20°C were brought to room temperature: the Paddock Aspirin suppository, zinc-carnosine 150 mg, aspirin 600 mg with zinc carnosine 75 mg, aspirin 600 mg with zinc-carnosine 150 mg, aspirin 600 mg, and the placebo fatty base suppository. One suppository of each formulation was placed in a 50 mL tube containing 30 mL of nutrient media and placed in a 37°C water bath. The tubes were vortexed at minute 10 to ensure a uniform suspension and again at minute 20 and removed at minute 30. The contents were then poured through a 70-micron filter in a biological safety cabinet that was HEPA-filtered. Sterile aliquots of 2.8 mL of liquid suppository were placed in each cell culture dish along with 7.2 mL of sterile DMEM media for 24 hours. Samples of the media and cell culture from each plate were taken and placed into vials. These vials were stored at -80°C for later use in the assay. Data was collected for all samples.
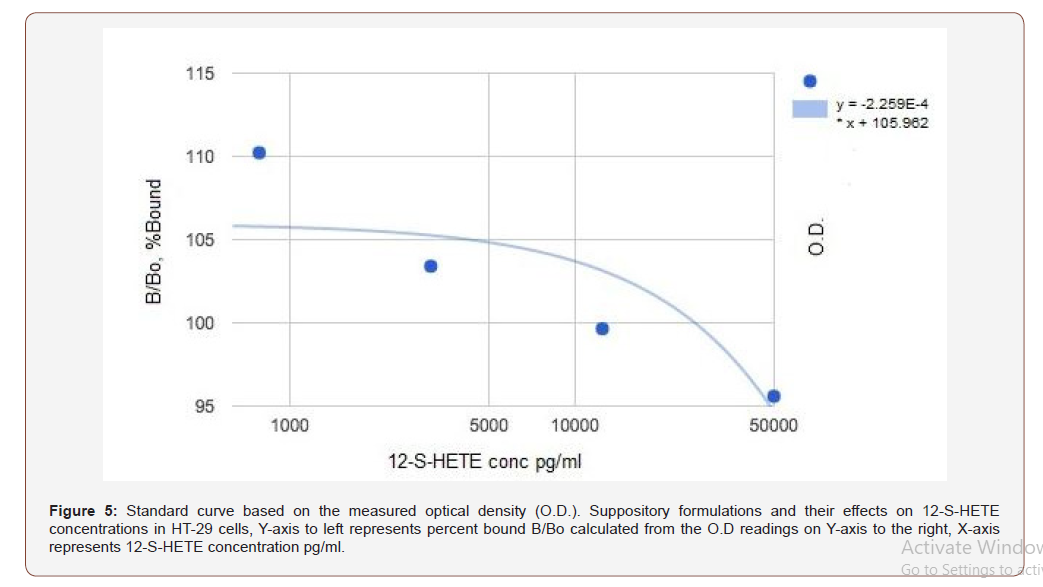
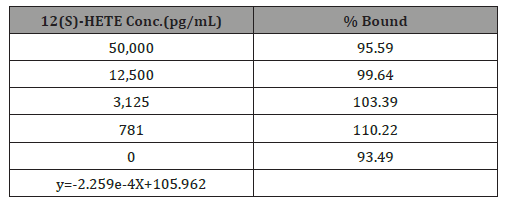
The effects of each suppository formulation on HT-29 cells was assessed using the Abcam® 12(S) HETE ELISA Kit [14]. In preliminary assays (n=2), optimal standard curves obtained using the 12(S)- HETE ELISA assay and spectroscopy in the ProMega Glomax (Figure- 5, Table 1) did not meet established criteria set out in the manufactured assay protocol (Figure- 6). No statistical analysis was performed on the raw data (Figure- 5 &6, Table 1).
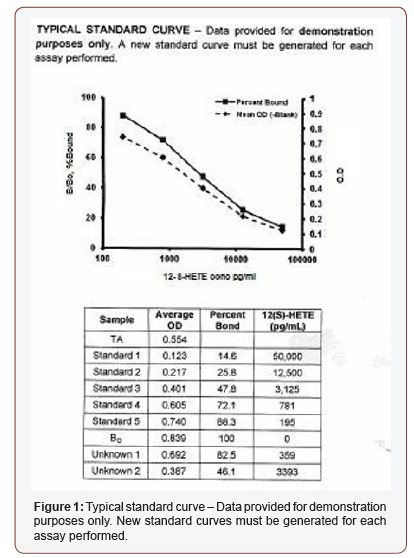
Table 1: Standard curve calculated values.
Results and Data Analysis
Based on the 5-arms of the study designed and the data planned to be collected, an ANOVA was selected to analyze the data along with descriptive statistics. The statistical analysis we planned to use was an ANOVA with a Dunnett’s post-test to determine where potential differences exist among the samples. Due to the complexity of the use of the kit which may have potentially introduced errors in the lab process, a reverse standard curve was obtained for the sample data when plotted. As discussed above, due to the inability to achieve standard curve, the null hypothesis could neither be rejected nor accepted. Although we were unable to determine our primary objective, activity was detected in the cells through spectroscopy of the samples recorded in units of optical density (Tables 2-5).
From this finding, we speculated that there was absorbance of aspirin into the HT-29 cells.
Table 2: Raw data representing optical density for undiluted test suppository formulations measured at 450 nm. For Tables 2-5, ASA refers to 5-acetyl salicylic acid, Zn to zinc-carnosin.
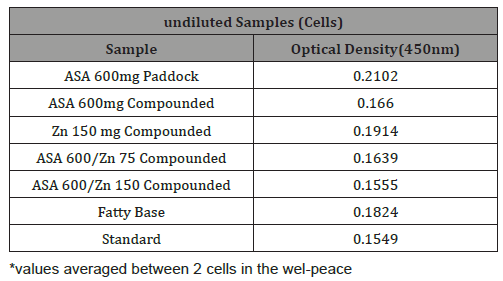
Table 3: Raw data representing optical density for diluted test suppository samples measured at 450 nm.
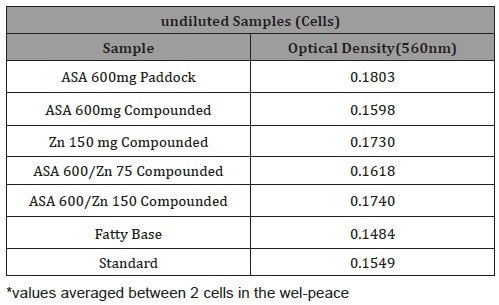
Table 4: Raw data representing optical density for undiluted test suppository samples measured at 560 nm.
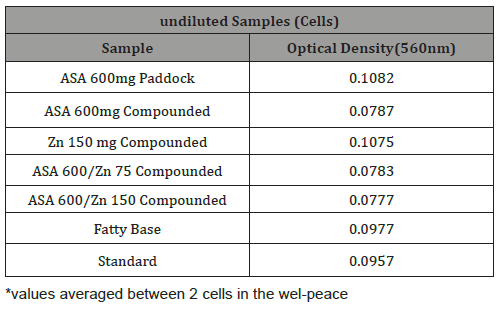
Table 5: Raw data representing optical density for diluted test suppository samples measured at 560 nm.
Discussion
Although results were limited, our novel formulation is still potentially equivalent, if not superior, to current products. Further studies will be conducted to evaluate such a relationship. In addition to the current assay, others will be considered that might improve on obtaining a standard curve with suppositories since they have such a unique formulation for in vitro testing. Our use of the 12(S)-HETE assay as a surrogate marker of aspirin absorption is unique and innovative. This particular assay is relatively new and methodologies for best practice of testing of a suppository formulation have not been established. Our work to establish such a methodology is beneficial to all who wish to test similar products. The substitution of the 0.5mM NaOH stop stock solution with the lab-produced equivalent was appropriate and demonstrated a solution to a potential complication of many assay types.
This described work and results present unique principles behind suppository formulation and testing in vitro. Many older drugs have outdated and inconsistent pharmacokinetic data, particularly suppositories, but because these drugs have years of evidence-based data, minimal research exists such fields. However, since a drug has a robust body of evidence-based data, does not exclude it from preclinical validation. It is even argued that such thinking has hampered the innovation of common drug products, such as aspirin, leading to suboptimal care and new innovative ways to perform drug analysis.
Conclusion
The objective of this study was to develop a novel aspirin suppository to enhance absorption in the rectum using a unique absorption enhancer called zinc-carnosine. From the data gathered, the conclusion about the relative efficacy of aspirin suppository formulated with zinc-carnosine cannot be compared with currently available aspirin suppositories. Given the inconsistencies in the data, further studies need to be conducted to evaluate the potential of these formulations to determine if they can compete with current marketed products. Although the data from this study is inconclusive, this study has established a foundational methodology to be used in further drug formulation research. Utilizing a more direct marker of aspirin absorption in cell lines, like COX-1 and 2 inhibition may facilitate and improve measurements in vitro. The absorption enhancer properties of zinc-carnosine make it a compound to include in these types of formulations, especially as it relates to optimizing absorption of drugs in the rectum. Future successful in vitro studies may lead to in vivo comparability studies that might help determine the safety and efficacy of this novel formulation.
Acknowledgement
This research received no specific grant from any funding agency in public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors report no conflict of interest with this research.
References
- Soni A (2005) Aspirin Use among the Adult U.S. Noninstitutionalized Population, with and without Indicators of Heart Disease. Medical Expenditure Panel Survey, Agency for Healthcare Research and Quality.
- Margaret M Nowak, Barbara Brundhofer, Milo Gibaldi (1974) Rectal absorption from aspirin suppositories in children and adults. Pediatrics [online] 54(1): 23.
- Stuurman-Bieze AGG, Moolenaar F, Schoonen AJM, Visser J, Huizinga T(1978) Biopharmaceutics of rectal administration of drugs in man. II. Effect of particle size on absorption rate and bioavailability. Int J Pharm [online] 1(6): 337-347.
- K Connolly, L Lam, O C Ward (1979) Rectal aspirin-- absorption and antipyretic effect. Arch Dis Child [online] 54(9): 713.
- Broome TA, Brown MP, Gronwall RR, Casey MF, Meritt KA (2003) Pharmacokinetics and plasma concentrations of acetylsalicylic acid after intravenous, rectal and intragastric administration to horses. Can J Vet Res 67(4): 297-302.
- Gerk PM, Yu AC, Shargel L (2016) Physiologic Factors Related to Drug Absorption. In: Shargel L, Yu AC (Eds.), Applied Biopharmaceutics & Pharmacokinetics, 7th edn. New York, NY: McGraw-Hill, USA.
- Suarez S, Marroum PJ, Hughes M (2016) Biopharmaceutic Considerations in Drug Product Design and In Vitro Drug Product Performance. In: Shargel L, Yu AC (Eds.), Applied Biopharmaceutics & Pharmacokinetics, 7th edn. New York, NY: McGraw-Hill; USA.
- Cynthia Wingert (2013) Assistant Professor of Biology, Cedarville University.
- Nate Hnatiuk (2013) Assistant Professor of Chemistry, Cedarville University.
- Halpern GM (2005) Zinc-Carnosine: Nature's Safe and Effective Remedy for Ulcers. Garden City Park, NY: Square One Publishers, USA.
- Brunnhuber, et al. (2017) Putting evidence into practice: Palliative care. United Health Foundation.
- Vincent GK, et al. (2017) The Next Four Decades: The Older Population in the United States: 2010 to 2050. U.S. Census Bureau.
- Schneider, Ryan et al. (2017) Modulation Of COX-1 And COX-2 Protein Levels by Selective And Non-Selective NSAIDS. FASEB J 29: 926.13.
- Abcam (2017) ab133034-12(S)-HETE ELISA Kit: Instructions for Use. Cambridge, Massachusetts: Abcam.
-
Miriam A Ansong* Rocco J Rotello, Danielle Eaton, Tiffany Hong, Mallory Thompson, et al. Evaluation of Novel Aspirin Suppository Formulation in HT-29 Human Adenocarcinoma Cells. Arch Phar & Pharmacol Res. 2(4): 2020. APPR.MS.ID.000544.
-
Aspirin, NPO, Suppository, Alternative, optimal medication therapy, gastroesophageal reflux disease, Drug, Acetylsalicylic acid.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






