 Research Article
Research Article
CYP4V2 Fatty Acid Omega Hydroxylase, A Druggable Target for The Treatment of Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease (NAFLD)
Nicholas Osborne1, Charles Leahy1, Yoon Kwang Lee1 and James P Hardwick1*
1Department of Integrative Medical Sciences, Northeast Ohio Medical Universities, USA
James P Hardwick, Department of Integrative Medical Sciences, Northeast Ohio Medical Universities, USA.
Received Date:September 13, 2021; Published Date: October 26,2021
Abstract
Fatty acids are essential in maintaining cellular homeostasis by providing lipids for energy production, cell membrane integrity, protein modification, and the structural demands of proliferating cells. Fatty acids and their derivatives are critical bioactive signaling molecules that influence many cellular processes, including metabolism, cell survival, proliferation, migration, angiogenesis, and cell barrier function. The CYP4 Omega hydroxylase gene family hydroxylate various short, medium, long, and very-long-chain saturated, unsaturated and polyunsaturated fatty acids. Selective members of the CYP4 family metabolize vitamins and biochemicals with long alkyl side chains and bioactive prostaglandins, leukotrienes, and arachidonic acids. It is uncertain of the physiological role of different members of the CYP4 omega hydroxylase gene family in the metabolic control of physiological and pathological processes in the liver. CYP4V2 is a unique member of the CYP4 family. CYP4V2 inactivation in retinal pigment epithelial cells leads to cholesterol accumulation and Bietti’s Crystalline Dystrophy (BCD) pathogenesis. In this commentary, we provide evidence of the role CYP4V2 has in metabolic syndrome and non-alcoholic fatty liver disease progression. This is accomplished by identifying its role in BCD, its control of cholesterol synthesis and lipid droplet formation in c. elegans, and the putative function in cardiovascular disease and gastrointestinal/hepatic pathologies.
Introduction
It has long-been thought that the role of omega hydroxylase role in the liver has long been thought to prevent toxic fatty acid overload by directing excessive fatty acids to chain-shorting β-oxidation [1,2]. Recent evidence has identified the paradox of omega hydroxylase’s role in lipogenesis and fatty acid oxidation through the production of acetate that serve as a substrate for de novo liver cholesterol and fatty acid synthesis or provides peripheral tissues acetyl-CoA for energy production, and /or epigenetic regulation via nuclear histone and non-histone proteins [3-5]. Present evidence indicates that different members of the CYP4 family have a distinct or unique substrate preference in the omega hydroxylation of short (CYP4B1, CYP4X1, CYP4Z1), medium (CYP4A), long-chain (CYP4F), and very-long-chain (CYP4F) fatty acids, which may serve as nuclear hormone receptors (NHR) ligands in the control of metabolism. The substrate preference of different members of the CYP4 family must be clearly defined in the metabolism of other chainlength fatty acids, bioactive lipids (prostaglandins, leukotrienes, eicosatetraenoic hydroxy acids), alkyl long-chain vitamins (vitamin E, K, A), and biochemical metabolites. These CYP4 family members have important roles in disease processes and possibly the initiation and progression of NAFLD. It has not been clearly defined as to the role of CYP4V2 function in fatty acid and drug metabolism and its physiological role in lipid metabolism and progression of non-alcoholic fatty liver disease (NAFLD).
CYP4V2
Cytochrome p450, family 4, subfamily v2 (CY4V2) is a monooxygenase belonging to the cytochrome p450 family 4, a collection of enzymes responsible for the ω-hydroxylation of fatty acids (FA.s) [6]. CYP4V2 has been designated as an “orphan” P450, as its profile of physiologic functions has yet to be fully determined [7]. Although defined as an orphan P450, CYP4V2 seems to have preferential catalytic activity towards medium-chain-length FAs (MCFA) like myristic acid, lauric acid, and palmitic acid, and polyunsaturated fatty acids (PUFAs), including arachidonic acid (AA), docosahexaenoic acid (DHA), eicosapentaenoic acid (EPA), and others [8]. CYP4V2 has a low affinity for the following MCFAs: myristic (Km=65 μM, kcat=35), lauric (Km=140, kcat=26 μM), and palmitic acids (Km=430 μM, kcat=8), suggesting that the optimal substrate has not been identified.
The CYP4V2 gene is highly expressed throughout the body, with prominence in retinal pigment epithelial cells (RPE) of the eye and tissues such as the liver, kidney, thyroid, adrenal glands, gall bladder, and small intestines [8,9]. On a cellular level, this enzyme is present in microsomes and peroxisomes, with metabolic interactions between mitochondrial and peroxisomal beta-oxidation [10]. The cyp4V2 gene shows a divergence from other family 4 P450s located on chromosome 4 instead of chromosomes 1 and 19. Another peculiarity of the CYP4V2 gene is its low sequence identity to those in its enzymatic family. These genetic peculiarities may account for its high degree of polymorphic variants, with many variants linked to pathologies of the eye and cardiovascular system.
Physiologically, the function of CYP4V2 remains elusive, although its Caenorhabditis elegans ortholog, CYP37A1, has been linked to dafachronic acid-like hormone production and the regulations of development and fat storage [11]. On pathology, CYP4V2 has been indicated in the pathogenesis of Bietti’s Crystalline Dystrophy (BCD) and deep venous thrombosis (DVT) and correlated with favorable prognosis of carcinomas such as hepatocellular carcinoma (HCC) [12].
Through analysis of metabolic associated fatty liver disease (MAFLD) human tissue samples and mouse modeling of the MAFLD spectrum (normal liver, steatosis, non-alcoholic steatohepatitis (NASH), cirrhosis, and HCC), we have linked CYP4V2 to have a potential role in the regulation in MAFLD progression [13].
In this commentary, we will highlight CYP4V2’s involvement in BCD, control of C. elegans’ dafachronic ascaroside pathways, and CYP4V2 potential role in MAFLD explored to highlight parallels and overarching themes in its function. Upon examining these parallels and themes, this commentary will offer future research into the functional role of the CYP4V2 gene in metabolic-associated fatty liver disease (MAFLD).
Bietti’s Crystalline Dystrophy
BCD is an autosomal recessive ophthalmic disease characterized by progressive chorioretinal degeneration and cholesterol accumulation in RPEs. (Figure 1) Cholesterol crystals develop in the cornea and fundus, appearing yellow. Once accumulation progresses, RPEs atrophy is followed by the atrophy of photoreceptors. The progression of this disease ends in blindness for the patient with no current treatment available treatment to alleviate the disease course [12,14,15]. Despite CYP4V2’s extensive expression throughout organ systems, no other organ system disorders have been reported in BCD patients [14].
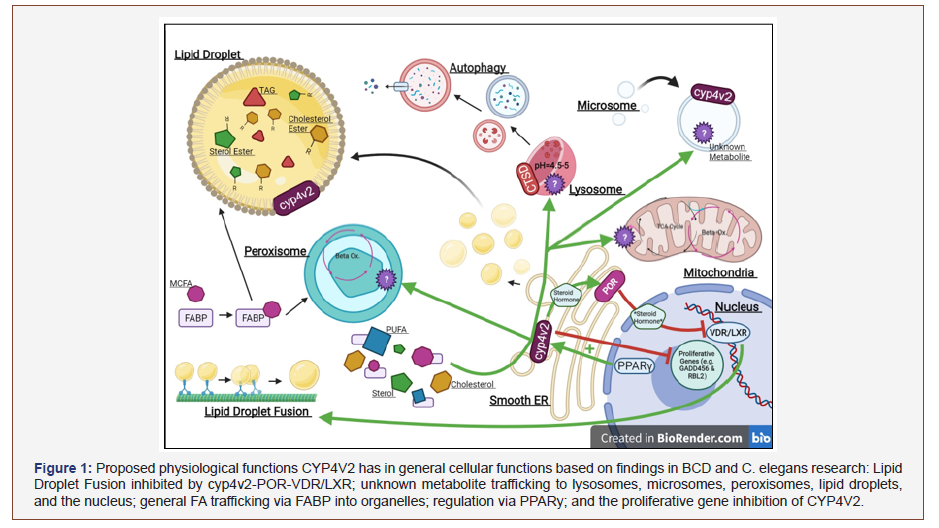
More than 60 CYP4V2 variants have been linked to the development of this disease [12]. The most common of these mutations is the c.802-8_810del17insGC variant [17]. These variants are mainly missense mutations, followed by nonsense, deletion, splice site, and insertion-deletion mutations [9,17]. Despite the sheer amount and variety of these variants, each of them causes a reduction in CYP4V2’s ω-hydroxylation activity, leading to an increase in intracellular FA, triglyceride, and cholesterol levels [17].
The exact mechanisms of BCD pathogenesis and progression are unknown due to few disease models. Despite this limitation, the utilization of induced pluripotent stem cells (iPSCs) has uncovered various cellular changes in cyp4V2 knockout cells [12,15]. The Hata group demonstrated that cells underwent abnormal vacuolation of the cytoplasm, lysosomal dysfunction, and autophagosome flux impairment [12]. Cell death was acclerated by dysfunction of autophagosome flux, while an alkalized pH characterized lysosomal dysfunction due to impairment of cathepsin D (CTSD). CTSD has been linked to homeostatic maintenance of cellular reactive oxygen species (ROS), cell turnover, and apoptotic death, thus defects of these parameters could account for BCD cellular changes [18,19]. The lysosomal dysfunction in BCD is comparable to Niemann- Pick and Gaucher’s disease due to the marked accumulation of glucosylceramide and free cholesterol [14]. The Hata group was also able to demonstrate cholesterol reduction via cyclodextrins, and delta-tocopherol treatment ameliorated the BCD phenotype. These findings link CYP4V2 to cholesterol metabolism that may regulate cell degradative and recycling pathways.
Adding to the Hata group’s findings, the Zhang group demonstrated that BCD iPSCs had a significant accumulation of PUFAs, leading to mitochondrial stress and ROS generation [15]. Eicosapentaenoic (EPA) and Arachidonic acids (AA) were shown to promote ROS generation, triggering p53-indpendent apoptosis. After induction of CYP4V2 in BCD iPSCs, they observed a decrease in ROS generation and the subsequent rescuing of cells. These investigators further explored the relationship of cholesterol in BCD and concluded that cholesterol alone could not account for the apoptotic changes in BCD cells. They, in turn, discovered an upregulation of pro-apoptotic genes (e.g., BBC3) and downregulation of anti-apoptotic genes (BCL2, BCL2L1, BIRC2), including the proto-oncogenes TP53, and cytochrome c release from the mitochondria.
Despite the localization of symptomatology in BCD patients to the chorioretinal cells, other studies reported cholesterol crystals accumulated in circulating lymphocytes and skin fibroblasts [17]. This aligns with the findings of CYP4V2’s wild-type expression in human THP1 macrophages [20]. CYP4V2 mutant cells were also discovered to have elevated TGs free cholesterol, lacking two unnamed fatty acid-binding proteins (FABPs), and decreased metabolism of pro-inflammatory PUFAs. Thus CYP4V2’s presence in immune cells and its metabolism of pro-inflammatory PUFAs, suggest that it serves a regulatory role over the immune system and inflammatory response. The lack of FABPs responsible for the trafficking of FAs throughout many organelles and the nucleus expands CYP4V2’s potential role in FA metabolic pathways [21].
The Jiao group also explored the structural variations between the BCD-causing CYP4V2 mutants through predictive softwarebased modeling [17]. They determined that the mutations (especially the missense) primarily impacted the transmembrane domain by altering hydrogen bonds and subsequent distortion of the alpha helixes. They influenced the porphyrin ring, changing the positioning of the heme group and the enzyme’s catalytic site.
Other work with BCD-causing CYP4V2 mutants explored the decreased lauric acid metabolism in RPE cells due to hydrogen bond alterations in the catalytic site [22]. It was shown that cyp4V2 variants, H331P and G410C, had 20-30% reduced lauric acid oxidation capacity compared to wild type. In addition, it was also suggested that RPE CYP4V2 might be regulated by peroxisome proliferator-activated receptors (PPARs). PPAR-regulation of cyp4v2 has also been described in THP1 macrophages and other immune cells [12,20].
Cyp4v2 and C. elegans Life Cycle
CYP4V2’s C. elegans ortholog, CYP37A1, plays a crucial role in regulating lipid droplet fusion and Dauer entry [11]. The mechanisms for these two functions are highly complex, with many analogous pathways to human physiology. To best understand cyp37A1’s role, it is crucial to have a fundamental knowledge of C. elegans ascaroside (ASCRs) metabolism and the stages of their life cycle, both of which are regulated in a balancing fashion by dafachronic acids (DAs) and ascaroside (ASCR) pheromones [11,23] (Figure 2).
Dauer diapause is a stress-resistant stage of life for c. elegans and related nematodes. It is a stage typically characterized by longevity (non-aging), cold and heat tolerance (15-34oC), fasting resistance, and reproductive development arrest [11,23,24]. Its induction is driven by ASCRs when their concentration is greater or equal to DAs; both ASCRs and DAs each inhibit the other’s effect [23]. ASCRs are produced in a condition of perceived stress (fasting) and behavioral/physiological needs (i.e., predator repulsion, mating, foraging, detoxification, etc.). They are synthesized from varying chain-length fatty acids through the ω-oxidation and a conserved peroxisomal β-oxidation pathway, MOAC-1- DHS-28- DAF-22 [24-26] (Figure 1). In contrast, DAs are synthesized when food is readily available and favorable environmental conditions via insulin and TGF- β signaling [27]. DAs are steroid hormones and are thus derived from cholesterol. Many pathways in DA synthesis remain unknown due to the wide variety of DAs. However, the enzymes STRM-1, EMB-8 (CYP450 oxidoreductase-POR), DHS-16 (ortholog to 3-HSD), and DAF-9 (CYP22A1) are all components of the DA synthesis pathway [11,27]. In addition to the pathways described above, a DA-like steroid hormone is produced by a pathway consisting of CYP37A1, DAF-12, and EMB-8 (analogous to human cyp4v2, VDR/LXR, and POR), identifying possible physiological parallels between C. elegans and higher eukaryotes of the DA synthesis pathways [11].
ASCRs and DAs signaling pathways converge upon the nuclear hormone receptor, DAF-12; DAs bind to it, displacing the inhibitory coregulator, DIN-1S/SHARP, and allow for reproductive development and aging while ASCRs inhibit the DA: DAF-12 formation, preserving DIN-1S/SHARP: DAF-12 complex [11,23,27]. (Figure 2). Upon DA: DAF-12 formation, let-7-microRNAs are transcribed to initiate the developmental changes through a series of positive-feedback loops for irreversible adult development. DAF-12 defective mutants are Dauer constitutive if the phenotypic change occurs on the receptor’s ligand-binding domain, while other DAF-12 defective mutants display an inability to enter Dauer diapause. Without DAF-12’s ability to bind DAs, DIN-1S/SHARP remains bound, and Dauer entry follows. DAF-9 defective mutants are also Dauer constitutive, as DAs cannot be synthesized. However, other independent pathways exist that trigger Dauer development, as seen with DAF-2 mutants (defective insulin receptor) being Dauer constitutive [27].
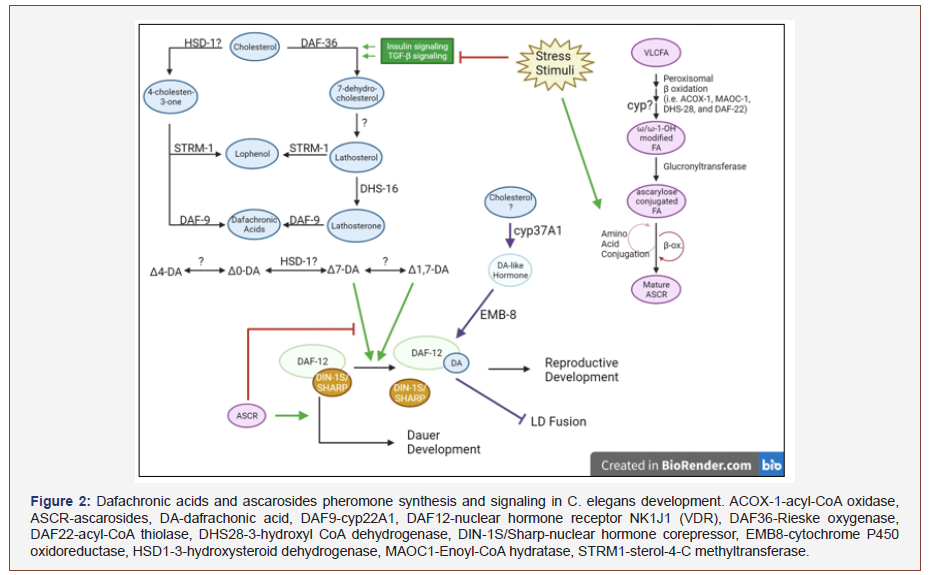
Peroxisome fatty acid chain-shortening has proven vital for the successful processing and signaling of both ASCRs and DAs. In addition to ASCRs’ Dauer inducing properties, the inflammatory response produced via free radical production under the ER stress response may inhibit and/or overwhelm their antiinflammatory properties [24]. Daumone [(2)-(6R)-(3.5-dojydroxy- 6-methyltetrahydropyran-2-yloxy)hepatanoic acid]) was shown to be anti-inflammatory and anti-carcinogenic in mouse models via the inhibition of TNF-α induced NF-κβ phosphorylation [24,28]. Peroxisomal β-oxidation dysregulation in c. elegans was compared to human peroxisomal disorders, Zellweger syndrome, and X-linked Adrenoleukodystrophy (ALD), and neurodegenerative disorders [24,26,29]. Mutations in the conserved MAOC-1/DHS-28d/ DAF-22 pathway of peroxisome β-oxidation and their respective consequences are comparable to human diseases leading to accumulations of triacylglycerols (TAG)s, very-long-chain fatty acids (VLCFAs), and PUFA, contributing to inflammation with increased lipid droplet size [26]. These lipid-toxic effects in humans have led to developmental defects, sensory impairment, demyelination of neurons in the brain and spinal cord cortex, hepatocyte injury, and male sterility [24,26,29]. These translational parallels warrant the need for further investigation into whether humans produce ASCR and DA-related molecules and their functions.
Cyp37A1 and Lipid Droplets
Lipid droplets (LDs) are storage organelles for lipids such as TAGs, FAs, and sterol esters. Under normal conditions, lipid molecules trafficked in and out of LDs in response to metabolic demand [30]. Pathological dysregulation (e.g., in foam cells of atherosclerosis or hepatocytes during steatosis) can alter the structure and storage properties. Defects in Peroxisomal β-oxidation can accumulate TAGs, cholesterol (free and esterified) and increase the size of LDs. The large LDs in the case like defects in peroxisome β-oxidation there is a resistant to fasting-induced lipolysis due to defective TAG lipases-triggered lipolysis [11,26,27] (Figure 3).
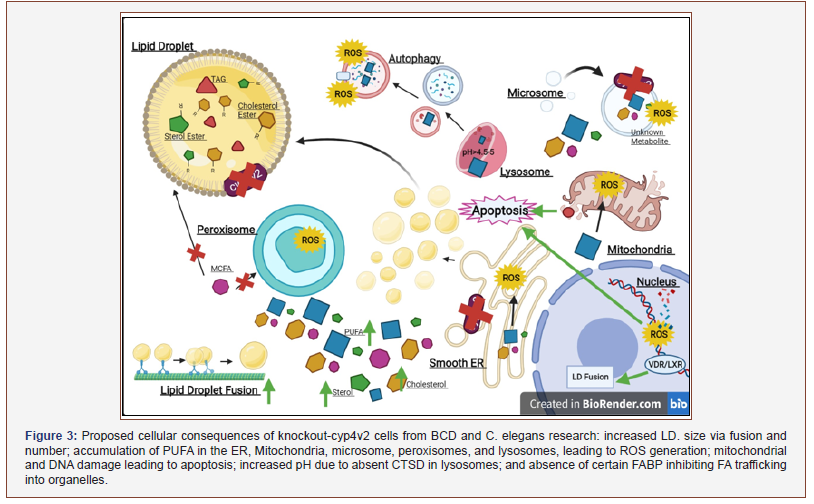
In c. elegans, LDs have been observed to be regulated by DAs in response to their environment. LDs are stored in the hypodermis and intestines of c. elegans, with fat stores primarily regulated by insulin, TGF-β, serotonin, and the mechanistic target of rapamycin (mTOR) [11,27]. There is evidence of crosstalk between LDs and other organelles (i.e., peroxisomes, lysosomes, and ER) to carry out metabolic and different signaling pathways, although more detailed research is needed to understand these types of interactions [11,26].
CYP37A1 is a regulator of c. elegans LDs’ size and thermosensitive fusion. This regulation occurred through the CYP37A1-DAF-12- EMB-8 nuclear hormone receptor pathway. Interestingly, although DAF-12 is a critical binding site for DAs to elicit developmental responses, CYP37A1 does not produce a specific DA, but produces a DA-like steroid hormone that binds to DAF-12 [11]. This further demonstrates the many pathways that intersect through DAs and ASCRs. Outside of development and aging, DA: DAF-12 also promotes FA oxidation for energy production [11,26]. This regulation of fat metabolism links CYP37A1’s product hormone to LD. Regulation [11].
The CYP37A1-DAF-12-EMB-8 pathway is important in regulating lipid metabolism or LD size regulation. CYP37A1 is essential because it is the only delineated p450-hormone nuclear receptor pathway with a well-appreciated physiological function: DAF-9-DAs-DAF-12-DIN-1. This pathway is analogous to human bile acid synthesis pathways for farnesoid x receptor (FXR) signaling. Li and others described these pathways as similar in that they regulate LDs and lipid metabolism but differ in critical steps: CYP37A1 synthesizes a hormone ligand that is not a DA; DAF-12 does not require DIN-1 to exert LD fusion, and DAF-9-DAs-DAF-12 regulate LD. Growth and lipogenesis rather than LD. Fusion [11].
CYP37A1’s pathway possesses negative regulation over LD fusion, with dysregulation causing thermo-dependent increased LD size. This could explain the accumulation of cholesterol and other lipids in BCD patients. This lipid droplet fusion is analogous to human temperature-dependent interconversion between uniocular white fat cells and multilocular brown fat cells. This poses the question: do CYP4V2, VDR/LXR, POR make up a conserved pathway in humans to regulate LD fusion?
The mechanism of how LD fusion occurs is not clear. LDs communicate and fuse with other organelles through physical contact, as seen with DAF-22 mutants displaying LD accumulation around peroxisomes [26]. However, the fusion is probably neither a mere biophysical fusion nor asymmetric sharing of TAGs and steroid-esters, as neither is known to be temperature-dependent. The Li team explains this unlikely fusion pattern due to the CYP37A1-DAF-12-EMB-8 mutant displaying first-order kinetics with an optimal temperature for reaction and the lack of increase in TAGs or changes phospholipid levels with LD fusion. An enzymatic reaction mechanism is indicated in this fusion [10]. This further emphasizes the importance of further investigation in CYP4V2’s LD regulation in human diseases: can its dysregulation cause lipid accumulating diseases such as steatosis in MAFLD.
Cyp4v2 and Cardiovascular Disease
CYP4V2 variants have been linked to BCD in Asian populations and deep vein thrombus (DVT) in European people. As is the recurring theme with CYP4v2, little is known about its mechanics in DVT development. In addition to increased DVT risk, there is an increased risk of tamoxifen-induced venous thrombosis. There is evidence CYP4v2 rs13146272, the variant responsible for high DVT risk, causes the substitution of glutamine with lysine at position 259 (from neutral to a basic amino acid), perhaps leading to a change in its substrate specificity [12].
As mentioned previously, CYP4V2 is highly expressed in THP1 macrophages [20]. More intriguing there is a possible association with atherosclerosis. Mutant CYP4V2 has been reported to cause cholesterol crystal formation and accumulation in circulating lymphocytes, including macrophages [17,20]. With this accumulation of lipids in macrophages, foam cells develop, a key hallmark of atherosclerosis. The potential for CYP4V2’s involvement in foam cell development is further supported by the lysosomal dysfunction observed in BCD patients. Lysosome dysfunction via cholesterol accumulation is a critical component of foam cell development in atherosclerosis [14,31]. Regardless, the expression of CYP4V2 in monocytes, healthy macrophages, and foam cells need to be studied carefully to confirm this relationship [20].
THP1 macrophages and CYP4v2 also led to the discovery of a critical regulator of this enzyme, PPARγ. PPARγ antagonist GW6471 was able to reduce CYP4V2 expression while PPARγ agonist rosiglitazone increased its expression. PPARγ is, of course, prominently expressed in macrophages and foam cells alike to regulate the metabolism of carbohydrates and lipids, serving as another route to explore CYP4v2’s role in atherosclerosis [20].
CYP4V2 in Gastrointestinal and Hepatic Pathologies
Despite CYP4V2 high expression throughout the gastrointestinal system (e.g., bladder, liver, and small intestine), CYP4V2 has not been systematically studied in the pathologies of these systems. CYP4V2 has, however, been observed in several GI malignancies. CYP4v2’s expression was reported to be useful as a colorectal prognostic factor, as its expression changes as the primary tumor progresses [32,33].
In hepatocellular carcinoma, CYP4V2 was established to be a favorable prognostic factor [34]. Patients with decreased CYP4V2 expression had higher-grade tumors in terms of histological and TNM staging. CYP4V2 was then negatively correlated with cellcycle- associated genes (e.g., GADD45G, CDC14B, RBL2, and more) [6,12].
The findings of CYP4V2 expression in these two types of cancers call into question the role CYP4V2 variants and their potential function in potentiating tumor development and progression due to their decreased catalytic activity. Are their CYP4V2 variants correlated with colorectal and HCC progression? Do BCD patients have higher occurrences of these malignancies.
Presently, CYP4V2 has not yet been linked to other GI pathologies. However, its linkage to lipid droplet regulation in c. elegans and atherosclerosis, along with the proposed disease mechanics of BCD patients, makes it a worthwhile candidate for metabolic associated fatty liver disease (MAFLD) pathogenesis and pathophysiology studies [17,20,35].
CYP4V2 and MAFLD
Although our research data of CYP4V2 in yet to be published data, we have discovered that CYP4V2 is elevated in steatosis and slightly elevated in NASH in human tissue samples. During steatosis, the elevation of CYP4V2 is significantly increased in lipid droplet isolates. Additionally, we observed expression of CYP4V3 (Human CYP4V2 mouse ortholog) change in mice under different dietary conditions. Namely, we observed the impact of fasting in male and female mice on its expression and the altered expression under other nutritional and metabolic conditions: wild type (wt), high fat (HF), methionine/choline deficiency diet (MCD), and mice with leptin deficiency (OB/OB). Under fasting conditions, there is a clear trend in increasing CYP4V3 expression as fasting duration continues up to 72 hours. Under the varying diets and metabolic mouse models, CYP4V3 expression was increased in mice on the MCD diet and reduced in HF and OB animal models. The MCD diet model of NASH in mice, shows a similar trend compared to samples from human NASH patients; NASH had a slight increase in CYP4V2 expression in humans but a dramatic increase in CYP4V3 for mice. Interestingly, the HF diet and OB/OB mice showed opposite results compared to human steatosis samples with decreased expression of CYP4V3.
The fasting conditions and the increased expression of CYP4V3 may be explained by the elevated fat accumulation in the liver during times of prolonged fasting, more available substrates to upregulate its expression. The elevations of CYP4V2 in human steatosis and NASH (MCD for mice) may reflect experimental findings of CYP4V2 physiological function from BCD studies. We have observed increased ROS species increase as MAFLD progresses from simple steatosis to HCC, with the highest levels of total lipid peroxidases in steatosis and HCC. It could be that CYP4V2’s expression is increased via induction by accumulating substrate. The accumulation of FAs, particularly PUFAs, may lead to toxic lipid effects such as endoplasmic reticulum (ER) stress, mitochondrial stress, and more, triggering a compensatory feedback mechanism to increase CYP4V2’s expression. It is known that different fatty acids serve both as an agonist and selective antagonist of PPARϒ, which is elevated in NAFLD [36] [Figures 2 and Figure 3].
These preliminary data have given us hints into the regular physiological role of cyp4V2 but also raises potential implications of CYP4V2 polymorphic variants. If functional impairment of CYP4V2 is similar in hepatocytes as in the RPE of BCD patients, there may be potential increased susceptibility to developing MAFLD and its progression. CYP4V2 is shown to be decreased in both cirrhosis and HCC. While most of the cyp450 enzymes we have observed decrease in cirrhosis, the decline in its expression could account for or allow disease progression. The is underscored by the fact that CYP4V2 this is a favorable prognosis factor for HCC.
Future research will need to explore the role CYP4V2 has in the metabolism of cholesterol, PUFAs, and its other lipid substrates. It is essential to identify where the metabolites of these substrates are trafficked to and the cellular reactions and mechanisms, they become a part of. It will be necessary to determine whether humans produce ASCRs and DAs and determine the degree of control CYP4V2 has on lipid droplet fusion. With the correlation between increased lipid droplets in size and potentially quantity and CYP4V2 expression, it is essential to investigate the role of CYP4V2 in regulation of lipid droplet mechanics. It is also crucial to determine whether there are polymorphic variants of CYP4V2 that influence MAFLD development and progression.
Summary
CYP4V2 is one of twelve fatty acid omega hydroxylases identified in humans. Inactivation mutation in the CYP4V2 gene leads to Bietti’s Crystalline Dystrophy (BCD), characterized by accumulation of cholesterol in retinal pigment cells and deep vein thrombus (DVT). Over 60 CYP4V2 variants have been identified, and these may be linked to different eye and cardiovascular system pathologies. CYP37A1, the C. elegans ortholog of CYP4V2, has a crucial role in lipid droplets and C. elegans entry into the Dauer phase by balancing the production of dafachronic acid and ascaroside pheromones. Ascarosides (ASCRs) are synthesized during fasting from ω-hydroxylation of different chain fatty acids produced by CYP37A1, while Dafachronic acids (DAs) are made during the fed cycle. The fed and fasting cycles are reciprocally regulated by DAs and ASCRs, which function as ligands for nuclear hormone receptors (NHRs). Mutations in the peroxisome β-oxidation pathway of ascarosides synthesis led to accumulation of TAGs, VLCFA, cholesterol, and increasing lipid droplet size like the pathologies associated with BCD. CYP37A1 is a regulator of C. elegans LD size and fusion, which may explain the accumulation of cholesterol and other lipids in BCD patients. The increased expression of CYP4V2 in macrophages and reduced expression in foam cells due to cholesterol-induced lysosomal dysfunction is a critical component of foam cell development in atherosclerosis. The discovery that PPARγ agonist increases CYP4V2 expression may reflect a functional role of PPARγ drugs in treating metabolic syndrome and NAFLD progression. Understanding the role of the CYP4V2 p450 in producing molecules like C. elegans ascarides may provide crucial insights on LD droplet formation in macrophages and the progression of steatosis in NAFLD. These molecules may offer new opportunities in the development of drugs to treat atherosclerosis and fatty liver disease.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Hardwick JP (2008) Cytochrome P450 omega hydroxylase (CYP4) Function in fatty acid metabolism and metabolic diseases. Biochemical Pharmacology 75(12): 2263-2275.
- James P Hardwick, Katie Eckman, Yoon Kwang Lee, Mohamed A Abdelmegeed, Andrew Esterle, et al. (2013) Eicosanoids in Metabolic syndrome. Immunopharmacology Advances in Pharmacology Academic Press Elsevier Inc 66: 157-266.
- Paula Rote, James P Hardwick (2019) Cytochrome P450 Fatty acid omega-hydroxylase, Avenues for fatty acid oxidation or fatty acid synthesis in NAFLD progression. Integrated pathways of disease in NASH and NAFLD Keystone Symposia on Molecular and Cellular Biology Santa Fe New Mexico.
- Xiao Zhang, Ting Gao, Senwen Deng, Lin Shang, Xiaocui Chen, et al. (2021) Fasting induces hepatic lipid accumulation by stimulating peroxisomal dicarboxylic acid oxidation J Biol Chem 296: 100622.
- Huabo Wang, Jie Lu, Xiaoguang Chen, Marie Schwalbe, Joanna E Gorka, et al. (2021) Acquired deficiency of peroxisomal dicarboxylic acid catabolism is a metabolic vulnerability in hepatoblastoma. J Biol Chem 296: 100283.
- Hyuk Soo Eun, Sang Yeon Cho, Byung Seok Lee, In-Ock Seong, Kyung-Hee Kim (2018) Profiling cytochrome P450 family 4 gene expression in human hepatocellular carcinoma. Mol Med Rep 18(6): 4865-4876.
- Kelly EJ, Nakano M, Rohatgi P, Yarov-Yarovoy V, Rettie AE (2011) Finding homes for orphan cytochrome P450s: CYP4V2 and CYP4F22 in disease states. Molecular interventions 11(2): 124-132.
- (2021) Consortium European Bioinformatics Institute Protein Information Resource SIB Swiss Institute of Bioinformatics Cytochrome. Uni Prot 450-452.
- US National Library of Medicine (nd.) CYP4V2 cytochrome P450 family 4 SUBFAMILY V MEMBER 2 [Homo Sapiens (human)] - gene - NCBI. National Center for Biotechnology Information.
- Mariko N, Edward J Kelly, Allan ER (2009) Short Communication: Expression and Characterization of CYP4V2 as a Fatty Acid omega-Hydroxylase. Specific regulation of thermosensitive lipid droplet fusion by a nuclear hormone receptor pathway 37(11): 2119-2122.
- Jarrar Y, Lee Su (2019) Molecular Functionality of Cytochrome P450 4 (CYP4) Genetic Polymorphisms and Their Clinical Implications. Int J Mol Sci 20(17): 4274.
- Hata (2017) Reduction of lipid acumulation rescues Bietti's crystalline.
- Nicholas Osborne, Elizabeth Olah, Paula Rote, Charles Leahy, Takhar Kasumov, et al. (2021) Orphan Omega fatty acid hydroxylase CYP4 gene expression in Human NAFLD: Polymorphic variants of CYP4V2 gene in hepatic steatosis. KEYSTONE SYMPOSIA Fatty Liver Disease and Multi-System Complication and Hepatobiliary Cancer: Pathobiology and Translational Advances 22-25.
- Zhang (2020) PSCs Reveal PUFA-Provoked Mitochondrial Stress as a Central Node Potentiating RPE Degeneration in Bietti's Crystalline Dystrophy.
- US National Library of Medicine (2020) Bietti crystalline DYSTROPHY: MedlinePlus Genetics. MedlinePlus.
- Jiao, et al. (2017) Identification and population history of CYP4V2 mutations in patients with Bietti crystalline corneoretinal dystrophy.
- Su S, Zhu X, Lin L, Chen X, Wang Y, et al. (2017) Lowering Endogenous Cathepsin D Abundance Results in Reactive Oxygen Species Accumulation and Cell Senescence. Molecular & cellular proteomics MCP 16(7): 1217-1232.
- Mijanovic O, Petushkova AI, Brankovic A, Turk B, Solovieva AB, et al. (2021) Cathepsin D—Managing the Delicate Balance. Pharmaceutics 13(6): 837.
- Yi M, Shin, Lee, (2017) Expression of CYP4V2 in human THP1 macrophages and its transcriptional regulation by peroxisome proliferator-activated receptor gamma 330:100-106.
- Smathers RL, Petersen DR (2011) The human fatty acid-binding protein family: Evolutionary divergences and functions. Hum Genomics 5(3): 170-191.
- Yazun Bashir Jarrar, Jae-Gook Shin, Su-Jun Lee (2020) Identification and functional characterization of CYP4V2 genetic variants exhibiting decreased activity of lauric acid metabolism 84(5): 400-411.
- Park, Park, Paik (2020) A Molecular Basis for Reciprocal Regulation between Pheromones and Hormones in Response to Dietary Cues in elegans 21(7): 2366.
- Park, Hyoe-Jin Joo, Saeram Park, Young-Ki Paik (2019) Ascaroside Pheromones: Chemical Biology and Pleiotropic Neuronal Functions 20(16): 3898.
- Zhang, et al. (2016) Structural characterization of acyl-CoA oxidases reveals a direct link between pheromone biosynthesis and metabolic state in Caenorhabditis elegans.
- Zhang SO, Box AC, Xu N, Le Men J, Yu J, et al. (2010) Genetic and Dietary regulation of Lipid droplet expansion in elegans 107(10): 4640-4645.
- Watts, Ristow (2017) Lipid and Carbohydrate Metabolism in Caenorhabditis elegans. 207(2): 413-446.
- Aguilaniu (2016) The Role of Dafachronic Acid Signaling in Development and Longevity in Elegans: Digging Deeper Using Cutting-Edge Analytical Chemistry.
- Park JH, Ha H (2015) Short-term Treatment of Daumone Improves Hepatic Inflammation in Aged Mice. Korean J Physiol Pharmacol 19(3): 269-274.
- Park, Paik, (2017) Genetic deficiency in neuronal peroxisomal fatty acid beta-oxidation causes the interruption of dauer development in elegans.
- Olzmann JA, Carvalho P (2019) Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol 20(3): 137-155.
- Andre RA Marques, et al. (2021) Lysosome (Dys) function in Atherosclerosis—A Big Weight on the Shoulders of a Small Organelle.
- Kumarakulasingham, Rooney PH, Dundas SR, Telfer C, Melvin WT, et al. (2005) cytochrome p450 profile of colorectal cancer: Identification of Markers of Prognosis 11(10): 3758-3765.
- Alnabulsi, Swan R, Cash B, Alnabulsi A, Murray GI (2017) The differential expression of omega-3 and omega-6 fatty acid metabolising enzymes in colorectal cancer and its prognostic significance 116(12): 1612-1620.
- Soo Eun, Cho SY, Lee BS, Seong IO, Kim KH (2018) Profiling cytochrome P450 family 4 gene expression in human hepatocellular carcinoma 18(6): 4865-4876.
- Jung Eun Park, Chunki Kim, Mikang Lee, Yanqiao Zhang, James P Hardwick, et al. (2017) Hairy and Enhancer Split 6 mediates hepatic lipid homeostasis through inhibition of Pparg2 expression. Hepatology Communications 1(10): 1085-10980.
-
Nicholas Osborne, Charles Leahy, Yoon Kwang Lee, James P Hardwick. CYP4V2 Fatty Acid Omega Hydroxylase, A Druggable Target for The Treatment of Metabolic Syndrome and Non-Alcoholic Fatty Liver Disease (NAFLD). Arch Phar & Pharmacol Res. 3(1): 2021. APPR. MS.ID.000552.
-
Cellular homeostasis ,Fatty Acid Omega Hydroxylase.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






