 Research Article
Research Article
Applications of Invasomal Drug Delivery System
A Krishna sailaja1*and T Meghana1
Department of pharmaceutics, RBVRR Women’s college of pharmacy, Affiliated to Osmania University, India.
A Krishna Sailaja, Department of pharmaceutics, RBVRR Women’s college of pharmacy, Affiliated to Osmania University, India.
Received Date:September 30, 2021; Published Date: October 22,2021
Abstract
The transdermal route is an important pathway for localized or systemic effects. The stratum corneum, the outer layer of the skin, is an essential skin permeation barrier for many drugs. To overcome this barrier, several techniques have been developed, including the use of methods that change the stratum corneum (SC) continuity, such as ultrasound, electroporation, and iontophoresis, and the use of the vehicle and nanocarriers to improve drug penetration. Recently, different types of nanocarriers have been designed to improve the dermal and transdermal delivery of medicines. Vesicular systems appear to be suitable carriers owing to their physicochemical properties, such as deformability, size, and charge, which can be modified by altering lipid constituents and preparation methods. Invasomes are novel and flexible vesicles containing a mixture of soy phosphatidylcholine (PC), terpenes, lyso PC, and ethanol with improved skin penetration in comparison with liposomes. Furthermore, invasomes have the same structural constituents as liposomes but contain terpene in their structure. Terpenes are hydrocarbon compounds and are known to be the primary constituents of essential oils from many plants. Addition of terpenes creates deformable vesicles, which can increase the fluidity of the lipid bilayers of the skin. The ability to permeate through skin layers enhances the activity of invasomes, which exert their effects by fluidizing the bilayer structure of SC lipids and disturbing lipids and intracellular protein interactions. In this article the structure of invasomes, their applications in drug delivery were discussed in detail.
Keywords: Invasomes, Terpene, Thin film hydration technique, Characterization
Introduction
Liposomal vesicular systems can incorporate both lipophilic and hydrophilic drugs to assist in the penetration of the incorporated agents. However, conventional liposomes are not approved as appropriate systems for transdermal delivery of drugs as they are unable to permeate the inner layers of skin and, therefore, their effects remain limited to the upper layers. Novel elastic vesicles containing penetration enhancers are superior to conventional liposomes due to their improved interactions with skin and better drug penetration. The primary deformable or elastic vesicles, named Transferosomes, were developed by Cevc et al. in the 1990s. These vesicles are composed of phospholipids and edge activators, such as polysorbate or sodium cholate, producing elastic carriers for improved transdermal drug delivery. The encouraging results seen with Transferosomes led to the development of other novel elastic vesicles via alterations in the vesicular composition. In previous examinations, elastic vesicles such as niosome (prepared mostly by non-ionic surfactant and cholesterol) and ethosome (containing high amount of ethanol in their structure) have displayed potential as a drug carrier.
Definition: Invasomes are liposomal vesicles embodying small amounts of ethanol and terpenes or terpene mixtures, which act as potential carrier with increased skin penetration. Invasomes have higher penetration rate through the skin compared to liposomes and ethosomes. Invasomes provide a number of advantages including improving the drug efficacy, enhancing patient compliance and comfort [1,2].
Advantages of Invasomes
-Non-invasive technique of drug delivery.
• Enhanced permeation of drug through the skin for transdermal drug delivery.
• Delivery of hydrophilic and lipophilic drugs is possible.
• Contains non-toxic raw material in formulation.
• Patient compliance as the drug can be administered as semisolid form (gel or cream).
• Simple method for drug delivery in comparison to iontophoresis and phonophoresis and other complicated methods.
Disadvantages of Invasomes
• It’s high production cost.
• Leakage and fusion of encapsulated drug/ molecule.
• The phospholipid present may undergo hydrolysis/oxidation, thus affecting stability of Invasomes.
Methods of Preparation
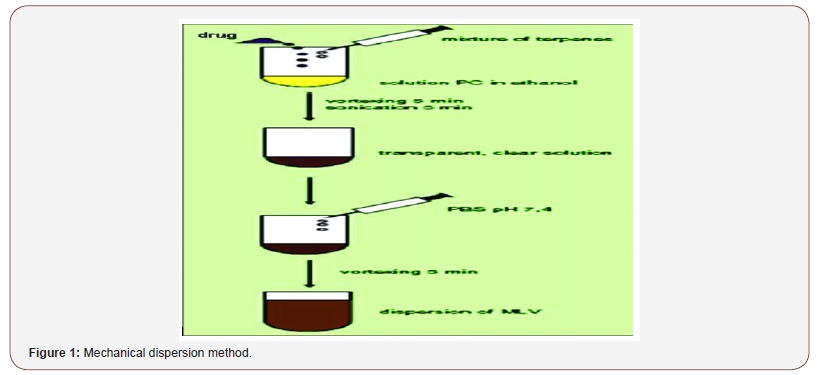
Mechanical Dispersion Method Drug and terpene or mixtures of terpenes are dissolved in ethanolic phospholipid solution. The mixture is vortexed for 5 min and then sonicated for 5 min in order to obtain a clear solution. Phosphate buffer saline (PBS) (pH: 7.4) is added to the solution by a syringe under constant vortexing. The vortexing is continued for an additional 5 min to obtain final invasomal preparation [3,4] (Figure 1).
Thin Film Hydration Method
Invasomes can also be prepared by the conventional film method. Phospholipids in ethanol are dissolved in methanol: Chloroform (2:1, v/v). This mixture is dried to a thin film by slowly reducing the pressure from 500 to 1 mbar at 50°C using the rotary flash evaporator (Figure 2).
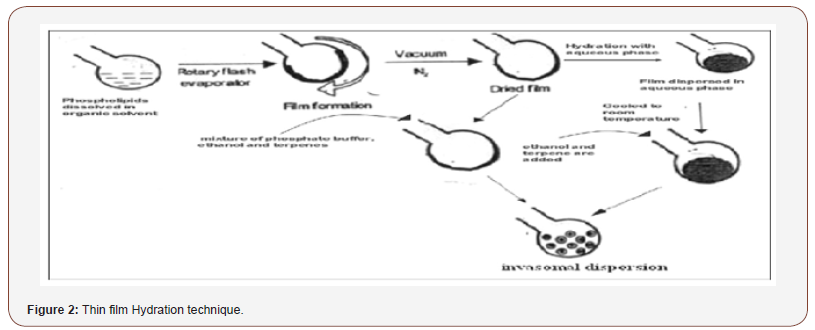
The film is kept under vacuum (1 mbar) for 2 h at room temperature and subsequently flushed with nitrogen. Then, the film deposited is either hydrated for 30 min at lipid phase transition with a mixture of phosphate buffer (pH: 7.4; PBS) containing ethanol and terpenes or it is hydrated using PBS (pH: 7.4) and after cooling to room temperature, ethanol and a single terpene or a terpene mixture are added in order to obtain Invasomes.
Characterization Of Invasomes
• Entrapment Efficiency.
• Surface Morphology.
• Stability Studies.
• Drug Content.
• Vesicular size.
• Ex Vivo Permeation Studies.
Entrapment Efficiency [5,6]
Entrapment efficiency was studied by ultracentrifugation method. 1ml of invasomal formulation was transferred to Eppendorf tubes, centrifuged at 15000 rpm, 4°C for 15 min in two cycles to separate the unentrapped drug. The clear fraction was used for determination of free drug. Percentage entrapped is calculated indirectly from the amount of free drug from the formula
Entrapment Efficiency (%) = 𝑡𝑜𝑡𝑎𝑙𝑑𝑟𝑢𝑔 −𝑓𝑟𝑒𝑒𝑑𝑟𝑢𝑔𝑡𝑜𝑡𝑎𝑙𝑑𝑟𝑢𝑔 × 100.
Surface morphology
It was studied by placing a drop of preparation on clear glass slide, air dried, coated with gold using sputter coater (Polaron E5100, Watford, UK) and visualized under scanning electron microscopy. Stability Studies Optimised invasomal formulation was sealed in 10ml glass vial and stored at refrigeration temperature (4 - 8°C) and room temperature for one month. Entrapment efficiency, physical appearance was determined at regular intervals.
Drug Content
Drug content of the invasomes can be determined by using ultraviolet spectrophotometer. This can be quantified by a modified high-performance liquid chromatographic method.
Vesicular Size and Shape
Invasomes can be visualised by using Transmission Electron Microscopy (TEM) and by Scanning Electron Microscopy (SEM). Vesicle size and zeta potential particle size of the invasomes can be determined by Dynamic Light Scattering (DLS) and photon correlation spectroscopy.
Ex Vivo Permeation Studies
The permeation of invasome formulations was determined by using the Franz diffusion cell. The effective surface area of cell was 2.0 cm² and has a receptor volume of 20ml. The skin was mounted on the receptor compartment with the stratum corneum side facing upwards into the donor compartment. The top of the diffusion cell was covered with lid. The donor compartment was applied with invasomal preparation and 20 ml of pH 7.4 phosphate buffer saline maintained at 37°C was used as receptor medium. Aliquot amounts were withdrawn and replaced by fresh media to maintain a sink condition. Samples were analysed using UV spectrophotometer.
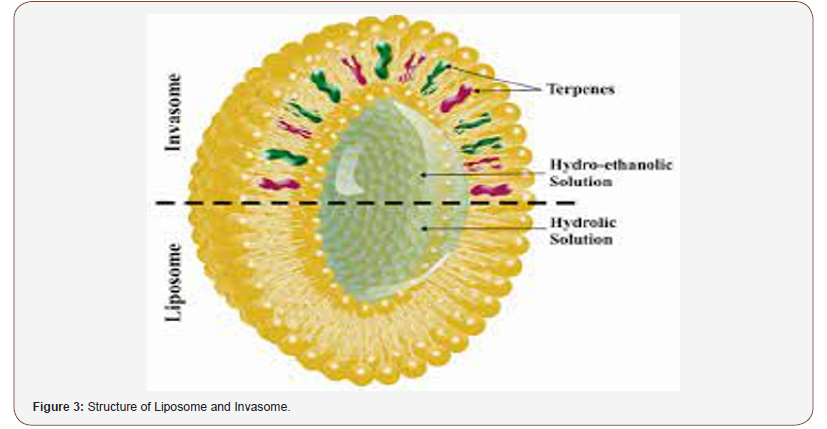
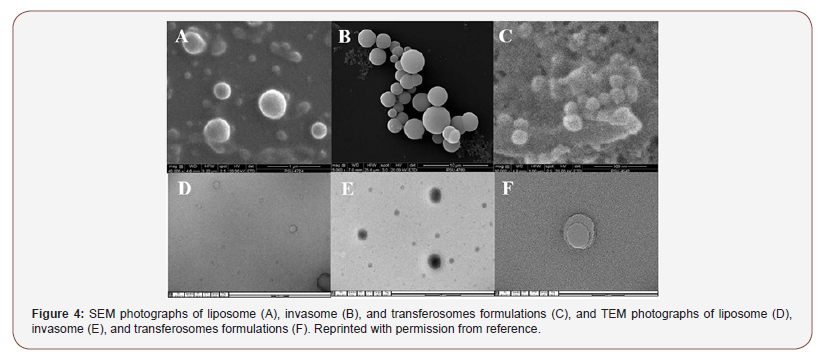
Liposomes are phospholipid-based vesicular structures composed of anionic, cationic, and neutral lipids and cholesterol that improve the encapsulation of lipophilic, hydrophilic, and amphiphilic drugs. Lipophilic drugs are placed in the inner part of the lipid bilayer, hydrophilic drugs in the aqueous core, and amphiphilic types in the interlayer of the vesicles. Contrary to this, invasomes are flexible liposomes consisting of phospholipids, ethanol, and one terpene molecule or a mixture of terpenes. Ethanol increases the fluidity of lipids in the vesicle structure, creating a soft structure less rigid than conventional liposomes and, therefore, enhancing its permeability into the skin. Similarly, terpenes have also been shown to improve penetration by disrupting the tight structure of the SC lipids. Microscopic image of invasome and its differences from liposome and transfersome are shown figure. SEM photographs indicated that all vesicles displayed smooth surface and spherical structure TEM photographs showed the surface morphology of liposomes and invasomes were unilamellar while unilamellar to multilamellar was revealed in the case of transfersomes [7,8] (Figure 3,4).
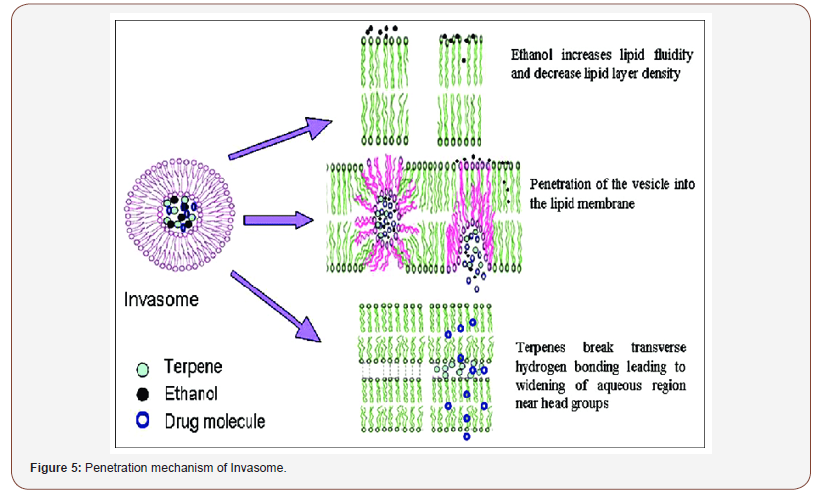
Penetration Mechanism of Invasomes [9,10]
Terpenes and ethanol in the invasomes cause deformability of the vesicles, disrupt the SC bilayer skeleton, and act as penetration enhancers, improving the permeability of the invasomes. According to Dragicevic-Curic et al., during penetration of the invasome, one part of the vesicle disintegrates and releases its components, such as terpenes, phospholipid segments, and single phospholipid molecules, which enhance the penetration and fluidize the SC lipids. Smaller invasome vesicles, which do not disintegrate, penetrate through the SC intact. According to Verma and group, upon penetration, intact invasomes may reach the inner parts of the SC by the follicular transport pathway or via the narrow hydrophilic channels existing in the intercellular region of the SC. Honeywell-Nguyen et al. revealed that smaller intact invasomes can penetrate the deeper part of the SC through the channel-like areas. This was deduced by the flexible vesicles of various sizes that were discovered at the channel-like areas in the deeper layer of the SC and skin surface vesicles. In general, a number of invasomes disintegrate when penetrating the SC, whereas smaller vesicles and flexible invasomes penetrate the deeper layers intact (Figure 5).
Effect of Composition on the Physicochemical Characteristics of Invasomes
Effect of Ethanol
The addition of ethanol in the formulation of lipid nanovesicles is an effective strategy to increase the fluidity of the lipid bilayer of the skin. The interaction of ethanol with the lipid elements in the polar group area of the SC leads to alterations in the structure of the keratinized or lipophilic domains, decreased transition temperature of lipids, and consequently fluidization and disruption of the tightly packed SC lipids. Ethanol-based nanocarriers can fluidize and disturb the SC lipids. The presence of ethanol increases the flexibility of the intercellular lipid matrix due to the rotating freedom of the lipid acyl chains. Thus, ethanol increases the fluidity of lipids in the vesicle structure, resulting in a structure that has softer and less rigid properties than conventional liposomes. In addition to enhanced penetration ability, ethanol creates a net negative surface charge and limited vesicle aggregation due to electrostatic repulsion, leading to increased stability of invasomes under storage conditions [11].
Effect Of Terpenes
Effect of Terpene on Penetration
X-ray diffraction and differential scanning calorimetry (DSC) results showed that terpenes lead to increased drug penetration by disrupting the tight bilayers and lipid packing in the SC. Furthermore, breaking the hydrogen bonds and extracting SC lipids, enhancing the partition into the SC by improving lipid fluidity and increasing diffusion via the intercellular lipids are another mechanisms that have been reported to increase drug permeability by terpenes. Dragicevic-Curic et al. revealed that various types of terpenes have a synergistic effect on the permeation of temoporfin. Invasomes containing a 1% mixture of three types of terpenes (citral, cineol, and limonene) demonstrated higher temoporfin permeability than invasomes containing 1% citral alone. In another study, Dragicevic-Curic et al. demonstrated the relationship between the permeated amount of temoporfin and the amounts of terpenes in the invasomes. They indicated that vesicles containing 1% terpenes have a 1.7-fold higher temoporfin penetration effect than vesicles containing 0.5% terpenes. Therefore, incorporation of temoporfin in vesicles containing 1% terpenes could lead to deeper penetration.
Effect of Terpene on the Size of the Invasomes
Examination of particle size demonstrated that the size of the invasomes is directly correlated to the number of terpenes; the size of the invasomes increases as the amount of terpene increases. The size of vesicles containing 1% terpenes was 124 nm, whereas the size of vesicles with 0.5% terpenes was 93.0 nm.Prasanthi et al. showed that the size of finasteride-loaded invasomes was influenced by the molecular size of terpene and the concentration of the added terpene. The size of invasomes containing nerolidol (molecular size 222 g/mol) was around 11 to 13 μm. The vesicle sizes of nimesulide-loaded liposomes containing citral, limonene, and cineole were 194 nm, 216 nm, and 244 nm, respectively.
Effect of Terpene on the Shape of the Invasomes
The results of cryo-transmission electron microscopy (cryo- TEM) were in agreement with the DSC and ESR results, indicating the influence of terpenes on the shape of the invasomes, i.e., in addition to spherical vesicles, malformed vesicles of varied shapes also existed in invasomal dispersions.Dragicevic-Curic et al. used cryo-electron microscopy to observe the lamellarity and shape of invasomes with various percentages of terpenes. Their results revealed that invasomes with Nanomaterials 2020, 10, 341 5 of 13 0.5% terpenes were mostly unilamellar and bilamellar or oval and spherical in shape; however, in the invasomal formulation with 1% terpenes, the invasomes appeared to be unilamellar and bilamellar. Therefore, the combination of 1% terpenes with the invasomes increased the membrane elasticity of invasomes, the percentage of terpenes, and the number of deformed vesicles.
Synergistic Effects
A synergistic effect between phospholipids, ethanol, and terpenes on dermal absorption has been visibly observed. Dragicevic-Curic et al. suggest that one part of the invasome disintegrates during permeation in the upper layers of skin and releases the phospholipids and terpenes, which act as permeation enhancers that fluidize the intercellular lipids. Furthermore, the ethanol in the invasome fluidizes the intercellular lipids and enhances the penetration of flexible vesicles. Verma et al. indicated that invasomes increased the transdermal permeation of cyclosporine A compared to an ethanolic solution. The improved efficiency of invasomes compared to an ethanolic solution suggests a synergistic effect of phospholipid, terpenes, and ethanol. Dragicevic-Curic et al. demonstrated that the improved permeation of temoporfin (mTHPC) with 1% terpenes was due to the concentration of terpenes and the synergistic effects of terpenes and ethanol.Thus, the results from the aforementioned studies point toward the synergistic effect of phospholipid, terpenes, and ethanol in the reformed activity of invasomes in comparison with liposomes.
Invasome Stability
The storage temperature has a significant effect on the physical stability of invasomes, i.e., the size of the particles and the polydispersity index (PDI) value. During storage at room temperature, all invasomes show an increase in the particles size and the PDI value, demonstrating physical instability, i.e., aggregation or fusion of the vesicles. In the case of the Dragicevic- Curic et al. study, the PDI of the invasomes stored at 4 °C was stable during storage for 12 months; however, after six months of storage, the invasomes showed a significantly increased particles size and PDI value. With regard to drug content, Lakshmi et al. determined that there was a loss of 10% of the encapsulated drug after one month of refrigeration. The loss of encapsulated drug increased to 50% when stored at room temperature.
Potency Versus Toxicity of Terpenes as One of the Main Components of Invasome
Different approaches have been examined to progress the transdermal permeation of drugs. Among them, use of effective and safe classes of penetration enhancers such as natural terpenes are the most popular methodology. Despite proper performance of penetration enhancers in transdermal delivery, few of them have been approved for clinical application due to their skin irritation and toxicity. In general, there is balance between the potency of penetration enhancers and their toxicity.
Terpenes present high penetration potency for both hydrophilic and lipophilic compounds even at small concentrations. The strategic mechanism for terpene penetration occurs through interaction with SC intercellular lipids. Terpenes are applied alone or in combination with other drug delivery systems as permeation enhancers for therapeutic applications. But beside the potency of terpenes in comparison to different penetration enhancers, the toxicities of terpenes should be considered. The toxicity assessment of terpenes in two skin cell lines using 3-(4,5- dimethylthiazol-2- yl)-2,5-diphenyltetrazolium bromide (MTT) assay and evaluation of transepidermal water loss (TEWL) indicated that terpenes achieved from natural sources are usually considered to be safe in comparison to synthetics [12].
Applications of Invasomes
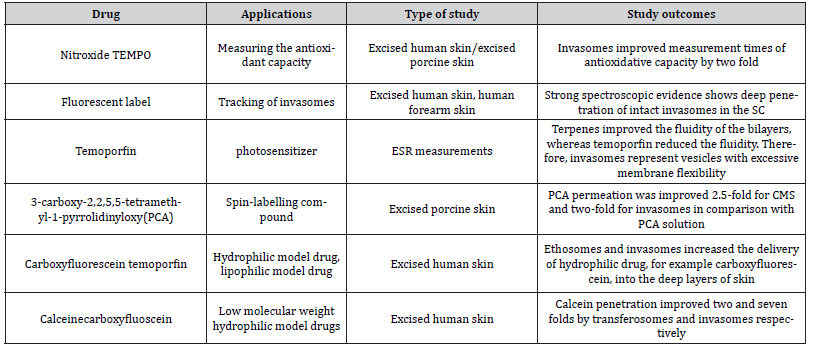
In this section, we have enumerated several applications of invasomes. An overview of various studies on the therapeutic applications and skin permeability enhancement of invasomes is given in Tables 1 and 2 respectively.
Table 1: Therapeutic Applications of Invasomes.
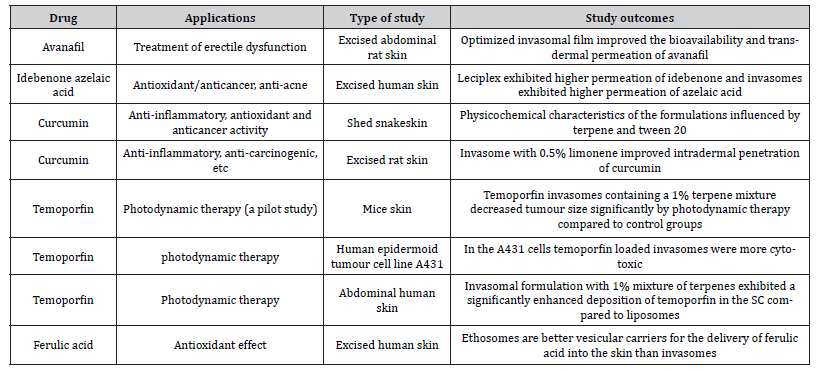
Table 2: Enhanced Skin Permeabilty of Invasomes.
Pharmaceutical Applications of Invasomes
Immunosuppressive drug delivery [13,14]
Immunosuppression is the primary approach to treating autoimmune diseases. Also, it is useful for the clinical application of existing immunosuppressive agents that have been suffered from various drug side effects. The nanotechnology centered approaches can correct the major limitations by enhancing immunosuppressant delivery to target cells of the immune system. Also, reducing the recommended dose for therapeutic function and reducing drug distribution to non-target tissues can be a key alternative to immunosuppressive drug delivery. From the sub-structure of lipidic vesicles in drug delivery, it receives the primary consideration of the investigators to develop advanced nanosized vesicles. A literature survey mentioned that cyclosporine A (CsA, CyA) is a lipophilic drug, and it exhibits poor penetration efficiency into the skin layers (partition coefficient: 4000). The topical applications of CsA can be a suitable alternative for the management of psoriasis and other dermatological diseases.
Initially, Verma were synthesized the invasomes nanocarrier for delivery of CsA, CyA using unsaturated soybean phosphatidylcholine (10% w/v), ethanol (3.3% w/v), citral:cineole:D-limonene mixtures (0.5:1.0:1.5% w/v) and PBS up to 100% w/v using mechanical dispersion. In vitro penetration study concluded that due to the presence of ethanol and terpenes, prepared invasome vesicles showed a higher amount of CsA in the deeper layer of skin (viable epidermis and dermis) as compared with the aqueous/ ethanolic drug solution and liposomes (without ethanol and terpenes). Besides, the increasing concentration of terpene (0.5 to 1.5%) significantly increased the amount of CsA in the deeper skin layer and subcutaneous layer. It shows the direct correlation between the amount of drug added into the terpene mixture and the amount of drug found in the skin layer. Owing to excellent findings of immunosuppressive agent–loaded invasomes, it can be used for treating autoimmune diseases. Taken as a whole, it confirmed that the invasomes can be an effective substituent for hydrophobic drug delivery to the deeper skin layers.
Anticancer drug delivery
From its inception, cancer treatment is still a challenging field in the era of biomedical science. Due to the ineffectiveness of currently engaged therapeutic strategies, a large number of deaths have been occurring each year. Therefore, there is an urge to develop an advanced substitute to resolve cancer ineffective treatment issues. Interestingly, the temoporfin is a potent (secondgeneration) photosensitizer. It shows high tumor selectivity and residual photosensitivity of only 2 weeks. Thus, this could be an effective anticancer agent in photodynamic therapy of early or recurrent carcinomas. On this account, Dragicevic-Curic and coauthors were reported that the deposition of the highly hydrophobic photosensitizer (temoporfin) using invasomes into the skin layer (SC). Briefly, temoporfin-loaded invasomes have been synthesized using the mechanical dispersion method. In that, the temoporfin and 1%w/v terpene (limonene/citral/cineole) were dissolved in ethanolic phospholipid solution (phosphatidylcholine:ethanol: 75:25 w/w) and subjected to vertexing followed by sonication for 5 min.
Finally, PBS was added into the above-mentioned clear solution with constant vortexing for 5 min. The drugloaded invasome (1%, w/v cineol) showed about 105.4 nm particle sizes and about 0.066 PDI. After plastic surgery, the human female abdominal skin was obtained, and penetration was carried out using the nominal surface of the Franz cells (3.14 cm2). The use of cineole (1% w/v) showed the highest penetration ability followed by a mixture (1% w/v) of cineole: citral: D-limonene (45:45: 10 v/v). Experimental outcomes revealed that the single terpene could make an efficient delivery of temoporfin, and a combination of terpenes could lead to the synergistic effect of active penetration to the subcutaneous and deeper skin layers. Besides, the stability study indicated that the invasomes containing 1% w/v cineole Nangare and Dugam Future Journal of Pharmaceutical Sciences (2020) 6:123 Page 10 of 21showed a small increase in particle size and PDL value and can be considered a physically stable form for 12 months. In the future, invasome can be an efficient carrier for the delivery of hydrophobic active molecules with effective concentration to the systemic/local site. However, there is no thumb rule for the use of terpene and its mixture towards penetration [15]. As abovementioned, temoporfin is a second-generation synthetic photosensitizer used for the treatment of oral carcinomas, refractory oral carcinomas, non-melanomatous tumors of the skin of the neck, and head. For photodynamic therapy, there is a need for sufficient skin penetration of photosensitizer. Dragicevic- Curic et al. reported that the invasome can promote the efficient delivery of temoporfin drug. In this context, they have prepared the temoprofin-loaded invasomes via mechanical dispersion method. In brief, temoporfin and 1% w/v of terpenes mixture [cineole, citral and D-limonene] were dissolved in ethanolic phospholipid solution (phosphatidylcholine: ethanol;75:25 w/w). Further, the mixture was subjected to vortexing and sonication to obtain a clear solution. After that PBS was added followed by constant stirring (5 min) to obtain multilamellar vesicles and then extruded using an Avestin hand-extruder via polycarbonate membranes (400 nm, 200 nm, 100 nm, 50 nm).
The particle size and PDI of invasomes (containing 1% citral) were found to 129.5 nm and 0.087 respectively. Furthermore, the use of terpene mixture (citral, cineol, and limonene) provides a higher penetration efficacy of photosensitizer due to the synergistic penetration effect. Besides this, the drug-loaded invasome photodynamic efficacy was carried out through topical application onto the skin of mice (bearing the subcutaneously implanted human colorectal tumor HT29) followed by photoirradiation. The result revealed that the use of photodynamic therapy for temoporfinloaded invasome formulation developed the significantly (p < 0.05) smaller size tumor HT29 as compared with the control (ice without any treatment and mice only photoirradiation) [33]. Hence, invasomal (with 1% terpene mixture) topical application opens the new door as promising tools for photodynamic therapy of psoriasis/ superficial skin tumors. As we know, anastrozole is an efficient aromatase inhibitor for the management of postmenopausal women with breast cancer. However, anastrozole shows many side effects and extensive first-pass metabolism. In 2019, Vidya and coauthors have developed the anastrozole-loaded invasomes using the film hydration method and then converted into invasomal gel. In this study, the phospholipids have been dissolved in ethanol: chloroform (2:1, v/v) mixture followed by drying to obtain thin film rotary flash evaporator.
In that, the pressure was reduced from 500 to 1 mbar at 45 °C and finally placed at 1 mbar for 24 h. Finally obtained film was hydrated using PBS, ethanol, drug, and fenchone for 30 min. The final invasomal vesicles were ultrasonicated and extruded using a polycarbonate membrane. Interestingly, a combination of anastrozole and fenchone has been shown high entrapment of the anastrozole. Besides, the high fencing concentration provides a low boiling point and lipophilicity and high concentration. Lowering the boiling point of terpene provides the weak cohesiveness of the molecules, and due to that, they can easily associate with lipids of SC and modifies the barrier property. The drug-loaded invasome particle size, PDI, and zeta potential were found to be 226.4 nm, 0.540, and − 20.9 mV, respectively. Due to the presence of ethanol in the sample, the negative zeta potential was found on the invasome vesicle, which helps to prevent the vesicle aggregation due to exhibited electrostatic repulsion. It would be more beneficial for the ensuring stability of invasomal dispersion. The ex vivo permeation and skin deposition of invasomes on male Wistar rat skin showed superior permeation, enhanced transdermal flux, and 73% skin deposition of drugs. The repulsion forces of lipophilic vesicles and the hydrophilic nature of terpene resulted in the enhancement of percent entrapment, penetration, and accumulation rate of actives as a compared drug without vesicle carrier. The cytotoxic study on the Michigan Cancer Foundation MCF-7 cancer cell line showed the cytotoxic effect of optimized formulation (at 5 μL/mL) [34]. These findings indicate that the characteristic of anastrozole skin deposition can be improved with invasomes to targeted drug action in the treatment of breast cancer in postmenopausal women to resolve the problem of oral administration of the active. Hence, it can pave the pathway for breast cancer treatment in postmenopausal women using anastrozole invasomal gel.
Delivery of vitamin analog
Isotretinoin is a vitamin A analog and used to treat eosinophilic pustular folliculitis. In recent attempts, Dwivedi and co-investigators have been revealed the synthesis of isotretinoin invasome using the mechanical dispersion method. In brief, isotretinoin dissolved eugenol was added into ethanolic egg lecithin and subjected to vortexing (60 min) which gives the homogeneous suspension. After that, hydration of vesicles has been performed using PBS (pH 6.8), which provides the yellowish translucent invasomes containing suspension. Finally, this suspension has been shifted to sonication (3 cycles/15 min), which provides excellent invasomes. The free isotretinoin has been separated from invasomes using a dialysis bag. In this study, the critical determinant revealed that the various factors of formulation affected the penetration rate of invasomes. The particle size, PDI, zeta potential, and entrapment efficiency of the optimized batch was found to be 148 nm, 0.16, − 69.2 mV, and 85.78%, respectively. Amusingly, the increase in the concentration of egg lecithin increased the size of invasomes. On the other hand, the concentration of eugenol does not affect the vesicle size of invasomes. At a higher concentration of egg lecithin, the single hydrophobic chain with a polar head group of egg lecithin resulted in the highly positive curvature in membranes, which increased the invasome vesicle size.
Besides this, the more lipid concentration increased the entrapment efficiency of the isotretinoin, because of the more lipids available to entrap the isotretinoin. In addition to this, the use of eugenol increased the solubility and the entrapment of isotretinoin. Similarly, the high concentration of eugenol and lecithin showed high deformability of invasome vesicles. Finally, the ex vivo permeation on rat-shaved skin has been carried out using a Franz diffusion cell. It showed 85.94% cumulative isotretinoin permeation and about 78.82 μg/h/cm2 topical flux (Jmax). The prepared invasome gel showed 97.12% drug content and zero-order release with a 1.135 diffusion constant. Also, it accomplished 85.21% of cumulative isotretinoin permeation within 8 h. Furthermore, invasomes gel arrested the cell growth up to 82% with an insignificant difference. Overall, invasome gel would be capable of delivering the isotretinoin to the follicular unit and accordingly to achieve pilosebaceous targeting. In the future point of view, clinical trials (phases I, II, and III) will be necessary before the use of human patients.
Used in alopecia treatment
There is a tremendous need for progress in engaged therapies to efficient alopecia treatment. The literature says that the use of invasomes yields long-term effects that are self-satisfactory. Today, patients also use alternative and complementary therapies to try to find a safe, natural, and efficient cure for hair restoration. In this context, the FDA approved the finasteride, a 5α reductase inhibitor, and now this is commonly preferred for the treatment of alopecia. Despite this, there are needs to develop a novel carrier for the delivery of finasteride across the dermis layer. Herein authors have been prepared the invasome of finasteride using the combination of a terpene (limonene, nerolidol, and carvone: 0.5%, 1.5%, and 1%, accordingly) through mechanical dispersion. In brief, soya phosphatidylcholine (10% w/v) was added into ethanol (40% w/v) and vortexed (5 min) to obtain the ethanolic lipid mixture. After that, the finasteride (0.35% w/v) has been added into terpene with different concentrations (0.–1.5% w/v) followed by constant vortexing and sonication (5 min) to which gives the clear solution. the hydration of vesicles has been achieved using PBS (up to 100% w/v) with constant stirring (5 min). Finally, drug-loaded multilamellar invasome vesicles were subjected to probe sonication (5 cycles/5 min) at 4 °C. The 0.5% limonene containing optimized invasomes showed about 84.56% of entrapment efficiency, − 69.1 mV of zeta potential, 5.81 μm of particle size, and 5.94 μg/ cm2 of cumulative amount permeated. Herein, finasteride-loaded invasomes showed a negative charge on the surface of vesicles due to the presence of ethanol. Besides, it resulted in electrostatic repulsion, which provides better stability to the invasomal dosage form. The sonication of invasome gives the high rotation energy that results in a high negative charge/potential.
Besides that, ethanol concentration showed a negligible change in the negative charge with different terpene phases and resulted in the progress of the finasteride penetration rate. The concentration of terpene and entrapment efficiency was found to be inversely proportional to each other. Concisely, hydrophobic limonene showed the maximum entrapment ability of finasteride. On the other hand, hydrophilic nerolidol showed the minimum entrapment of finasteride. Owing to the presence of hydrocarbon and ketone group in limonene and carvone which gives the more hydrophobic nature, provides more entrapment of actives. Furthermore, limonene, nerolidol, and carvone act by lipid extraction of SC increasing the diffusion coefficient of active and disruption of highly ordered intercellular lipid structure of SC, respectively. The in vivo study of transdermal invasomal gel using a rabbit model exhibited a maximum concentration of 656.53 ± 25.03 ng/mL. Besides this, the AUC0–72 was 3.04 times higher than that of the oral route (5630.58 ± 361.50 ng h/ml). Additionally, the bioavailability of finasteride was enhanced by 303% using invasomes. The use of the current invasomal drug-loaded formulation demonstrated hemorrhage, edema, congestion, infiltration of mononuclear cells, thickening of epidermis degeneration, and fatty changes in the dermis as compared with the well-woven structures with distinct SC in control. In conclusion, the iontophoresis of invasome formulation revealed the enhancement in the penetration rate of finasteride.
Hence, in the future, these new lipid vesicles can be alternative carriers for iontophoretic transdermal delivery of finasteride. The minoxidilloaded invasome formulation using soya lecithin and different penetration enhancers via film hydration method reported in 2009 by Mura et al. In this pioneered study, three penetration enhancers namely transcutol, labrasol, and cineole have been used. Out of this, cineole and transcutol are well-known nontoxic, biocompatible penetration enhancers, whereas labrasol is a safe, non-ionic hydrophilic surfactant. Briefly, soya lecithin, minoxidil, dicetylphosphate, and penetration enhancers have been dissolved into the chloroform. After that, the lipid-minoxidil mixture has been deposited as a thin film using a roto-evaporator. After the removal of chloroform, the hydration has been done using distilled water under constant mechanical stirring at room temperature. Then, vesicle suspension was subjected to sonication and after that freeze-drying (− 20 °C for 12 h). As compared with the average diameter of cineole vesicle (144 nm), labrasol (202 nm), and transcutol (171 nm) showed a larger diameter. It may due to hydrophilic molecules increase vesicle surface energy and that shows vesicle enlargement.
Also, it exhibited homogeneous and monodisperse suspension with an excellent PDI < 0.3, which confirms the stability of invasomes formulation. Furthermore, it showed negative zeta potential (from − 52 to − 58 mV), which ensured the prevention of vesicle aggregation. Furthermore, an in vitro diffusion study using newborn pigskin showed a statistically significant improvement of minoxidil deposition in the upper skin in comparison with drug liposomes and drug ethanolic solutions. Additionally, it avoids the transdermal delivery of minoxidil which helps to improve the cutaneous minoxidil bioavailability. Therefore, the use of penetration enhancers improved deformable vesicles than conventional ones. In vitro penetration revealed that the use of penetration enhancers increases the deposition of minoxidil into the skin membrane than the alcoholic solution of minoxidil. Therefore, it can be a potential innovative carrier for improving the specific topical delivery and accordingly bioavailability of minoxidil.
Delivery of anti-acne agent
Acne is a widespread skin disorder worldwide in recent times. Dapsone is an exceptionally active pharmaceutical ingredient for leprosy treatment. It has significant potential for acne therapy due to its anti-inflammatory action. As essential for the delivery of topical drugs, an effective carrier must be established for the delivery of dapsone to the specific site. In consequence, El-nabarawi et al. prepared the dapsone-loaded invasome by a film hydration technique using terpene (limonene, cineole, citral, or fenchone) and phosphatidylcholine for the treatment of mild to moderate acne. Briefly, dapsone (20 mg) and terpenes with different concentrations were mixed with a clear solution of phosphatidylcholine (200 mg) in methanol/chloroform, 2:1 v/ v.
After that, this mixture was subjected to rotary evaporation to remove the organic solvent at 120 rpm (60 °C) for 15 min. Finally, thin-film was hydrated using 3% v/v ethanolic: water mixture at 120 rpm (60 °C) for 1 h and further filtered (pore size 25 μm) to separate the drug crystals from invasomes. The high concentration of terpene showed the superior entrapment efficiency of dapsone. Furthermore, the limonene containing invasomes showed the highest entrapment efficiency followed by cineole, fenchone, and citral. Possibly, it may because of the lipophilicity of the used terpenes. The optimized drug-loaded unilamellarinvasome exhibited uniform spherical discreet shape, and − 37.5 mV zeta potential that confirms the stability of invasome vesicles. The differential scanning thermogram revealed that the invasomecontaining drug was converted into the amorphous region with uniform distribution. Interestingly, the in vivo permeation study on Wistar rats resulted in the enhancement of penetration rate of invasome by 2.5-fold as compared with the solution form. Besides, they found the in vivo rat skin deposition amount of dapsone to be 4.11 μg/cm2 for invasomes, which was high than the drug-alcoholic solution (1.71 μg/cm2).
Particularly, the Wister albino rats have been shown invasome increases the 2-fold AUC0–10 than the solution of dapsone. Overall, invasomes significantly increased the skin deposition of dapsone. Therefore, invasome can be potential vehicles for the delivery of an anti-acne agent. A literature survey reported that capsaicin is a major pungent photo component and widely studied in the pharmaceutical arena. Particularly, it is used for the management of oral and topical pain. In 2016, Duangjit et al. synthesized capsaicin (0.15%) loaded invasomal formulation using phosphatidylcholine and D-limonene. The optimized capsaicin-loaded invasomes showed narrow size distribution (0.01–0.30), smaller than 100 nm in size. Furthermore, negative (− 20 mV) zeta potential confirmed the stability of drug-loaded invasomes. Furthermore, it gives excellent skin permeability over the conventional liposome formulation and commercial product (0.15% capsaicin in ethanolic solution). Hence, the optimized capsaicin invasomes can be used as a substitute for transdermal drug delivery.
Delivery of anti-hypertensive agent
Anti-hypertensives are used to treat elevated hypertension. It has numerous problems such as low aqueous solubility, low bioavailability, short biological half-life, low permeability, and a list of undesirable side effects. This can be waved off using a suitable route for delivery and formulation. A calcium channel blocker, isradipine is generally used for the treatment of hypertension. Unfortunately, it has low oral bioavailability, and it suffers the firstpass metabolism. Kamran et al. accomplished the development of invasome using Phospholipon90G (2% w/v), b-citronellene (0.1% w/v, terpene), and ethanol 10% w/v through conventional film hydration technique and used as an efficient carrier for the delivery of isradipine via the transdermal route. In brief, isradipine, terpene, and Phospholipon90G were dissolved in chloroform: methanol (2:1 v/v).
Then, the organic solvents were removed through rotary evaporation and organic solvent traces were collected separately using a vacuum cabinet overnight. The hydration of isradipineinvasomal lipid film has been performed using PBS: ethanol at 60 rpm using rotary evaporator for 1 h and then subjected to probe sonification (4o C) at 40% output frequency. The particle size, polydispersity index, entrapment efficiency, and transdermal flux through rat skin of isradipineinvasomes were found to be 194 nm, 0.272, 88.46%, and 22.80 mg/cm2 /h, respectively. Because of the presence of ethanol and terpene, it provides particle deformability and enhances the penetration rate of isradipine. Interestingly, enhancement in the deformability of the invasomes and lipidic bilayer of the SC disruption facilitates the penetration of isradipineinvasome vesicles. Hence, the prepared isradipineinvasomes follows the transepidermal osmotic gradient into and through the skin SC and deliver the antihypertensive agent to the systemic circulation.
In short, invasomes delivery systems could be a potential supplier of isradipine through transdermal for hypertension treatment. Abundant literature surveys reported that the hydrophobic olmesartanmedoxomil exhibited low oral bioavailability and short biological half-life. It is preferably used to treat hypertension, but it also offers the aforementioned limits for oral administration of olmesartan. In 2016, Kamran et al. investigated nano-sized invasome for the delivery of olmesartanmedoxomil through the transdermal route using the film hydration method. In brief, olmesartan (10 mg) and Phospholipon90 G mixture were dissolved into 1: 2 v/v of methanol: chloroform (10 mL). Then, thin layer lipid films have been obtained using rotary evaporation which removes the organic solvent when applied vacuum with appropriate time at a temperature more than lipid transition temperature. Moreover, the film hydration was carried out using PBS and ethanol that gives the multilamellar invasome vesicles. Finally, these obtained invasome vesicles have been subjected to obtain the nanosized invasomes. The vesicle’s size, entrapment efficiency, and transdermal flux of invasomes were found to be 83.35 nm, 65.21%, and 32.78 mg/ cm2 / h, respectively. In this study, the terpene (i.e., bcitronellene) has shown admirable entrapment and penetration capacity of olmesartan invasomes. The bcitronellene and phospholipid concentration are directly proportional to the size of invasomes and entrapment efficiency invasome vesicles, respectively. The nanosized invasomal gel showed a smooth, homogeneous appearance and texture and basic pH and exhibited no risk of skin irritation. Furthermore, the olmesartan release kinetics from invasomal gel follows the Higuchi matrix model (R2: 0.986), which confirmed that the invasomal gel exhibited the diffusion process– based olmesartan release. Moreover, the rat skin showed that the Olmesartan invasome eventually distributed and permeated deep into the rat skin. Besides, olmesartan invasome improved bioavailability (1.15 times) in Wister rats [40]. It indicated that nano-invasomes were a competent transdermal delivery system for olmesartan. Thus, invasomes could be a more superior carrier for the delivery of other antihypertensive drugs via the transdermal route.
Treatment of erectile dysfunction
Erectile dysfunction is an inability to start or maintain the required penile erection during satisfying sexual intercourse and millions of men’s population are affected by erectile dysfunction. Whereas about 30 million of the men’s population has been added to the erectile dysfunction each year, and only 2 lakhs men pursue treatment from a physician. Therefore, there is an urge to investigators from the suffered population to treat erectile dysfunction. Owing to this, there is a need to deliver the drug efficiently using a suitable carrier which can overcome the limitations of previously engaged techniques. Avanafil is a selective phosphodiesterase type 5 inhibitor (FDA approved), generally used for oral administration in the treatment of erectile dysfunction.
Although, the oral bioavailability of the avanafil is challenging due to poor aqueous solubility, extensive presystemic metabolism. Additionally, there is a chance of alteration of the absorption of drugs in the presence of food. In 2019, Ahmed et al. were prepared the avanafil-loaded invasomes for the treatment of erectile dysfunction using Phospholipon90G, D-limonene via film hydration method. Briefly, avanafil-loaded invasomes have been synthesized by dissolving avanafil (100 mg) and Phospholipon90G in methanol/ chloroform mixture (1:2 v/v) followed by removal of organic solvent using rotary evaporation. The deposited avanafil lipid films have been kept in a vacuum cabinet to remove the organic solvent overnight. Further, film hydration has been carried out using PBS/ethanol mixture (7:3) in rota vapor at 60 rpm (25 °C) for 1 h and then shifted to sonication in an ice bath to obtain nanosized invasome vesicles. The optimized avanafilloadedinvasome vesicles (10.47% phospholipid, 2.00% ethanol, and 1.50% D-limonene) resulted in about 96.98% entrapment efficiency. Moreover, the vesicular size and PDI of invasomes was found to be 109.92 nm and < 0.3, respectively. Interestingly, the PDI value confirmed the homogenous and mono-dispersedness of avanafil invasomes. Then, the optimized avanafil invasomal formulation has been further incorporated into the hydroxypropyl methyl cellulose-based transdermal film.
They have reported that the change in the β-citronellol to D-limonene reduced the size of the invasome and it could be an effect of molecular weight and lipidic nature of terpene. Additionally, the β-citronellol has less lipophilicity than the Dlimonene, which increased the force among vesicles and terpene and therefore, caused the enlargement of invasome size. The exorbitant amount of lipids leads to an increase in the lipid particles forming each vesicle, and these raise the possibility of the drug to incorporate with a lipidic portion of the invasomes. The optimized AVA invasomal film enhanced ex vivo permeation with an enhancement factor of 2.514 which was performed on excised abdominal Wistar rat skin using a Franz diffusion cell and compared with avanafil raw film. The relative bioavailability of invasome was found to be more than 4-fold increased as compared with the raw avanafil-loaded film. The above-mentioned findings of drug loaded invasomal transdermal film could be taken as a promising avanafil delivery method to resolve the enlisted problems such as poor aqueous solubility, extensive presystemic metabolism, etc., faced by the oral drug absorption. Therefore, invasomal formulation provides the capability to enhance the skin permeation and bioavailability of avanafil. Consequently, it can be used as an innovative carrier for drug delivery in the treatment of erectile dysfunction.
Antioxidant
Nowadays, ferulic acid (antioxidant) is gaining much attention from research scholars due to its therapeutic effects such as anticancer, anti-skin disorders, antidiabetes, anti-cardiovascular disease, anti-inflammatory, etc. Ferulic acid is normally located in many of the plant cell walls. Unfortunately, it takes a short half-life of removal and needed many dosages with regular administration. In this regard, appropriate transdermal vesicles may also be a safer choice for ferulic acid delivery. Chen and co-investigators prepared the terpene (limonene, citral, cineole-1:4.5:4.5 v/v) based invasomes for the delivery of ferulic acid using the film hydration method and compared with conventional liposomes, ethosomes, Tween 80-based deformable liposomes. In brief, soybean phosphatidylcholine (133 mg/mL), ferulic acid (12 mg/ mL), and terpenes (10 mg/mL) were dissolved in methanol and chloroform mixture (1:2 v/v) and then subject to removal of organic solvent using rotary evaporation under specified vacuum condition for the appropriate time at 43 °C. The thin lipid film hydration was accomplished using PBS (pH 7.4) and ethanol (10% v/v). Further, ferulic acid-loaded invasomes were subjected to sonication for 15 min (5 min/cycles) in an ice-water bath that gives the nanosized invasomes. Finally, obtained invasomal suspension has been shifted to sized using polycarbonate membrane (pore diameter: 100 nm), which gives the uniform ferulic acid invasomes.
The ferulic acid invasomes exhibited about – 39-mV zeta potential, 129.1-nm vesicle size, and PDI < 0.2. It confirmed that the developed suspension of ferulic acid invasomes had a stable, uniform, nanosized, and homogeneous form. In addition to this, the in vitro permeation of ferulic acid from ethosomes through the human skin was found to be high as compared with the other formulations. It may be because of the high concentration of ethanol in ethosomes. On the other hand, conventional liposomes have been expected to be effective for the delivery of drugs into the upper layers of the skin. Besides, ferulic acid invasomes showed better permeation because of deformable vesicles and penetration enhancer’s interaction with lipid lamellae and skin layer.
In present pioneering attempts, Shah and co-authors investigated the penetration rate of antioxidant/anticancer and anti-acne agents, for example, idebenone and azelaic acid, respectively, in the original carrier systems (cationic LeciPlex, invasomes, and conventional liposomes). In brief, initially, idebenone/ azelaic acid was dissolved in 3 mL organic solvent (chloroform: methanol) and further mixed with an ethanolic solution of soybean phospholipid (75%). After that, a thin lipid film was developed using rotary evaporation and then subjected to nitrogen drying (15 min). Finally, a mixture of terpenes, PBS, and ethanol with lipid film vortexed to obtain multilamellar invasome vesicles. The homogenous and uniform nanosized invasomes have been achieved using polycarbonate membranes (100 nm). The particle size, PDI, and entrapment efficiency of idebenone and azelaic acid invasome vesicles were found to be 140 nm, < 0.1, and more than 90%, respectively, which is much smaller than other formulations (cationic LeciPlex, and conventional liposomes). Particularly, single-step mixing without homogenization showed higher vesicle sizes and PDIs of LeciPlex formulations. In the case of drug-loaded invasomes, the existence of phosphatidylinositol and phosphatidic acid invasomes exhibited negative zeta potential (− 13 mV), which confirmed the stability of invasomes. Idebenone and azelaic acid invasomes were demonstrated in unilamellar vesicles along with a mixture of the small and large unilamellar vesicles, which confirmed that incorporating drugs into vesicles does not affect the vesicle shape. On the other hand, an ex vivo human skin penetration study revealed that the interaction of idebenone with the phospholipid membrane.
Idebenone has been delivered by LeciPlex significantly high as compared with the invasomes. Besides this, the azelaic acid– based invasome showed a higher penetration ability for azelaic acid as compared with idebenone, while the interactions between carboxylic groups of the azelaic acid and the cationic surfactants resulted in low penetration of azelaic acid–loaded LeciPlex. Azelaic acid interacts with phospholipid containing phosphate and weak interaction with fatty acid chains. Due to the presence of phospholipid, the invasome exhibited in the L-lamellar phase and showed a high penetration ability. In conclusion, for azelaic acid delivery invasome is a suitable carrier while for idebenoneLaciPlex. This hypothesis was confirmed by the in vivo effectiveness analysis of azelaic acid formulations. Intracutaneous delivery of active pharmaceutical agents is developed based on pharmaceutical properties of drug, formulation variable characteristics, and formulation properties benefit of the entire. Therefore, during formulation development, the property of the drug, formulation, and its components has held the major account.
Miscellaneous applications of invasomes
Curcumin is an active phytoconstituents obtained from Curcuma longa Lin. It has high potential in the treatment of various common and serious health issues such as hepatic disorders, hypocholesterolemic, anticoagulant, Alzheimer’s disease, rheumatism, anorexia, anti-carcinogenic, anti-inflammatory, antispasmodic, antibacterial, etc. Nevertheless, curcumin is a poorly water-soluble active phytoconstituent and it has poor bioavailability. Owing to that several attempts have been made to overcome these issues using different innovative strategies. In 2014, Lakshmi et al. have been developed the curcumin-loaded cyclodextrin and hydroxypropyl β-cyclodextrin complex for solubility enhancement and then this complex loaded into the invasomes. On this account, various types of terpene with different concentrations (limonene, fenchone, nerolidol: 0.5, 1.0, 1.5%) have been used as a penetration enhancer. In brief, the complexation of curcumin has been achieved using curcumin: cyclodextrin and hydroxypropyl βcyclodextrin (1:2) through the co-precipitation method.
Then, the curcumin complex was loaded into invasomes via mechanical dispersion using ethanolic solution soya phosphotidylcholine (1–3% w/v) followed by constant vortexing (5 min) with the addition of curcumin complex and terpenes. Then, the obtained solution has been subjected to sonication (5 min) and further hydration of lipid thin film has been achieved by adding PBS (10% w/v) under constant vortexing (5 min). Finally, it was converted to the gel form of invasomes. Interestingly, the vesicle size and PDI of curcumin invasomes were found to be 134.7 nm and < 0.2, respectively. Moreover, due to the presence of ethanol, it showed negative zeta potential (− 33.7 mv), which confirm the stability of the invasomes. Owing to the high concentration of hydrophobic groups in limonene, invasome showed greater entrapment of curcumin. The entrapment efficiency of the curcumin complex with terpene was found in limonene > fenchone > nerolidol order. Additionally, due to the presence of ethanol, it gives noticeable release (22.31 μg/ cm2 /h1/2) from invasomes as compared with other limonene concentrations (1% and 1.5%). The ex vivo skin permeation study on abdominal skin (male Wistar rat) has been carried out using Franz diffusion cell.
The 4% of ethanol and 0.5% limonene-based curcumin invasomes accomplished the superior permeation (8.11 times) as compared with the control. The synergetic effect of ethanol and terpene enhances the permeation of curcumin across the membrane. Consequently, for solubility enhancement of curcumin, the cyclodextrin and hydroxypropyl βcyclodextrin complex can be a suitable approach, and 0.5% limonene containing invasome can be used as a carrier for curcumin delivery through the skin. It is well known that microneedles are majorly used for the delivery of drug molecules, proteins, vaccines, etc. along with various forms. Generally, microneedles are significantly increased the skin permeability and deliver the drug across the SC. In 2009, Badran and group claimed that the invasion is a more effective technique for the delivery of a hydrophilic drug to the local/systemic applications. In this study, the drug loaded (carboxyfluorescein and radiolabelled mannitol, as a model drug) invasomes has been prepared using 1% w/v terpene (cineole: citral: D-limonene 45:45:10 v/v) mixture as a penetration enhancer and 13.3% w/v ethanolic phospholipid solution (equivalent to 10% soybean phosphatidylcholine) via mechanical dispersion. Herein, the terpene mixture vortexed (5 min) with ethanolic phospholipid at room temperature to obtain a clear solution. After that, the aqueous phase was added into the organic phase under constant vertexing that resulted in large multilamellar invasomes.
Finally, the drug-loaded invasomes extruded using a polycarbonate membrane (400–50 nm) that provides the nanosized uniform invasome vesicles. Interestingly, the average size of the drug (labeled mannitol and carboxyfluorescein) loaded invasomes was found to be 123.6 nm and 105.5, respectively. Furthermore, the PDI value was found to be 0.08 for both drug-loaded formulations, which indicated that the high homogeneity of invasomes. The skin penetration study was performed on human abdominal skin obtained after plastic surgery that divulged the use of a dermaroller with invasome increased penetration and permeation rate of a hydrophilic drug as compared with the aqueous drug solution.
Due to the presence of terpenes and ethanol increase, the interaction with the SC lipidic layers, which enhanced the drug penetration rate. The hydrophilic agent was successfully encapsulated into the core and the outer phase was water. Thus, the flexible invasome enhanced the penetration rate of active dissolved in the external water phase. Without treatment of a dermaroller, it showed less penetration of invasome-loaded mannitol across the membrane as compared with a dermaroller. The appropriate microneedle length selection gives an excellent penetration rate. In conclusion, the microneedles assembly and its geometry actively influence the penetration of microneedles and consequently show an effect on the delivery of active to the selected site of application. Hence, using a dermaroller with appropriate geometry for the delivery of invasome-loaded active pharmaceutical ingredients would be a promising tool for the efficient delivery of hydrophilic/ hydrophobic molecules through the skin.
Conclusions
Invasomes is considered as novel vesicular drug delivery system with more penetration when compared to other drug delivery systems. The effect of terpene in invasomal drug delivery system was discussed. Invasomal drug delivery system was having enormous number of applications. Invasomal drug delivery system can be designed for the drugs with poor permeability and for local action.
Acknowledgement
None.
Conflict of Interest
None.
References
- Atsang AKG, Dzeufiet DPD, Foyet HS, Dimo T, Kamtchouing P (2014) Analgesic and Anti-inflammatory Effect of the Aqueous Extract of Dichrostachys glomerata (Forssk.) Hutch Fruits. European Journal of Medicinal Plants 4: 964-978.
- Anonymous (1990) Cancer pain relief and palliative care; report of a WHO expert committee. World Health Organization Technical Report Series, 804. Geneva, Switzerland: World Health Organization pp. 1-75.
- Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, et al. (2003) "Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations". Archives of Neurology 60: 1524-1534.
- Youngstedt, Shawn D, Kripke Daniel F (2007) "Does bright light have an anxiolytic effect? – an open trial". BMC Psychiatry. 7: 62.
- Rajaram C, Reddy KR, Chandra KB (2015) Evaluation of anti-arthritic activity of Caesalpinia pulcherrima in freund's complete adjuvant induced arthritic rat model. J Young Pharm 7: 128-132.
- Shi F, Zhou D, Ji Z, Xu Z, Yang H (2015) Anti-arthritic activity of luteolin in Freund's complete adjuvant-induced arthritis in rats by suppressing P2X4 pathway. Chem Biol Interact 226: 82-87.
- Bhalekar MR, Upadhaya PG, Nalawade SD, Madgulkar AR, Kshirsagar SJ (2015) Anti-rheumatic activity of chloroquine-SLN gel on wistar rats using complete freund's adjuvant (CFA) model. Indian J Rheumatol 10: 58-64.
- Gomes RP, Bressan E, Silva TM, Gevaerd SM, Tonussi CR, et al. (2013) Standardization of an experimental model suitable for studies on the effect of exercise on arthritis. Einstein (São Paulo) 11: 76-82.
- Kapetanovic M, Lindqvist E, Geborek P, Saxne T, Eberhard K (2011) Long-term mortality rate in rheumatoid arthritis patients with disease onset in the 1980s. Scand J Rheumatol 40: 433-438.
- Adedapo AA, Sofidiya MO, Afolayan AJ (2009) Anti-inflammatory and analgesic activities of the aqueous 275 antianxiety agent" at Dorland's Medical Dictionary. J. Phys. Pharm. Adv 2(8): 269-276
- Sapolsky RM, Romero LM, Munck AU (2000) "How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions." Endocr Rev 21(1): 55-89.
-
A Krishna sailaja, T Meghana. Applications of Invasomal Drug Delivery System. Arch Phar & Pharmacol Res. 3(1): 2021. APPR. MS.ID.000551.
-
Invasomes, Terpene, Thin film hydration technique, Characterization.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






