 Research Article
Research Article
An Overview of Ocular Microbiology: Ocular Microbiota, the Effect of Contact Lenses and Ocular Disease
Mohammad AA Al-Najjar*, Maram Altah and DemaAljakhim
Faculty of Pharmacy, Applied Science Private University, Jordan
Mohammad AA Al-Najjar, Department of Pharmaceutical Sciences and Pharmaceutics, Applied Science Private University, Amman, Jordan.
Received Date:June 03, 2019; Published Date: July 12, 2019
Abstract
The human eye is an organ that is exposed to the environment continuously, which makes the ocular surface subjected to different types of pathogenic and non-pathogenic microorganisms. Different types of microorganisms can be found naturally in each layer of the eye, such as the Grampositive bacteria (i.e., the Coagulase-negative staphylococci including S. epidermidis and Bacillus sp), the Gram-negative bacteria (as Pseudomonas sp. including P. aeruginosa) and fungi. Apparently, bacteria are considered to be the major causative agent of ocular infections that are believed to be a global problem. Unfortunately, Ocular infections, if left untreated, may lead to damage in the structure of the eye with possible complications such as blindness and visual impairments. Scientists highlighted the importance of studying the ocular microbiome, which indeed has been accelerated recently. In this review, we aim to overview ocular microbiota and the factors that may affect it, the diagnosis of ocular infections, and the available treatment so far.
Keywords: Eye microbiota; Gut microbiota; Eye infection
Eye Microbiota
The human eye is a very complex organ which consists of three layers: tear film, lacrimal glands, and eyelids [1–3]. The outer section consists of the cornea and the sclera, where cornea protects the eye against infection [3]. The ocular surface is frequently exposed to the environment and to different types of microbes starting from birth time and throughout life. If a swab was taken from different parts of the ocular surface, many types of microbes can be isolated and examined by culture [4,5]. Interestingly, the microbial composition could be (i) gram-positive bacteria such as Staphylococcus aureus and staphylococciepidermidis, (ii) gram-negative bacteria such as Pseudomonas aeruginosa, and [4,5], (iii) fungi such as Aspergillus spp, Curvularia sp., Penicillium sp., Helminthosporium sp., Candida albicans, C. guilliermondii, C. parapsilosis, Saccharomyces cerevisiae, Hormodendrum sp., and Rhodotorula rubra [6–9] (Table 1 & 2).
Table 1: The tablet is a summary of the Ocular microbiota of conjunctiva, eyelid, and tears, adopted from [4-10].
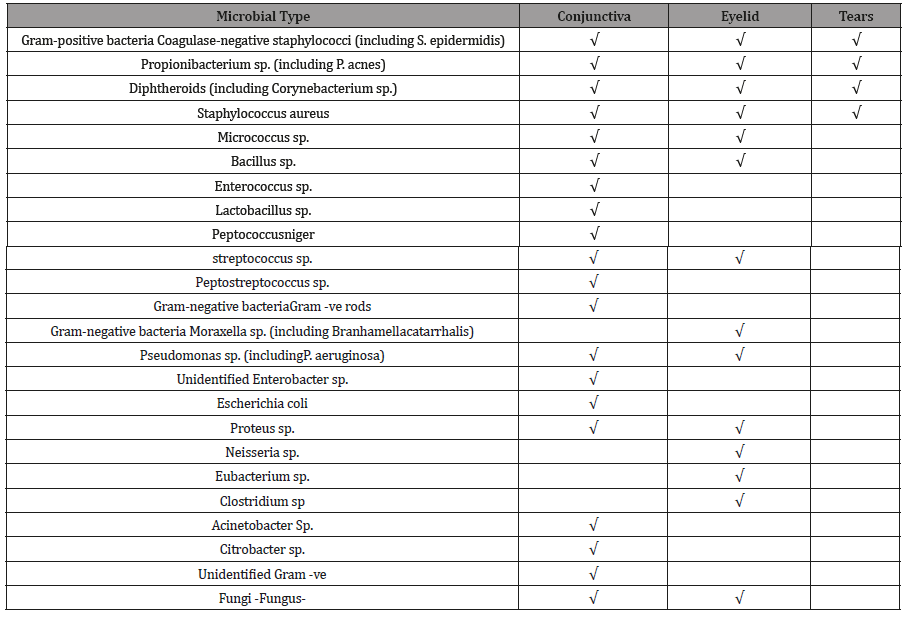
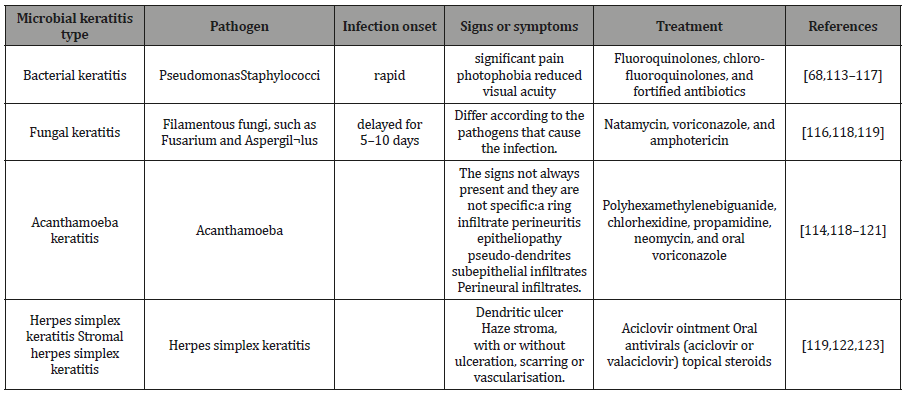
Table 2: Clinical features of keratitis types.
Effect of The Gut Microbiome on Ocular Disease
Relevant to ophthalmologist’s studies, gut bacteria can influence immunity at distant sites, including the eye. Some ocular conditions have been associated with gut microbiome abnormalities including Sjögren’s associated dry eye, glaucoma, and macular degeneration. Patients have infectious keratitis, Patients with bacterial keratitis had higher relative abundances of Proteobacteria and Firmicutes in the gut comparing them with healthy individuals [11].
Dry eye
Sjögren’s is a disease caused by lymphocytic infiltration of the lacrimal and salivary glands disorder which as consequence will cause dry eye and mouth [12]. Studies demonstrated that commensal bacteria variation in the gut provokes a worse dry eye phenotype, while the normalization of the improvement of the microbiome can cause improvement in the dry eye phenotype [13,14].
Autoimmune uveitis
The results of studies were done on patients with posterior segment uveitis have shown that these patients have gut microbiome disturbance [15,16]. Compared to healthy individuals there were increased abundances of Fusobacterium and Enterobacteriaceae [17]. This alteration in the commensal gut bacteria believed to alter the immune privilege status of the eye and thus prompted toward uveitis. A series of actions potentially relate the microbiome to uveitis; consist of loss of immune tolerance to commensal microbiomes increased inflammation and permeability of the gastrointestinal tract and translocation of microbial antigens to extraintestinal sites such as the eye [15,16,18].
Glaucoma
Studies investigated the link between the microbiome and different sub-types of glaucoma, as they revealed that alterations of gut microbiome were related to primary open angle glaucoma (POAG). This infection was correlated with the raise abundance of Bacteroides and Prevotella [19,20].
Age-related macular degeneration (AMD)
Age-related macular degeneration (AMD) is a deactivating eye condition that affects the slow decrease of central visualization [21,22]. This eye disorder influences a central part of the retina well-known as the macula, which controls critical central vision that is necessary for daily actions as reading, watching television, driving and facial recognition. Even though AMD does not normally indicate to absolute loss of sight (peripheral vision is retained), failure of central vision possibly will have the main effect to an individual’s conventionality and value of natural life [23]. Although in this disorder the exact pathogenesis of AMD is poorly understood, it is believed to be an inflammatory component related to innate immunity. These inflammatory pathways consist of complement and Toll-like receptors, pathways adjusted by the microbiome [17].
Stages of AMD: AMD has three stages all of which will appear in the eye retina. The early AMD in which the size of drusen is medium and the patient vision will not be lost. In the intermediate AMD, the drusen size will be larger; this stage is asymptomatic although the patient may have an abnormality in the retinal pigment. The last stage is the late AMD, which is characterized by having two types the wet AMD and the dry AMD, and at this stage, the size of drusen is large too but the patient may loss his central vision [23]. Patients with neovascular AMD had to gut microbiota that was supplemented with Anaerotruncusspp., Oscillibacter spp., Ruminococcus torques, and Eubacteria ventriosum, whereas the microbiota of a healthy person was dominated by Bacteroideseggerthii. The latter microorganism could be a defensive anti-immune-mediated disease, which could be due to the variations in bacterial genes associated with type of metabolic pathways. Especially those involving the diminished amount of genes included in fatty acid elongation and supplementation of genes associated with L-Alanine fermentation, glutamate degradation, and Arginine biosynthesis [24].
Preventive treatment of AMD: There has been wider agreement on the relevance of food supplements (i.e., vitamins) in the improvement and restriction of AMD. For example, antioxidant and mineral supplementation may be used in early and intermediate AMD [25]. These supplements, such as vitamin E, may contain (α-tocopherol) and C (ascorbic acid), zinc, glutathione, and especially macular carotenoids, such as lutein and zeaxanthin [26–28]. Recently studies have discussed that lutein and zeaxanthin may be more effective in the suppression of lipofuscin pigments photo-oxidation than α-tocopherol because they have the capability to quench singlet oxygen. This represents a potential protective effect for these two-component against harmful photo-oxidative processes proceeding in the path of lipofuscinogenesis with its impact on AMD development [28,29].
Effect of Ocular Diseases on the Ocular Microbiota on the Eye
Previous studies demonstrated that the surface eyes microbiota has a vital role and it may affect the local immune response and pathogenesis. Thus, many eye disorders can be caused if there was any imperfection in the microbiota [24].
Eye microbiota and contact lenses
Contact lenses, which worn on either daily disposable, daily wear or extended wear bases, usually made of many different types of polymers such as polymethyl methacrylate (PMMA) [30], hydroxyethyl methacrylate (HEMA) [31], or the silicone hydrogel materials [32]. Contact lenses are considered to be a risk factor for infection. Several studies were done in order to determine the types of microbiota isolated from contact lenses after wearing them especially their effect on the microbiota of the conjunctiva and lids [33].
The most isolated microbes from contact lenses after wear are coagulase-negative staphylococci, Propionibacterium sp. and Corynebacterium sp. Almost no difference was noticed on the eye microbiota when comparing the effect of different types of polymers and the wear schedule [5,34-38]. Even when examining the effect of long term extended wear of HEMA-based lenses, the results showed that this type of polymers did not change the conjunctiva or lid microbiota. However prolonged wearers were more commonly colonized on these two surfaces by pathogenic microbes that have the capability to cause infection (such as Gram-negative bacilli). Nevertheless, daily wear of HEMA-based lenses was associated with an increase in colonization incidence of the conjunctiva and lids by bacteria such as coagulase-negative staphylococci [39].
A new study shows that the eye microbiota of lens wearers is different from that of non-wearers. According to the result of cultures from contact lenses, the presence of Staphylococcus, Propionibacterium, Corynebacterium, Bacillus, Micrococcus, Rothia (previously assigned to Stomatococcus)[35,39–45], and Pseudomonas have been reported [46–48] .In more than half of the sample, the relative abundance of some “taxa” in contact lenses was detected was 1%. Streptococcus [49], Methylobacterium, and Acinetobacter, and members of families Oxalobacteraceae and Enterobacteriaceae were detected in these samples [50]. On the other hand, some studies state that the use of contact lenses does not change the ocular microbiota; due to the use anesthetic eye drops such as topical proparacaine hydrochloride anesthetic before sampling, which may decrease the detected ocular microbiota diversity. Usually, this topical anesthetic may cause dilution of the bacteria or be washed away from the ocular surface [50].
Contact lenses effect on eye bacterial biofilms: Biofilms are defined as a community of bacteria held together by a polymeric extracellular matrix [51-54]. They provide a defense mechanism against the environmental stress, antimicrobial agents, the host immune cells, and the invasion by other microorganisms. Contact lenses and the placement of intraocular lenses, which are two procedures used to manage those eye abnormalities and vision problems, provide the bacteria with a new surface on which many pathogens can form biofilms[51-53,55].Unfortunately, many types of bacteria that can form biofilms may lead to poor visual outcomes and occasionally loss of sight. Examples of these infections are acute bacterial endophthalmitis and corneal ulceration. Endophthalmitis infection is caused by Coagulase-negative staphylococci and Propionibacterium acnes and it can affect patients who intraocular lens and posterior capsules [56–64]. Keratitis which frequently may affect patients who use contact lenses caused by Staphylococci, Pseudomonas spp. [65–67], Fungi Acanthamoebaless [68].
Infectious diseases associated with contact lenses: Contact lenses use has been recognized as a risk factor for the development of eye infections such as giant papillary conjunctivitis and keratitis [69,70]. Tearing and the corneal epithelium are inherent protective mechanisms to protect the eye from the pathogenic microorganisms. The tear fluid and blinking remove pathogens from the cornea [71–73]; moreover, it contains antimicrobial components such as lysozyme and lactoferrin. In addition, the epithelial cells produce innately antimicrobial peptides and mucins [74], and their tight junctions work as a physical barrier to microbes [71,72,75]. Nevertheless, Contact lens wear disrupts some of these innate defenses and renders the cornea more susceptible to infection [76].Contact lens wear has also been shown to change the corneal epithelium, to carry organisms to the ocular surface and to limit natural clearance mechanisms [77,78]. In addition, biofilms which are less susceptible to the normal antimicrobials defense mechanisms of the tears will be formed on the posterior contact lens surface place bacteria close to the epithelium [75,78]. The contact lenses will cause mechanical damage of the epithelium, punctate epithelial erosions, and reduced tear exchange [79–82]. Microbial keratitis (MK), which is the most an ophthalmic emergency visionthreatening disease and severe complication [83], is a broad term that includes bacterial keratitis (BK), fungal keratitis (FK), and Acanthamoeba keratitis (AK) [84,85]. The MK in contact lens wearers is usually related to some risky behaviors owing to the “noncompliant “or bad hygienic practices [83,86]. However, daily disposable lenses do not reduce the risk of MK though; the use of this type may be a lower risk of vision loss when compared with the other type [87,88].
Bacterial keratitis: The bacterial infection is the most frequent cause of infectious keratitis. Usually, the underlying types of bacteria involved in the infection are staphylococci, Streptococcus pneumoniae, and Pseudomonas spp [89,90] Patients can experience pain, photophobia, blurred vision, corneal cloudiness or pus inside the eye [91]This type of infection can be treated with topical antibiotics include monotherapy of fluoroquinolones or combination therapy of aminoglycoside/cephalosporin [92,93].
i. Fungal keratitis: Fungal keratitis, which occurs because of Filamentous fungi, such as fusarium and Aspergil¬lus tend to be most often related with contact lens wear and trauma, [94,95], while those with the ocular surface disease are more prone to yeasts [96]. Fungal biofilms are the expected cause of Contact lens-related Fungal Keratitis, where the fungi can be strongly attached to the posterior side of the lens or extend into the lens matrix [97]. The clinical appearance of Fungal Keratitis depends on the causative pathogens. Corneal infections due to Candida often similar to Bacterial Keratitis [98].However, mycotic keratitis that is associated with Feathery borders, satellite lesion, and necrotic slough is due to Fusarium or Aspergillus[99].The management Fungal keratitis includes the use of antifungal agents such as Natamycin, voriconazole, and amphotericin [100].
ii. Viral keratitis: Herpes simplex keratitis can be classified according to the layer involved to epithelial, stromal, endothelial or mixed, epithelial herpes simplex characterized with a dendritic ulcer [101], and it typically can be treated with acyclovir ointment; however, at the early stage of the herpetic epithelial keratitis topical corticosteroids should be avoided[102]. On the other hand, endothelial keratitis is characterized by the presence of keratitis precipitates on the endothelium and corneal edema, while cloudiness Stromal herpes simplex keratitis characterized by the presence of cloudiness of the stroma, with or without ulceration, scarring or vascularization [101,103]. In the case of the stromal and endothelial keratitis, the treatment would be oral antivirals (acyclovir or valaciclovir) [102,104,105].
iii. Parasitic keratitis: Contact lens-wearing individuals who expose their lenses to water through swimming, hot tubs, trauma with contaminated water, or care for their lenses with water are at greater risk of infection [106]. The largest risk factor for contact lens-related AK is poor compliance with lens care which leads to subsequent biofilms formation [106,107].The signs of this infectious disease not always present and they are not specific but some signs such as a ring infiltrate perineuritis, epitheliopathy, pseudodendrites, subepithelial infiltrates and Perineural infiltrates may indicate the patient has the Acanthamoeba keratitis [108,109]. This type of keratitis can be treated with polyhexamethylenebiguanide, chlorhexidine, propamidine, neomycin, and oral voriconazole [69,108–112].
Ocular Infections
Infectious conjunctivitis
Conjunctivitis is the most events that reasons for the expansion of the conjunctival blood vessels and causes in inflammation. In which one or both eyes become red or pink and might be sticky or watery [124,125]. Method to detect and treats conjunctivitis, according to the signs and symptoms. Evaluation should involve examination visual perception and investigation with a torch or lamp. Fluorescein droplets must the conjunctival sac and the eye observed with the cobalt blue light of the fundoscopy, to investigate out any signs of corneal ulceration or infection. Cold sores should be searched for and the patient observed for cold sores or a vesicular rash incident the infection is due to herpes simplex or zoster virus [125,126].
Bacterial conjunctivitis: Bacterial conjunctivitis is a less common origin of conjunctivitis [127], is more frequent in kids and babies [128]. The most frequent bacteria are Haemophilus influenza, Streptococcus pneumoniae, and Staphylococcus aureus [129]. The Chlamydial conjunctivitis infection is one of the most common bacterial conjunctivitis [130], symptoms of this infection mostly include conjunctival hyperemia, mucopurulent discharge and lymphoid follicle growth [126]. Usually, treatment with oral antibiotics as Azithromycin or doxycycline is recommended to manage the infection [131].Gonococcal conjunctivitis, which Occurs via Neisseria gonorrhoeae, is a rare case of infection but it should be measured in neonates and sexually active adults[124].In order to treat the infection, Antibiotic treatment is the suggested therapy and the drug of choice ceftriaxone [132]. Also, patients should lavage the infected eye with saline [132-134].
Viral conjunctivitis: Pinkeye is the most common condition occurs by adenovirus; this infection is most common in adult than in children. Frequently, herpes simplex or zoster virus is responsible too. Usually, viral conjunctivitis is self-limiting and there is no need for antibiotics, for the relief they can use cold compresses, artificial tears or topical antihistamines [132], If there is a herpes simplex or zoster virus then antiviral should be given, such as acyclovir ointment or ganciclovir gel [126,132].
Infectious endophthalmitis
Is an inflammation inner eyes that can occur by infection thru microbes, involving bacteria or fungi [135]? It is an infrequent condition and its occurrence according to the reason. Risk factors for endophthalmitis involve cataract surgery, intravitreal injections (for age-related macular degeneration), and filtering bleb (for glaucoma) [136,137]. After the surgery is done, an ocular infection may be developed, the most frequent incident of infection is endophthalmitis, it caused by bacteria and it is found within seven days of surgery [137].
Conclusion
Ocular surface has a broad diversity of the composition of the microbiota, the distribution of the microbiota differ according to the ocular layers, Many factors may affect the microbiota and the ocular infection development, for example, contact lenses wearing considered to be a key factor for the biofilms formation and as a result the development of ocular eye infection. According to many types of research, both Gram-positive and Gram-negative bacteria may cause ocular infections and ophthalmic tissues damage in some cases. Still, Gram-positive bacteria are the main contributor of ocular infections, many types of ocular infection involves as conjunctivitis, keratitis, and endophthalmitis, some of these infection caused by the bad hygienic practice or the limited knowledge such as the infections caused by the contact lenses use, In spite of this, more investigation must be done in this field to find ways to prevent the infections, and create methods to eradicate the probability of biofilms formation and eliminate the microorganisms resistant.
Acknowledgement
The authors acknowledge the support from the Applied Science Private University.
Conflict of Interest
The authors declare that there is no any financial interest or any conflict of interest exist.
References
- Rolando M, Zierhut M (2001) The Ocular Surface and Tear Film and Their Dysfunction in Dry Eye Disease. Surv Ophthalmol 45(Sup2): S203-10.
- Conrady CD, Joos ZP, Patel BCK (2016) Review: The Lacrimal Gland and Its Role in Dry Eye. J Ophthalmol.
- Willoughby CE, Ponzin D,Ferrari S, Lobo A, Landau K, et al.(2010) Anatomy and physiology of the human eye: Effects of mucopolysaccharidoses disease on structure and function - a review. Clin Exp Ophthalmol 38(1): 2-11.
- Doan T, Akileswaran L,Andersen D,Johnson B, Ko N, et al.(2016) Paucibacterial Microbiome and Resident DNA Virome of the Healthy Conjunctiva.Invest. Ophthalmol. Vis Sci 57(13): 5116–5126.
- Willcox MDP (2013) Characterization of the normal microbiota of the ocular surface. Exp Eye Res 117: 99-105.
- Mitsui Y, Hanabusa J (1955) Corneal infections after cortisone therapy. Br J Ophthalmol 39(4): 244-250.
- Hammeke JC, Ellis PP (1960) Mycotic flora of the conjunctiva. Am J Ophthalmol 49: 1174-1188.
- Ainley R, Smith B (1965) Fungal flora of the conjunctival sac in healthy and diseased eyes. Br J Ophthalmol 49(10): 505-515.
- Sehgal SC, Dhawan S, Chhiber S,Sharma M, Talwar P (1981) Frequency and significance of fungal isolations from conjunctival sac and their role in ocular infections. Mycopathologia 73(1): 17-19.
- Thomas PA, Kaliamurthy J (2013) Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect 19(3): 210-220.
- Kalyana Chakravarthy S, Jayasudha R, Ranjith K, Dutta A, Pinna NK, et al. (2018) Alterations in the gut bacterial microbiome in fungal Keratitis patients. PLoS One 13(6).
- Tsigalou C, Stavropoulou E, Bezirtzoglou E (2018) Current Insights in Microbiome Shifts in Sjogren’s Syndrome and Possible Therapeutic Interventions. Front Immunol 9: 1106.
- Wang C, Zaheer M, Bian F,Quach D, Swennes AG, et al.(2018) Sjogren-Like Lacrimal Keratoconjunctivitis in Germ-Free Mice. Int J Mol Sci 19 (2).
- Zaheer M, Wang C, Bian F, Yu Z, Hernandez H, et al. (2018) Protective role of commensal bacteria in Sjögren Syndrome. J. Autoimmun 93: 45-56.
- Horai R, Zarate-Blades CR, Dillenburg-Pilla P, Chen J, Kielczewski JL, et al. (2015) Microbiota-Dependent Activation of an Autoreactive T Cell Receptor Provokes Autoimmunity in an Immunologically Privileged Site. Immunity 43(2): 343-353.
- Zarate-Blades CR, Horai R, Mattapallil MJ, Ajami NJ,Wong M, etal. (2017) Gut microbiota as a source of a surrogate antigen that triggers autoimmunity in an immune privileged site. Gut Microbes 8(1): 59-66.
- Lin P (2018) The role of the intestinal microbiome in ocular inflammatory disease. Curr Opin Ophthalmol 29(3): 261-266.
- Rosenbaum JT, Asquith M (2018) The microbiome and HLA-B27-associated acute anterior uveitis. Nat Rev Rheumatol 14(12): 704-713.
- Collins DW, Gudiseva HV, Trachtman B, Bowman AS, Sagaser A, et al. (2016) Association of primary open-angle glaucoma with mitochondrial variants and haplogroups common in African Americans. Mol Vis 22: 454-471.
- Ma J, Coarfa C, Qin X, Bonnen PE, Milosavljevic A, et al. (2014) mtDNA haplogroup and single nucleotide polymorphisms structure human microbiome communities. BMC Genomics 15: 257.
- Ehrlich R, Harris A, Kheradiya NS, Winston DM, Ciulla TA, et al. (2008) Age-related macular degeneration and the aging eye. Clin Interv Aging 3: 473-482.
- Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, et al. (2008) Vascular endothelial growth factor in eye disease, Prog Retin Eye Res 27(4): 331-371
- Taylor DJ, Hobby AE, Binns AM, Crabb DP (2016) How does age-related macular degeneration affect real-world visual ability and quality of life? A systematic review. BMJ Open 6(12): e011504.
- Baim AD, Movahedan A, Farooq AV, Skondra D (2018) The microbiome and ophthalmic disease. Exp Biol Med 244(6): 419-429.
- Weikel KA, FitzGerald P, Shang F, Andrea Caceres M, Bian Q, et al. (2012) Natural history of age-related retinal lesions that precede AMD in mice fed high or low glycemic index diets. Investig Ophthalmol Vis Sci 53(2): 622-632.
- Hogg R, Chakravarthy U (2004) AMD and micronutrient antioxidants. Curr Eye Res 29(6): 387-401.
- Kim SR, Nakanishi K, Itagaki Y, Sparrow JR (2006) Photooxidation of A2-PE, a photoreceptor outer segment fluorophore, and protection by lutein and zeaxanthin. Exp Eye Res 82: 828-839.
- Bone RA, Landrum JT, Cao Y, Howard AN, Alvarez-Calderon F (2007) Macular pigment response to a supplement containing meso-zeaxanthin, lutein and zeaxanthin. Nutr Metab (Lond) 4:12.
- Wiktorowska-Owczarek A, Nowak JZ (2004) Lutein and zeaxanthin–two carotenoids serving as the protection against age-related macular degeneration (AMD). Okulistyka 7: 34-39.
- McMahon TT, Zadnik K (2000) Twenty-five years of contact lenses: the impact on the cornea and ophthalmic practice.Cornea 19: 730-740.
- Wichterle O, Lim D (1960) Hydrophilic gels for biological use. Nature 185: 117.
- Musgrave CSA, Fang F (2019) Contact Lens Materials: A Materials Science Perspective. Mater. (Basel, Switzerland) 12(2): 261.
- Borazjani RN, Levy B, Ahearn DG (2004) Relative primary adhesion of Pseudomonas aeruginosa, Serratia marcescens and Staphylococcus aureus to HEMA-type contact lenses and an extended wear silicone hydrogel contact lens of high oxygen permeability. Contact Lens Anterior Eye 27: 3-8.
- Campos MS, De Q Campos e Silva L, Rehder JR, Lee MB, O Brien T, et al. (1994) Anaerobic flora of the conjunctival sac in patients with AIDS and with anophthalmia compared with normal eyes. Acta Ophthalmol 72: 241-245.
- Elander TR, Goldberg MA, Salinger CL, Tan JR, Levy B, et al. (1992) Microbial changes in the ocular environment with contact lens wear. CLAO J 18: 53-55.
- Graham JE, Moore JE, Jiru X, Moore JE, Goodall EA,et al .(2007) Ocular pathogen or commensal: a PCR-based study of surface bacterial flora in normal and dry eyes. Invest. Ophthalmol Vis Sci 48(12): 5616-5623.
- McClellan KA, Cripps AW, Clancy RL, Billson FA (1998) The effect of successful contact lens wear on mucosal immunity of the eye.Ophthalmology 105: 1471-1477.
- Ozkan J, Zhu H, Gabriel M,Holden BA, Willcox MDP (2012) Effect of prophylactic antibiotic drops on ocular microbiota and physiology during silicone hydrogel lens wear. Optom Vis Sci 89(3): 326-335.
- Callender MG, Tse LS, Charles AM, Lutzi D (1986) Bacterial flora of the eye and contact lens. Cases during hydrogel lens wear.Am. J Optom Physiol Opt 63: 177-180.
- Fleiszig SM, Efron N (1992) Microbial flora in eyes of current and former contact lens wearers. J Clin Microbiol 30(5): 1156-1161.
- Hovding G (1981) The conjunctival and contact lens bacterial flora during lens wear. Acta Ophthalmol 59: 387-401.
- Iskeleli G, Bahar H, Eroglu E, Torun MM, Ozkan S (2005) Microbial changes in conjunctival flora with 30-day continuous-wear silicone hydrogel contact lenses. Eye Contact Lens 31: 124-126.
- Larkin DF, Leeming JP (1991) Quantitative alterations of the commensal eye bacteria in contact lens wear. Eye (Lond) 5(Pt 1): 70-74.
- Stapleton F, Willcox MD, Fleming CM, Hickson S, Sweeney DF, et al. (1995) Changes to the ocular biota with time in extended- and daily-wear disposable contact lens use. Infect Immun 63: 4501-4505.
- Willcox MD, Power KN, Stapleton F, Leitch C, Harmis N,et al. (1997) Potential sources of bacteria that are isolated from contact lenses during wear. Optom Vis Sci 74: 1030-1038.
- Sankaridurg PR, Vuppala N, Sreedharan A, Vadlamudi J, Rao GN (1996) Gram negative bacteria and contact lens induced acute red eye. Indian J Ophthalmol 44(1): 29-32.
- Holden BA, La Hood D, Grant T, Newton-Howes J, Baleriola-Lucas C,et al (1996) Gram-negative bacteria can induce contact lens related acute red eye (CLARE) responses. CLAO J 22(1): 47-52.
- Cavanagh HD, Ladage PM, Li SL, Yamamoto K, Molai M, (2002) Effects of daily and overnight wear of a novel hyper oxygen-transmissible soft contact lens on bacterial binding and corneal epithelium: a 13-month clinical trial. Ophthalmology 109: 1957-1969.
- Sankaridurg PR, Sharma S, Willcox M, Naduvilath TJ, Sweeney DF, et al. (2000) Bacterial colonization of disposable soft contact lenses is greater during corneal infiltrative events than during asymptomatic extended lens wear. J Clin Microbiol 38(12): 4420-4424.
- Shin H, Price K, Albert L, Dodick J, Park L, et al. (2016) Changes in the eye microbiota associated with contact lens wearing. MBio 7: 1-6.
- Donlan RM, Costerton JW (2002) Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15(2): 167-193.
- Hooshangi S., Bentley WE (2008) From unicellular properties to multicellular behavior: bacteria quorum sensing circuitry and applications. Curr Opin Biotechnol 19(6): 550-555.
- Stewart PS, Franklin MJ (2008) Physiological heterogeneity in biofilms. Nat Rev Microbiol 6: 199.
- Watnick P, Kolter R (2000) Biofilm, city of microbes. J Bacteriol 182: 2675–2679.
- Donlan RM, Costerton JW, Annous BA, Fratamico PM, Smith JL (2009) Biofilms: survival mechanisms of clinically relevant microorganisms. J Food Sci 74: 167-193.
- Adan A, Casaroli-Marano R, Gris O, Rafael Navarro, Rafael Navarro, et al. (2008) Pathological findings in the lens capsules and intraocular lens in chronic pseudophakic endophthalmitis: An electron microscopy study. Eye(Lond) 22: 113-119.
- Baillif S, Casoli E, Marion K, Roques C, Pellon G, et al. (2006) A Novel In Vitro Model to Study Staphylococcal Biofilm Formation on Intraocular Lenses under Hydrodynamic Conditions. Invest Ophthalmol Vis Sci 47(8): 3410-3416.
- Baillif S, Ecochard R, Casoli E, Freney J, Burillon C, et al. (2008) Adherence and kinetics of biofilm formation of Staphylococcus epidermidis to different types of intraocular lenses under dynamic flow conditions. J Cataract Refract Surg 34(1): 153-158.
- Garcia-Saenz MC, Arias-Puente A, Fresnadillo-Martinez MJ, Matilla-Rodriguez A (2000) In vitro adhesion of Staphylococcus epidermidis to intraocular lenses. J Cataract Refract Surg 26: 1673-1679.
- Griffiths PG, Elliot TS, McTaggart L (1989) Adherence of Staphylococcus epidermidis to intraocular lenses. Br J Ophthalmol 73(6): 402-406.
- Sawusch MR, Michels RG, Stark WJ, Bruner WE, Annable WL, et al. (1989) Endophthalmitis due to Propionibacterium acnes sequestered between IOL optic and posterior capsule. Ophthalmic Surg 20: 90-92.
- Okajima Y, Kobayakawa S, Tsuji A, Tochikubo T (2006) Biofilm formation by Staphylococcus epidermidis on intraocular lens material. Invest Ophthalmol Vis Sci 47(1): 2971-2975.
- Shimizu K, Kobayakawa S, Tsuji A, Tochikubo T (2006) Biofilm formation on hydrophilic intraocular lens material. Curr Eye Res 31(12): 989-997.
- Teichmann KD (2000) Propionibacterium acnes endophthalmitis requiring intraocular lens removal after failure of medical therapy. J Cataract Refract Surg 26(7): 1085-1088.
- Han DP, Wisniewski SR, Wilson LA, Barza M, Vine AK,et al. (1996) Spectrum and susceptibilities of microbiologic isolates in the Endophthalmitis Vitrectomy Study. Am J Ophthalmol 122(1): 1-17.
- Benz MS, Scott IU, Flynn HWJ, Unonius N, Miller D(2004) Endophthalmitis isolates and antibiotic sensitivities: a 6-year review of culture-proven cases. Am J Ophthalmol 137(1): 38-42.
- Schimel AM, Miller D, Flynn HWJ (2013) Endophthalmitis isolates and antibiotic susceptibilities: a 10-year review of culture-proven cases. Am. J. Ophthalmol 156: 50-52.
- Bispo P, Haas W, Gilmore M, Zimmerman A, Nixon A, et al. (2015) Biofilms in Infections of the Eye, Pathogens 4(1): 111-136.
- Szczotka-Flynn LB, Pearlman E, Ghannoum M (2010) Microbial contamination of contact lenses, lens care solutions, and their accessories: a literature review. Eye Contact Lens 36(2): 116-129.
- Keay L, Stapleton F, Schein O (2007) Epidemiology of contact lens-related inflammation and microbial keratitis: a 20-year perspective. Eye Contact Lens 33(6 Pt 2): 346-353.
- Fleiszig SMJ, Kwong MSF, Evans DJ (2003) Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect Immun 71(7): 3866-3874.
- Kwong MSF, Evans DJ, Ni M, Cowell BA, Fleiszig SMJ (2007) Human tear fluid protects against Pseudomonas aeruginosa keratitis in a murine experimental model. Infect Immun 75(5): 2325-2332.
- McDermott AM (2013) Antimicrobial compounds in tears. Exp Eye Res 117: 53-61.
- Evans DJ, Fleiszig SMJ (2013) Why does the healthy cornea resist Pseudomonas aeruginosa infection? Am J Ophthalmol 155(6): 961-970.e2.
- McNamara NA, Andika R, Kwong M, Sack RA, Fleiszig SMJ (2005) Interaction of Pseudomonas aeruginosa with human tear fluid components. Curr. Eye Res 30(7): 517-525.
- Ladage PM, Jester JV, Petroll WM, Bergmanson JPG, Cavanagh HD (2003) Vertical movement of epithelial basal cells toward the corneal surface during use of extended-wear contact lenses. Invest Ophthalmol Vis Sci 44(3): 1056-1063.
- Willcox MDP, Carnt N, Diec J, Naduvilath T, Evans V, et al. (2010) Contact lens case contamination during daily wear of silicone hydrogels. Optom Vis Sci 87(7): 456-464.
- Fleiszig SMJ, Evans DJ(2010) Pathogenesis of Contact Lens-associated microbial keratitis. Optom Vis Sci 87(4): 225-232.
- Alarcon I, Tam C, Mun JJ, LeDue J, Evans DJ, et al. (2011) Factors impacting corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Invest Ophthalmol Vis Sci 52(3): 1368-1377.
- Fleiszig SMJ (2006) The pathogenesis of contact lens-related keratitis. Optom Vis Sci 83: E866-E873.
- Lin MC, Yeh TN, Graham AD, Truong T, Hsiao C, et al. (2011) Ocular surface health during 30-day continuous wear: rigid gas-permeable versus silicone hydrogel hyper-O2 transmitted contact lenses. Invest. Ophthalmol Vis Sci 52(6): 3530-3538.
- Choo JD, Holden BA, Papas EB, Willcox MDP(2009) Adhesion of Pseudomonas aeruginosa to orthokeratology and alignment lenses Optom Vis Sci 86: 93-97.
- Dart JK, Stapleton F, Minassian D (1991) Contact lenses and other risk factors in microbial keratitis. Lancet (London, England) 338(8768): 650-653.
- Furlanetto RL, Andreo EGV, Finotti IGA, Arcieri ES, Ferreira MA, et al. (2010) Epidemiology and etiologic diagnosis of infectious keratitis in Uberlandia, Brazil. Eur J Ophthalmol 20(3): 498-503.
- Shah VM, Tandon R, Satpathy G, Nayak N, Chawla B, et al. (2010) Randomized clinical study for comparative evaluation of fourth-generation fluoroquinolones with the combination of fortified antibiotics in the treatment of bacterial corneal ulcers. Cornea 29(7): 751-757.
- Poggio EC, Glynn RJ, Schein OD, Seddon JM, Shannon MJ, et al. (1989) The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. N Engl J Med 321(12): 779-783.
- Stapleton F, Keay L, Edwards K, Naduvilath T. Dart JKG, et al. (2008) The incidence of contact lens-related microbial keratitis in Australia. Ophthalmology 115(10): 1655-1662.
- Dart JKG, Radford CF, Minassian D, Verma S, Stapleton F (2008) Risk factors for microbial keratitis with contemporary contact lenses: a case-control study. Ophthalmology 115(10): 1647-1654.e1-3.
- Greenn M, Apel A, Stapleton F (2008) Risk factors and causative organisms in microbial keratitis. Cornea 27(1): 22-27.
- Sharma A, Taniguchi J (2017) Review: Emerging strategies for antimicrobial drug delivery to the ocular surface: Implications for infectious keratitis. Ocul Surf 15(4): 670-679.
- Thomas PA, Geraldine P (2007) Infectious keratitis. Curr Opin Infect Dis 20(2): 129-141.
- Alam M, Bastakoti B (2015) Therapeutic Guidelines: Antibiotic. Version 15 Aust Prescr 38(4): 137.
- Rattanatam T, Heng WJ, Rapuano CJ, Laibson PR, Cohen EJ (2001) Trends in contact lens-related corneal ulcers. Cornea 20(3): 290-294.
- Iyer SA, Tuli SS, Wagoner RC (2006) Fungal keratitis: emerging trends and treatment outcomes. Eye Contact Lens 32(6): 267-271.
- Srinivasan M(2004) Fungal keratitis. Curr Opin Ophthalmol 15(4): 321-327.
- Gower EW, Keay LJ, Oechsler RA, Iovieno A, Alfonso EC, et al. (2010) Trends in fungal keratitis in the United States, 2001 to 2007. Ophthalmology 117(12): 2263-2267.
- Zhang S, Ahearn DG, Stulting RD, Schwam BL, Simmons RB, et al. (2007) Differences among strains of the Fusarium oxysporum-F. solani complexes in their penetration of hydrogel contact lenses and subsequent susceptibility to multipurpose contact lens disinfection solutions. Cornea 26(10): 1249-1254.
- Klotz SA, Penn CC, Negvesky GJ, Butrus SI (2000) Fungal and parasitic infections of the eye. Clin Microbiol Rev 13(4): 662-685.
- Thomas PA (2003) Fungal infections of the cornea. Eye (Lond) 17(8): 852-862.
- Prajna NV, Krishnan T, Mascarenhas J, Rajaraman R, Prajna L, et al. (2013) The mycotic ulcer treatment trial: a randomized trial comparing natamycin vs voriconazole. JAMA Ophthalmol 131(4): 422-429.
- Holland EJ, Schwartz GS (1999) Classification of herpes simplex virus keratitis. Cornea 18: 144-154.
- White ML, Chodos J (2014) Herpes Simplex Virus Keratitis: A Treatment Guideline-2014. AAO.
- Hamrah P, Pavan-Langston D, Dana R (2009) Herpes simplex keratitis and dendritic cells at the crossroads: lessons from the past and a view into the future. Int Ophthalmol Clin 49(1): 53-62.
- Knickelbein JE, Hendricks RL, Charukamnoetkanok P (2009) Management of herpes simplex virus stromal keratitis: an evidence-based review. Surv Ophthalmol 54(2): 226-234.
- Barron BA, Gee L, Hauck WW, Kurinij N, Dawson CR, et al. (1994) Herpetic Eye Disease Study. A controlled trial of oral acyclovir for herpes simplex stromal keratitis. Ophthalmology 101(12): 1871-1882.
- Radford CF, Minassian DC, Dart JKG (2002) Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol 86(5): 536-542.
- Joslin CE, Tu EY, Shoff ME, Booton GC, Fuerst PA, et al.(2007) The association of contact lens solution use and Acanthamoeba keratitis. Am. J. Ophthalmol 144(2): 169-180.
- Bacon AS, Frazer DG, Dart JK, Matheson M, Ficker LA, et al .(1993) A review of 72 consecutive cases of Acanthamoeba keratitis, 1984-1992. Eye(Lond)( Pt 6) : 719-725.
- Dart JKG, Saw VPJ, Kilvington S (2009) Acanthamoeba keratitis: diagnosis and treatment update 2009. Am. J. Ophthalmol 148(4): 487-499.e2.
- Lorenzo-Morales J,Khan NA, Walochnik J (2015) An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite 22: 10.
- Ferrari G, Matuska S, Rama P (2011) Double-biguanide therapy for resistant acanthamoeba keratitis, Case Rep. Ophthalmol 2(3): 338-342.
- Chin J, Young AL, Hui M, Jhanji V (2015) Acanthamoeba keratitis: 10-year study at a tertiary eye care center in Hong Kong. Cont. Lens Anterior Eye 38(2): 99-103.
- Konda N, Motukupally SR, Garg P, Sharma S, Ali MH, et al .(2014) Microbial analyses of contact lens-associated microbial keratitis. Optom Vis Sci 91(1): 47-53.
- Annous BA, Fratamico PM, Smith JL, Zimmerman AB, Nixon AD, et al. (2017) Common eye infections.Aust. Prescr 6: 912-919.
- Preechawatmd P, Ratananikommd U, Lerdvitayasakul R, Kunavisarut S (2007) Contact lens-related microbial keratitis. J. Med Assoc Thai 90(4): 737-743.
- Ormerod LD, Smith RE (1986) Contact Lens-Associated Microbial Keratitis, Arch. Ophthalmol 104(1): 79-83.
- Shin H, Price K, Albert L, Dodick J, Park L, et al. (2016) Changes in the eye microbiota associated with contact lens wearing. MBio 7(2).
- Zimmerman AB, Nixon AD, Rueff EM (2016) Contact lens associated microbial keratitis: practical considerations for the optometrist. Clin Optom 8: 1-12.
- Sharma S, Gopalakrishnan S, Aasuri MK, Garg P, Rao GN (2003) Trends in contact lens-associated microbial keratitis in Southern India. Ophthalmology 10(1): 138-143.
- Bispo PJM, Haas W, Gilmore MS, Press D (2016) Contact lens associated microbial keratitis: practical considerations for the optometris: 1-12.
- Zimmerman AB, Nixon AD, Rueff EM (2016) Contact lens associated microbial keratitis: practical considerations for the optometrist. Clin Optom 8: 1-12.
- Guess S, Stone DU, Chodosh J (2007) Evidence-Based Treatment of Herpes Simplex Virus Keratitis: A Systematic Review. Ocul Surf 5 (3) 240-250.
- Nesburn AB (1980) Common Viral Eye Diseases and Latent Infections. Ophthalmology 87(12): 1202-1207.
- Morrow GL, Abbott RL (1998) Conjunctivitis. Am Fam Physician 57: 735-746.
- Azari AA, Barney NP (2013) Conjunctivitis: a systematic review of diagnosis and treatment. Jama 310: 1721-1730.
- Watson S, Cabrera-Aguas M, Khoo P (2018) Common eye infections. Aust Prescr 41(3): 67-72.
- Stenson S, Newman R, Fedukowicz H (1982) Laboratory studies in acute conjunctivitis. Arch Ophthalmol 100: 1275-1277.
- Hørven I (1993) Acute conjunctivitis: a comparison of fusidic acid viscous eye drops and chloramphenicol. Acta Ophthalmol 71: 165–168.
- Epling J, Smucny J (2005) Bacterial conjunctivitis. Clin Evid 756-761.
- Rönnerstam R, Persson K, Hansson H, Renmarker K (1985) Prevalence of chlamydial eye infection in patients attending an eye clinic, a VD clinic, and in healthy persons. Br J Ophthalmol 69: 385-388.
- Katusic D, Petricek I, Mandic Z, Petric I, Salopek-Rabatic J, et al. (2003) Azithromycin vs doxycycline in the treatment of inclusion conjunctivitis. Am. J. Ophthalmol 135(4): 447-451.
- Emptage NP, Collins N, Lum FC, S Garratt (2013) American Academy of Ophthalmology Cornea/External Disease Panel. Preferred Practice Pattern Guidelines. Conjunctivitis. San Fr CA Am Acad Ophthalmol, San Fr, CA, USA.
- Matoba AY, Harris DJ, Mark DB, Meisler DM (2003) American Academy of Ophthalmology Cornea/External Disease Panel, Preferred Practice Patterns Committee, Blepharitis. Am. Acad. Ophthalmol, San Fr, CA, USA.
- Rapuano CJ (2008) American Academy of Ophthalmology Cornea/External Disease Panel, Prefer. Pract. Pattern Guidel. Conjunctivitis.
- Durand ML (2017) Bacterial and fungal endophthalmitis. Clin Microbiol Rev 30: 597-613.
- H Cronau, RR Kankanala, T Mauger (2010) Diagnosis and management of red eye in primary care, Am Fam Physician 81: 137-144.
- L Keay, EW Gower, SD Cassard, JM Tielsch, OD Schein (2012) Postcataract surgery endophthalmitis in the United States: analysis of the complete 2003 to 2004 Medicare database of cataract surgeries, Ophthalmology 119: 914-922.
-
Mohammad AA Al-Najjar, Maram Altah, DemaAljakhim. An Overview of Ocular Microbiology: Ocular Microbiota, the Effect of Contact Lenses and Ocular Disease. Arch Phar & Pharmacol Res. 1(5): 2019. APPR.MS.ID.000522.
-
Eye microbiota; Gut microbiota; Eye infection
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






