 Research Article
Research Article
UNI-494 Lowers Acute Kidney Injury Markers When Administered Before Ischemia/Reperfusion in a Rat Model
Satya Medicherla*, Guru Reddy and Shalabh Gupta
Vice President, Pre-Clinical Pharmacology, Unicycive Therapeutics, Inc. USA
Satya Medicherla, Vice President, Pre-Clinical Pharmacology, Unicycive Therapeutics, Inc. USA
Received Date:November 26, 2024; Published Date:December 04, 2024
Abstract
Introduction: There are no effective, FDA-approved treatments for acute kidney injury (AKI), including AKI with delayed graft function (DGF).Inflammation-and reactive oxygen species-driven mitochondrial permeability transition pore (mPTP) opening causes mitochondrial dysfunction/
swelling and eventual cell death [1, 2]. This is implicated in a wide range of acute diseases, including AKI originating from ischemia-reperfusion (I/R) injury or DGF [3]. Nicotinamide adenine dinucleotide can suppress the frequency and duration of mPTP opening [4]. UNI-494 is a novel nicotinamide ester derivative and a selective mitochondrial ATP-sensitive potassium channel activator that reverses mitochondrial dysfunction by closing the mPTP. We present results from a study evaluating UNI-494’s efficacy when administered preventatively before I/R in an animal model of AKI with DGF.
Methods: In both intravenous (IV) and oral models, 48 rats were randomized to no I/R, vehicle (I/R only), and I/R with high/low UNI-494
doses administered 30 minutes before I/R. Rats were anesthetized, the right kidney removed, and I/R induced by clamping left renal vessels for
30 minutes. After 24 hours of reperfusion in metabolic cages, blood samples were collected for serum creatinine (sCr) and blood urea nitrogen
(sBUN) levels, and urinary samples were collected for albumin-creatinine ratio (uACR) and neutrophil gelatinase-associated lipocalin (uNGAL).
After necropsy, the clamped left kidney was collected and processed for histology for tubular injury scores.
Results: I/R induced significant increases of sCr, sBUN, uACR, uNGAL, and proximal tubular injury scores in the vehicle treated I/R group
compared to the no I/R sham group. Single doses of IV or oral UNI-494 reduced the kidney functional markers sCr and uACR, the tubular injury
marker uNGAL, and proximal tubular injury scores (p<0.05 for all except sCr and tubular injury score at 50 mg/kg oral dose).
Conclusions: The results indicate that UNI-494 effectively prevented AKI markers in an AKI rat model and is a potential candidate for preventing DGF and other AKI clinical conditions.
Keywords:Acute Kidney Injury; Delayed Graft Function; Mitochondrial ATP-sensitive Potassium Channel Activator; Ischemia Reperfusion Injury;UNI-494
Introduction
Acute kidney injury (AKI) is a clinical syndrome defined by the sudden loss of kidney function, resulting in an inability to maintain electrolyte, acid-base, and water balance [5]. Currently, there are no effective treatments approved for AKI; management of the condition is primarily supportive. As AKI affects 10-15% of hospitalized patients and often results in renal transplantation or lifelong dialysis [5], there is an urgent need for the development of new, effective treatments for AKI, including delayed graft function (DGF), a manifestation of AKI with attributes unique to the transplant process.
Mitochondrial dysfunction is a central factor in the development of AKI, as increased production of reactive oxygen species (ROS) by mitochondria contributes to cell death over time [6]. The mitochondrial permeability transition pore (mPTP) plays a critical role in mitochondrial dysfunction. First, increased ROS activates mPTP opening, which further increases ROS production [4,7]. Additionally, an inflammatory cascade elevates sustained mPTP opening in low-grade or chronic inflammation [4]. Thus, inflammation- and ROS-driven mPTP opening causes mitochondrial dysfunction/swelling [2]. This ruptures the outer mitochondrial membrane leading to subsequent apoptotic/necrotic cell death and is implicated in a wide range of acute diseases including AKI originating from ischemia-reperfusion (I/R) injury or DGF [3]. Next, the unresolved inflammation enhances sustained opening of mPTP [8], which eventually induces fibrotic lesions [9]. This is evident in a broad range of chronic diseases such as chronic kidney disease (CKD) originating from hypertension or hyperglycemia and/or inflammation-driven fibrosis [10].
Animal models of I/R injury, DGF, and AKI mimic many features seen in human kidney allografts. Proximal tubule mitochondrial dysfunction in renal cells plays a critical role in the pathophysiology of AKI [3]. The proximal tubule is the primary sensor and effector in AKI [11]. I/R injury induces AKI characterized by tubular injury, which is caused by mitochondrial dysfunction, changes in hemodynamics, decrease in oxygen tension, and depletion of adenosine triphosphate (ATP) [3,11]. Hence, I/R injury-induced rat model of AKI with acute tubular necrosis/injury is used in the studies hereunder. There is evidence that both the frequency and duration of mPTP opening can be suppressed by nicotinamide adenine dinucleotide (NAD+) [3]. UNI-494 is a novel nicotinamide ester derivative that is a selective mitochondrial ATP-sensitive potassium channel (KATP) activator, which reverses mitochondrial dysfunction by closing the mPTP. We present results from a preclinical study evaluating UNI-494 efficacy when administered preventatively before I/R in an animal model of DGF.
Methods
Study Designs
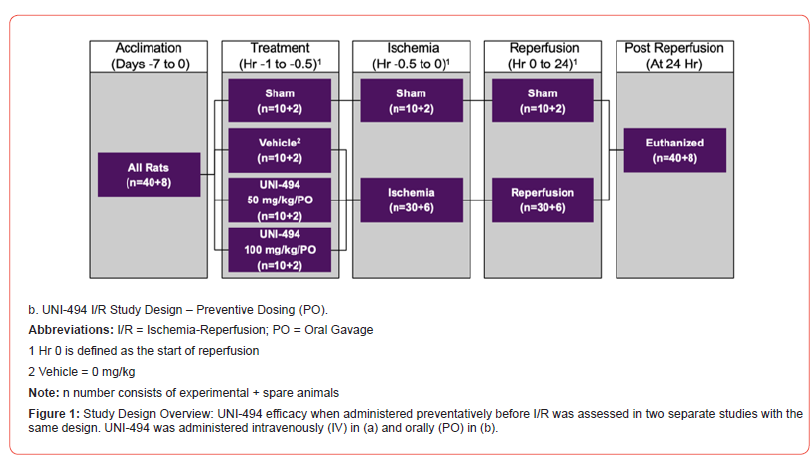
The efficacy of UNI-494 when administered preventatively before I/R was assessed in two separate studies with the same design (Figure 1). One study administered UNI-494 intravenously (IV) and the other administered UNI-494 orally (PO). In both models, 48 male Sprague-Dawley rats (40+8 spare rats) were randomly assigned to four groups to evaluate the in vivo efficacy of UNI-494 on kidney injury with a special focus on kidney functional markers, a tubular injury marker, and tubular damage through histological analysis (Jablonski scores).
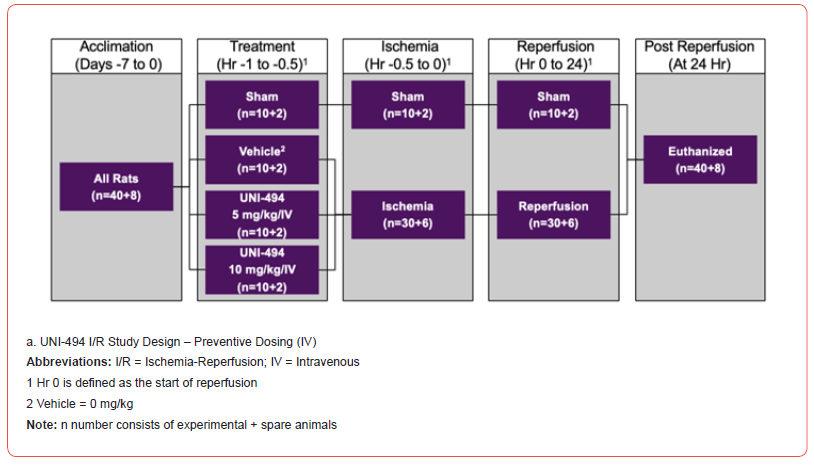
Study Treatments Intravenous
In the IV study, jugular vein cannulated Sprague-Dawley rats were randomly assigned to receive no treatment (sham), vehicle, 5 mg/kg/IV UNI-494, or 10 mg/kg/IV UNI-494. UNI-494 was administered 30 minutes prior to the induction of ischemia via IV route of administration. The vehicle for IV dosing consisted of Sterile Water for Injection, which was obtained by the Testing Facility from a commercial vendor.
Oral
In the PO study, non-jugular vein cannulated Sprague-Dawley rats were randomly assigned to receive no treatment (sham), vehicle, 50 mg/kg/PO UNI-494, or 100 mg/kg/PO UNI-494. UNI- 494 was administered 30 minutes prior to the induction of ischemia via oral route of administration. The vehicle for PO dosing consisted of a solution of 1% carboxymethylcellulose in Sterile Water for Injection, which was obtained by the Testing Facility as Sigma- Aldrich (St. Louis, MO) Catalog C488 (Carboxymethylcellulose sodium salt, medium viscosity).
I/R Surgery
Animals were anesthetized, and the abdomen was opened at the midline. In both the IV and PO studies, right nephrectomy was performed in 36 rats in vehicle (I/R) and UNI-494 (I/R) groups. The ischemia was induced in the left kidney using a small clamp (Sugita standard aneurysm clip holding force 145 g) that was placed on the renal vascular pedicle for 30 minutes as described by Shimizu et al. 2011 [12]. Removal of the clip was defined as the start of reperfusion. For the other 12 rats in the sham group (no I/R), sham operations were performed in a comparable manner, except that the right kidney was not removed, and renal vessels were not clamped.
Study Assessments
Study assessments were the same for both the IV and PO studies. At zero hours (or the equivalent for sham-operated animals), each rat was moved to an individual metabolic cage for the collection of urine. After 24 hours in the metabolic cage (i.e., 24 hours of reperfusion and urine collection), animals were euthanized, and terminal samples (blood and the left kidneys) were collected. The levels of selected markers were determined in the serum or urine samples collected from animals at termination.
A volume of blood sufficient to provide 5 mL of serum was collected into serum separator tubes; following centrifugation, the resulting serum from each animal was aliquoted to individual tubes (5 x 1 mL). The tubes were then flash-frozen on dry ice and stored at -20°C pending analysis. Subsequently, serum creatinine (sCr), blood urea nitrogen (sBUN), and serum neutrophil gelatinaseassociated lipocalin (sNGAL) were determined in the non-acetic acid-stabilized samples using instruments/kits according to the respective manufacturer’s instructions.
The volumes and pHs of the 24-hour urine samples were recorded. The urine samples were aliquoted to individual tubes, flash-frozen on dry ice, and stored at -20°C pending analysis. Subsequently, urine creatinine (uCr), urine albumin (uALB), and urinary neutrophil gelatinase-associated lipocalin (uNGAL) were determined using kits according to the respective manufacturer’s instructions. The uCr and uALB values were used to calculate the uALB to uCr ratio (uACR). Concentrations in experimental samples were generally determined by comparison to a standard curve generated using the respective purified analyte. Exceptions included sCr and sBUN analyses, where the analyzer’s internal calibrations were used, and uCr analyses, where a calibration curve was used.
At necropsy, the left kidney of each animal was fixed overnight in 10% neutral buffered formalin at room temperature and then stored in 70% ethanol at room temperature. Fixed tissues were processed, sectioned, and stained with hematoxylin and eosin. For histopathological evaluation, the slides were examined using a Nikon Ci Eclipse light microscope fitted with a Spot Digital Camera. Tissues were assessed for tubular injury using Jablonski et al 1983 proximal convoluted tubule scoring method (0=No change present; 1=Minimal; 2=Mild; 3=Moderate; 4=Marked) [13]. Representative images of histopathological changes were derived from images captured with 3DHistech Pannoramic digital scanner.
Statistical Analysis
Data were analyzed with a one-way ANOVA followed by Dunnett’s multiple comparison tests. A threshold of p<0.05 was used to determine a significant difference between groups. Statistical Package for the Social Sciences (SPSS) was used for all statistical analyses.
Results
Kidney Function Analyses Intravenous
I/R induced significant increases of sCr, sBUN, uACR, uNGAL, and proximal tubular injury scores in the vehicle treated I/R group when compared to the no I/R sham group (p<0.0001 for sCr, sBUN, uACR, and tubular injury score and p<0.01 for uNGAL, Dunnett’s multiple comparison tests after one-way ANOVA) (Figure 2). A single dose of UNI-494 at 5 mg/kg (low dose) or 10 mg/kg (high dose), administered intravenously before I/R, phenomenally reduced the kidney functional markers sCr (p<0.0001 at low and high doses), sBUN (p<0.0001 at low and high doses), and uACR (p<0.001 at low and high doses) when compared to the vehicle treated I/R group. In addition, UNI-494 was highly effective in lowering the tubular injury marker uNGAL and proximal convoluted tubular injury scores (p<0.0001 at low and high doses for both).
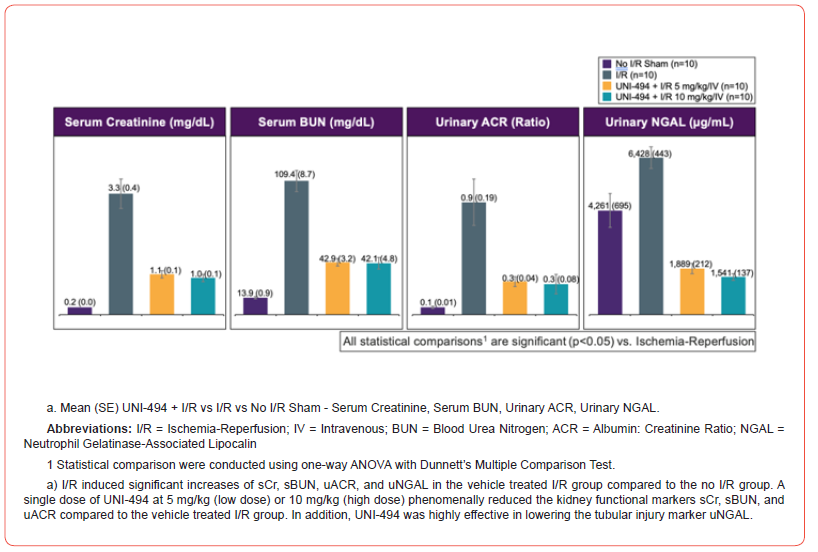
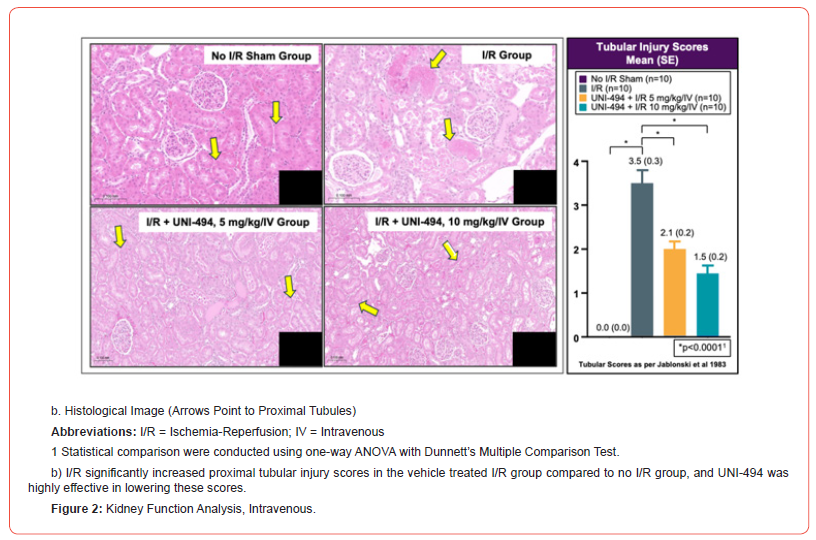
Oral
I/R induced significant increases of sCr, sBUN, uACR, uNGAL, and proximal tubular injury scores in the vehicle treated I/R group when compared to the no I/R sham group (p<0.0001 for sCr, sBUN, uACR, and tubular injury score and p<0.01 for uNGAL, Dunnett’s multiple comparison tests after one-way ANOVA) (Figure 3). A single dose of UNI-494 at 50 mg/kg (low dose) or 100 mg/kg (high dose), administered orally before I/R, significantly reduced the kidney functional markers sCr (p<0.05 at high dose) and uACR (p<0.001 at low dose and p<0.001 at high dose) when compared to the vehicle treated I/R group. In addition, UNI-494 was effective in lowering the tubular injury marker uNGAL (p<0.0001 at low and high doses) and proximal convoluted tubular injury scores at high dose (p<0.01).
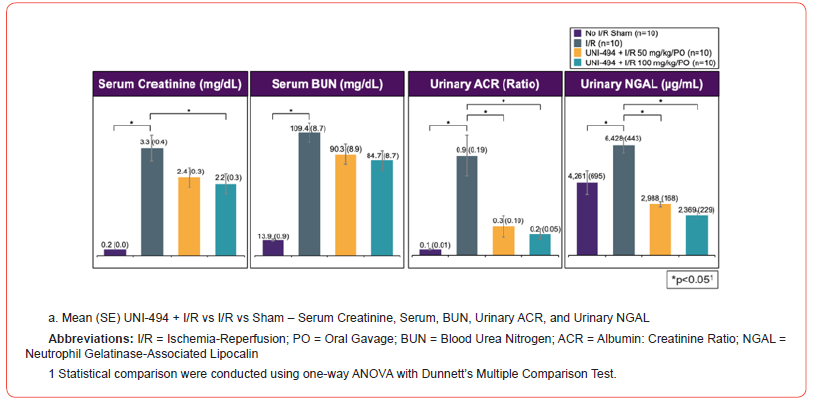
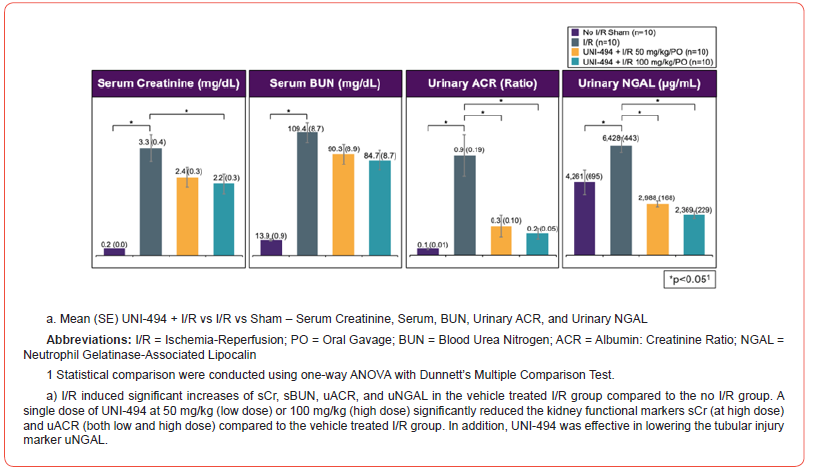
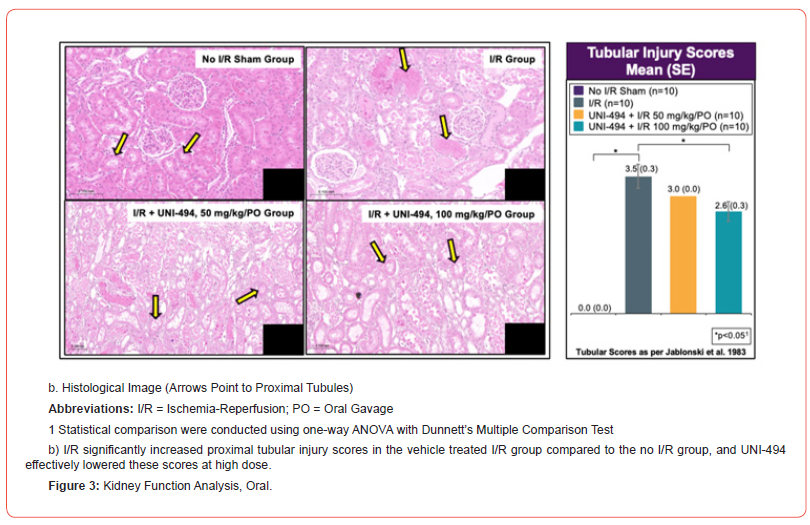
Safety
All rats survived to the scheduled euthanasia time point. No clinical observations were noted during any phase of the study.
Discussion
Currently, there are no effective, FDA-approved treatments for AKI. Mitochondrial dysfunction is central to the pathology of various acute diseases, including AKI [5]. The mPTP is crucial to mitochondrial dysfunction as, over time, inflammation- and ROSdriven mPTP opening leads to mitochondrial dysfunction and swelling [4, 10]. This ruptures the outer mitochondrial membrane and causes apoptotic/necrotic cell death, which is associated with various acute diseases, including AKI originating from I/R injury or DGF [3]. Additionally, persistent inflammation worsens ongoing mPTP opening [6], which is evident in CKD [9]. Therefore, therapies that mitigate mPTP opening are logical to treat AKI. UNI-494 is a novel nicotinamide ester derivative that is a selective mitochondrial KATP activator, which reverses the mitochondrial dysfunction by closing the mPTP. The objective of the study was to evaluate UNI- 494’s efficacy when administered preventatively before I/R in a rat model of IR/DGF. To our knowledge, this is the first preclinical study to assess whether preventative administration of UNI-494 can reduce AKI markers in a rat I/R model.
Study results clearly showed that I/R induction increased AKI markers and injured proximal tubules, and that both oral and IV administration of UNI-494 significantly reduced AKI markers and proximal tubular injury scores in a rat model. The reduction of uNGAL and proximal convoluted tubular injury scores by low and high doses of UNI-494 indicate that UNI-494 may have a renoprotective effect. 5 mg/kg of UNI-494 administered intravenously was sufficient to achieve a robust benefit comparable to the 10 mg/kg dose and follow-up studies with doses under 5 mg/kg of UNI-494 are warranted. Greater improvements in kidney function analyses were observed in the IV vs. PO study, suggesting that gastrointestinal enzymes decrease UNI-494 bioavailability. The main limitation was that this preclinical study evaluated UNI-494 in a rat model, not the target population of patients with AKI. However, preclinical animal studies are an established procedure in drug development. UNI-494 is a potential candidate for the treatment of AKI, including AKI with DGF. The mechanism of the renoprotective effect should be further investigated. Studies evaluating this novel treatment in the target population of patients with AKI should be conducted.
Conclusion
The data from this study clearly indicates that UNI-494 ameliorates AKI in a murine model. The reduction in kidney functional markers sCr, sBUN, and uACR for both IV and oral UNI- 494 doses indicates a renoprotective effect. Additionally, kidney functional data were very well supported by lower tubular injury marker uNGAL and proximal tubular injury scores. UNI-494 administered intravenously effectively reduced sCr, sBUN, uACR, uNGAL and tubular injury scores at both high and low doses, with tubular injury scores showing a trend towards a dose-dependent effect. UNI-494 administered orally effectively decreased uACR and uNGAL at high and low doses but reduced sCr and tubular injury scores at high dose only, indicating a dose dependency for these two markers. Our study results are consistent with previously published data by Shimizu et al. 2011 with nicorandil in a similar rat model of I/R [12]. UNI-494 is a potential candidate for the prevention of DGF and other AKI clinical conditions.
Funding Sources
This study was funded by Unicycive Therapeutics, Inc.
Author Contributions
Satya Medicherla, Guru Reddy, and Shalabh Gupta contributed to the study design, data acquisition, and data analysis. All authors have reviewed the manuscript, believe it represents valid work, and approved it for submission.
Data Availability Statement
The data that supports the finding of this study are available from the corresponding author upon reasonable request. Restrictions apply to the availability of data generated or analyzed during this study to preserve confidentiality or because they were used under license.
The corresponding author satya.medicherla@unicycive.com will on request detail the restrictions and any conditions under which access to some data may be provided.
Acknowledgment
Editorial support was provided by Xelay Acumen Group, Inc. (funded by Unicycive Therapeutics, Inc.). All the authors have authorized the submission of their manuscript via Xelay Acumen Group, Inc. and have approved all statements and declarations, including conflicting interests and funding.
Conflict of Interest
Satya Medicherla, Guru Reddy, and Shalabh Gupta are employees of Unicycive Therapeutics, Inc. Editorial support was funded by Unicycive Therapeutics, Inc.
References
- Hunter DR, Haworth RA (1979) The Ca2+-induced membrane transition in mitochondria. I. The protective mechanisms. Arch Biochem Biophys 195(2): 453-459.
- Kinnally KW, Peixoto PM, Ryu SY, Dejean LM (2011) Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta 1813(4): 616-622.
- Zhang X, Agborbesong E, Li X (2021) The Role of Mitochondria in Acute Kidney Injury and Chronic Kidney Disease and Its Therapeutic Potential. Int J Mol Sci 22(20): 11253.
- Kent AC, El Baradie KBY, Hamrick MW (2021) Targeting the Mitochondrial Permeability Transition Pore to Prevent Age-Associated Cell Damage and Neurodegeneration. Oxid Med Cell Longev: 6626484.
- Goyal A, Daneshpajouhnejad P, Hashmi MF, Bashir K (2023) Acute Kidney Injury. StatPearls [Internet], StatPearls Publishing, Treasure Island, Florida.
- De Giorgi F, Lartigue L, Bauer MK, Schubert A, Grimm S, et al. (2002) The permeability transition pore signals apoptosis by directing Bax translocation and multimerization. Faseb j 16(6): 607-609.
- Zorov DB, Juhaszova M and Sollott SJ (2014) Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94(3): 909-950.
- Endlicher R, Drahota Z, Štefková K, Červinková Z, Kučera O (2023) The Mitochondrial Permeability Transition Pore-Current Knowledge of Its Structure, Function, and Regulation, and Optimized Methods for Evaluating Its Functional State. Cells 12(9): 1273.
- Zhang S, Tan X, Chen Y, Zhang X (2017) Postconditioning protects renal fibrosis by attenuating oxidative stress-induced mitochondrial injury. Nephrol Dial Transplant 32(10): 1628-1636.
- Irazabal MV, Torres VE (2020) Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 9(6): 1342.
- Chevalier RL (2016) The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol 311(1): F145-F161.
- Shimizu S, Saito M, Kinoshita Y, Ohmasa F, Dimitriadis F, et al. (2011) Nicorandil ameliorates ischaemia-reperfusion injury in the rat kidney. Br J Pharmacol 163(2): 272-282.
- Jablonski P, Howden BO, Rae DA, Birrell CS, Marshall VC, et al. (1983) An experimental model for assessment of renal recovery from warm ischemia. Transplantation 35(3): 198-204.
-
Satya Medicherla*, Guru Reddy and Shalabh Gupta. UNI-494 Lowers Acute Kidney Injury Markers When Administered Before Ischemia/Reperfusion in a Rat Model. Arch Phar & Pharmacol Res. 4(4): 2024. APPR.MS.ID.000594.
-
UNI-494, Mitochondrial ATP-sensitive Potassium Channel Activator, Acute Kidney Injury, Delayed Graft Function, Ischemia Reperfusion Injury
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






