 Research Article
Research Article
Seborrheic Dermatitis: Exploring the Pathogenesis, Clinical Features, And Treatment Strategies
Rawan Nasser, Ana Paula Fonseca*
Polytechnic Institute of Coimbra, Coimbra Health School, Portugal.
Ana Paula Fonseca, Polytechnic Institute of Coimbra, Coimbra Health School, Portugal. LabinSaúde, Rua 5 de Outubro - SM Bispo, Apartado 7006, 3046-854 Coimbra Portugal.
Received Date:July 12, 2023; Published Date:July 18, 2023
Abstract
Introduction: Seborrheic dermatitis is a common chronic inflammatory skin condition that primarily affects sebum-rich areas such as the scalp,
face, and trunk. This abstract provides a comprehensive overview of seborrheic dermatitis, including its pathogenesis, clinical presentation, and
management strategies, The exact etiology of seborrheic dermatitis remains elusive, but a combination of genetic, environmental, and immunological
factors is believed to play a role. Malassezia yeasts, particularly Malassezia spp, are thought to contribute to the development of the condition by
triggering an abnormal immune response in susceptible individuals.
Objective: The aim of this study is to explore the pathology of seborrheic dermatitis, identifying the causes and symptoms of these diseases, in
addition to researching associated, pharmacological, and non-pharmacological therapies.
Material and methods: To conduct this literature study, articles were collected from “PubMed”, “NCBI” and “Google scholar” databases,
publications dates between 2015 and 2023.
Results and discussion: The results of this study demonstrate the effectiveness of various treatment modalities in managing seborrheic
dermatitis. Using topical and systematic treatments has shown significant efficacy in reducing inflammation, controlling the overgrowth of Malassezia
species, and alleviating symptoms associated with seborrheic dermatitis. However, long-term use may be necessary to maintain remission.
Conclusion: seborrheic dermatitis is a prevalent inflammatory skin disorder with complex etiology. Understanding its pathogenesis, recognizing
its clinical presentation, and implementing appropriate management strategies are vital for effective control of symptoms and improvement in the
patient’s quality of life. Further research is warranted to elucidate the underlying mechanisms and develop novel targeted therapies for this common
dermatological condition.
Keywords: Seborrheic Dermatitis; Associated Therapy; Malassezia spp; Corticosteroid medications
Introduction
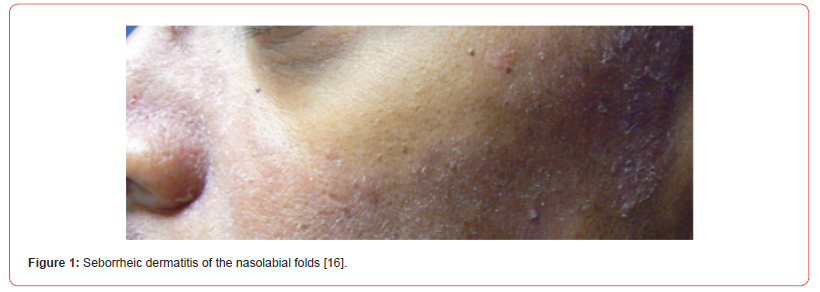
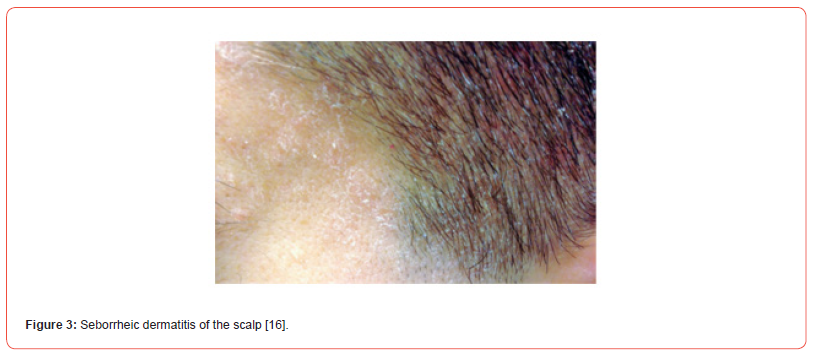
Seborrheic Dermatitis (SD) is a common dermatological problem that affects the seborrheic areas of the body [Figure 1,2]. SD considered the same basic condition sharing many features and responding to similar treatments, differing only in locality and severity [1]. The current usage of many terminologies for seborrheic dermatitis, such as seborrheic eczema, dandruff, and pityriasis capitis, indicates the complicated character of this widespread skin illness [2]. Whereas seborrheic dermatitis (SD) is a type of inflammatory skin disease that can affect the scalp [Figure 3], face, chest, upper trunk, external ear, axillae, and inguinal folds [3]. SD can affect up to 42% of people. SD most commonly happens during the first three months of life, adolescence, and between the ages of 40 and 60 [4]. The Symptoms in Adults include well-defined, red, raised skin lesions yellowish scales that look oily or greasy, white scales or flakes on the head or hair, mild itching, crusty, yellow material on the eyelash’s eyelid redness [5]. The second most prevalent clinical symptom in babies is cradle cap, it can appear on the scalp, face, retro-auricular region, body folds, and trunk; in rare cases, it might be generalized.

Another typical baby appearance is the “diaper” [6]. The pathogenesis is influenced by a number of intrinsic and extrinsic factors, including sebaceous secretion, an increase in triglycerides and cholesterol and a decrease in squalene and free fatty acids, the colonization of Malassezia yeasts on the skin’s surface, host factor susceptibility, and interactions between these factors. The majority of fungi that colonize human scalps are lipophilic yeasts, and Malassezia spp. are among the most significant representatives of yeast in the skin’s fungal microbiome [7]. Frequently males get seborrheic dermatitis more than females, most likely as a result of androgens stimulation of sebum production [8]. Seborrheic dermatitis manifests as marginated lesions or patches coated with persistent yellow flakes, with moist, greasy skin texture and obvious redness (surface erythema) as telltale symptoms of inflammation [9]. Additionally, seborrheic dermatitis therapy is traditionally achieved through the use of several classes of topically applied keratolytic, corticosteroid, and antifungal products; options for systemic treatment include antifungals like imidazole’s (itraconazole, fluconazole, and ketoconazole), as well as terbinafine, with variable outcomes and no gold standard among them. It has been hypothesized that the low-dose oral isotretinoin treatment may have a possible benefit in the eradication of Malassezia spp. colonization in the scalp of people with moderate to severe seborrheic dermatitis and/or seborrhoea. Malassezia spp is a lipophilic yeast that needs an adequate environment to develop [7]. Seborrheic dermatitis improves because of topical antifungal therapies’ reduction of Malassezia growth and the accompanying irritation. In most cases, corticosteroid medications are used to treat inflammation. A modest topical corticosteroid called hydrocortisone is used to treat inflammatory skin conditions including seborrheic dermatitis to lessen swelling, redness, and itching [10]. In general, the discovery of new therapeutic targets is guided by parameters indicating pathogen survival and absence from the human genome [11].
Objective
The aim of this study is to explore the pathology of seborrheic dermatitis, identifying the causes and symptoms of this disease, in addition to researching the associated therapy.
Materials and Methods
This study is a literature review, carried out between November 2022 and June 2023 and for its accomplishment, free access articles were collected from “Pubmed”, “NCBI” and “Google schoolar” databases. The keywords used in the research were “Seborrheic Dermatitis,” “Associated Therapy”, “Malassezia spp” and “corticosteroid medications”. This study includes all scientific articles and scientific reviews that are of interest to the topic in question, with their publication dates between 2015 and 2023.
Results and Discussion
Etiology
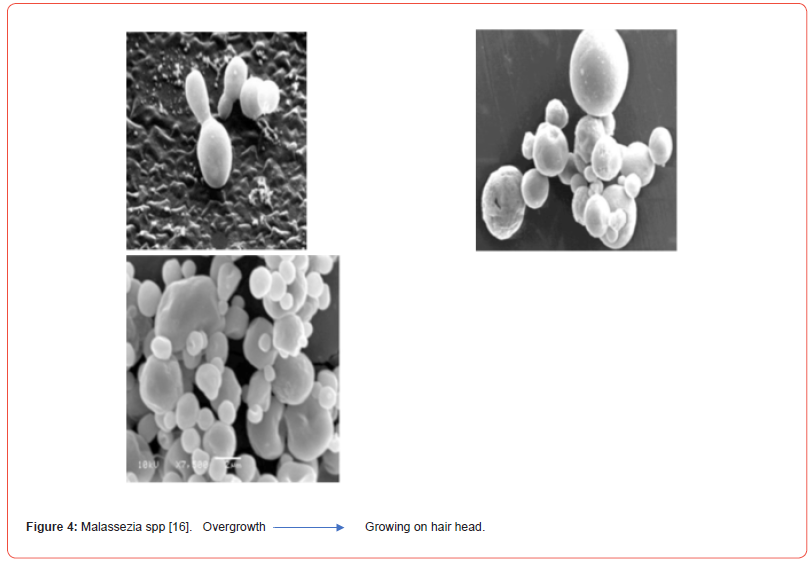
The etiology of the disease is multifaceted and linked to the presence of Malassezia yeast, hormonal variables, levels of sebum production, immunological response, neurogenic factors, and environmental factors. The presence of Malassezia spp [Figure 4], sebum secretion, and an individual’s propensity to acquire the condition are the three key etiological variables linked to the occurrence of SD. When an individual has a functioning immune system, they are asymptomatic and do not experience symptoms. Malassezia species appear to contribute through inducing innate immunity in the skin via intricate interactions between fungal cells and virulence factors. This results in an increase in lipase synthesis, an inflammatory response by releasing oleic acid and arachidonic acid, and the creation of bioactive components from sebum lipids. Unsaturated fatty acids and metabolites released have direct irritating and scaling effects on keratinocytes, as well as inducing an enhanced inflammatory response due to epidermal barrier function loss. Arachidonic acid, which is processed by cyclooxygenase, also acts as a source of pro-inflammatory eicosanoids and prostaglandins, which contribute to the damage in the corneum stratum. Concurrently, keratinocytes release proinflammatory cytokines such as IL-1, IL-6, IL-8, and TNF-, which augment and perpetuate the inflammatory response, therefore developing chronicity [12].
Epidemiology
SD prevalence varies greatly, ranging from 3 to 10% of the general population, and it is more common in males than in women. Some investigations found a high frequency among teens, up to 11%. According to other research, this syndrome affects between 2% and 5% of the population, with no racial bias. Regarding the age of presentation, a higher frequency of cases has been demonstrated in children, particularly those younger than three months, with cradle cap presentation affecting from 11.6% to 70.0% in some cohorts, as well as in adults aged 30 to 60, with an epidemiological peak between the third and fourth decade of life [12]. While SD affects people of all ages, it has a bimodal distribution, with a peak in infancy (2-12 months of age) and another high in early adulthood. Furthermore, the incidence is greater in HIV-infected and immunocompromised individuals, ranging from 30% to 83%. Gender, individual lipid composition, immune status, neuropsychiatric factors (including Parkinson’s disease (PD) and other neuropsychiatric diseases), and high environmental humidity and heat are all factors that influence an individual’s susceptibility to disease development. Several studies in recent years have revealed a genetic propensity, while further research is needed. While SD and dandruff have a close link to HIV, most SD patients have no substantial immune system problems [6]. Seborrheic dermatitis is at risk for developing because of the following factors: Increased sebaceous gland activity, Immunodeficiency, including (Lymphoma Renal transplantation, HIV-AIDS), Neurological and psychiatric disease, including (Stroke, Alzheimer dementia, Major depression, Autonomic dysfunction), drug treatment exposure, such as (Dopamine antagonists, Immunosuppressants, Psoralen/ PUVA, Lithium), low ambient temperature or ambient humidity, age and male sex [13].
Diagnosis Blood Tests
Previously common, severe, and refractory SD forms have been regarded a probable dermatological sign of acquired immunodeficiency syndrome (AIDS) in both adults and children at all stages of HIV infection.SD can occur in up to 40% of seropositive persons, whereas it varies from 30% to 83% in HIV patients (versus 3% in the seronegative population). In these individuals, a CD4 cell count decline to 450-550 cells/L is common at the outset of SD, with additional fall during disease deterioration and rise during clinical recovery. Severe and intractable SD forms may also be associated with hereditary (acrodermatitis enteropathy) or acquired neonatal/ adult zinc deficiency, as well as alcohol addiction, alcoholic chronic pancreatitis, hepatitis C virus infection, and nutritional deficiencies (riboflavin, pyridoxine, niacin, and essential fatty acids) [14].
Dermatoscopy
Dermatoscopy, also known as “dermoscopy” or “incidental light microscopy,” is a non-invasive technique that allows for quick and magnified in vivo observation of the skin, allowing for the discovery of morphologic traits that are not visible to the naked eye. It can be done manually (magnifications up to 10) or digitally (videodermatoscopy), which requires a video camera with optic fibers and lenses (magnifications up to x1000). The collected pictures may be seen on a display and saved on a personal computer to check for any potential changes over time. Dermoscopy may be used in clinical practice to distinguish scalp SD from other common scaling illnesses such as psoriasis or tinea capitis based on unique dermoscopy findings. Psoriasis dermoscopy observations include the presence of bushy capillaries with twisted loops in 100% of cases, whereas tinea capitis “comma” hair (C-shaped hair shaft with a sharp, slanting end and homogeneous thickness), There may be “corkscrew” hair (twisted or coiled, short, broken hair fragments), “zigzag” hair (hair shaft bent at many locations), and “morse code” hair (the presence of various transverse bands or gaps across the hair shaft).Unlike psoriasis and tinea capitis, SD can be distinguished by dotted vessels with a patchy distribution and fine white yellowish scales, as well as follicular plugs, orange-yellowish patches, whitish structureless areas, and linear branching vessels. Finally, certain clinical trials in both adults and children emphasize the benefits of dermoscopy as a technique for more accurate SD severity classification, as well as therapy monitoring and follow-up [14].
Histological Examination
There are differences between the acute and chronic stages of SD’s histopathologic features, which are nonspecific. Spongiosis and psoriasiform hyperplasia are frequently seen in the epidermis in the former, along with the classic finding of “shoulder parakeratosis” around follicular openings; superficial perivascular and perifollicular inflammatory infiltrates, primarily composed of lymphocytes and histiocytes, are also noticeable in the dermis. Conversely, a prominent psoriasiform rash is a feature of chronic SD. Parakeratosis and epidermal hyperplasia, together with dilated superficial dermal venules. Additional features, such as widespread parakeratosis and keratinocyte necrosis, may be typical of SD caused by HIV [14].
Pharmacological therapies
The expectations of various SD therapy approaches are predicated on minimizing Malassezia spp. proliferation and thereby limiting indications and symptoms, such as pruritus and scaling, as well as decreasing the inflammatory response to Malassezia spp [15]. The main objectives of treatment for seborrheic dermatitis in teenagers are to diminish the condition’s outward symptoms and to lessen pruritus and erythema. This treatment is the same as that for adults. Treatment options include topical antifungals, calcineurin inhibitors, and corticosteroids in addition to over-the-counter shampoos. Seborrheic dermatitis requires continuing maintenance medication because it is a chronic disorder [Table 1,2] [16].
Table 1:Treatments for Scalp Seborrheic Dermatitis in Adults and Adolescents.
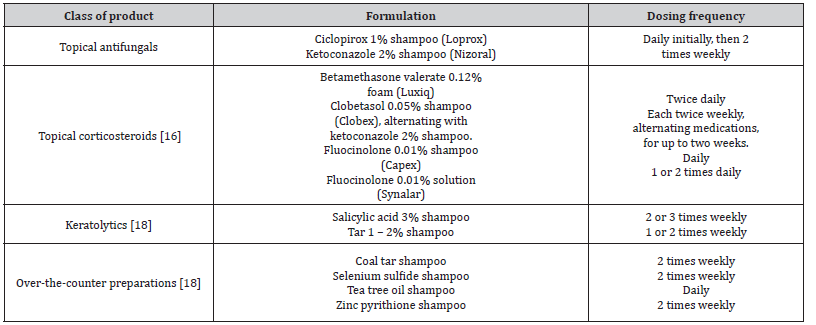
Table 2:Treatments for Facial and Body Seborrheic Dermatitis in Adults and Adolescents.
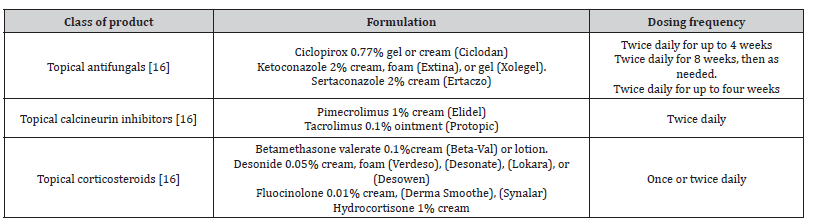
Topical treatments Antifungal agents
Antifungal agent development has not advanced as quickly as antibacterial agent development. Each medicine acts at a different place, resulting in variations in each drug’s effectiveness, course of therapy, and side effects [17].
Corticosteroid
In cases of severe SD, a topical corticosteroid of low-to-medium strength is advised. To control inflammation, primarily erythema and pruritus, this medication can be administered alone or in combination with an antifungal agent. Due to side effects such hypertrichosis, skin shrinkage, telangiectasia, perioral dermatitis, etc., prolonged usage is not recommended. Numerous trials have demonstrated that topical corticosteroid monotherapy does not outperform topical antifungal agents. Therefore, topical corticosteroids are the first-line treatment for moderate-to-severe SD whereas antifungal drugs are still considered the first-line treatment for mild SD [17].
Topical calcineurin inhibitors (TCIs)
The way that calcineurin inhibitors work is by preventing the release of cytokines from T cells, which reduces inflammation. Negative consequences are hardly ever connected to TCIs. However, due to a report of cancer in individuals who used topical calcineurin inhibitors for a longer period, continued usage is not indicated. Pimecrolimus cream and topical corticosteroids (betamethasone 17-valerate 0.1% cream or hydrocortisone acetate 1% cream) are equally effective in treating SD, according to several studies. Additionally, it was shown that pimecrolimus cream showed a lesser recurrence and a longer period of remission. It was determined that tacrolimus ointment was more effective than topical corticosteroids but not superior to zinc pyrithione shampoo. Due to its greasiness, tacrolimus ointment is typically not well received by patients when applied [17].
Systemic treatment Systemic antifungal agents
Deep mycosis (subcutaneous mycosis and systemic mycosis) and some superficial mycoses are treated with systemic antifungal agents. Several factors influence agent selection, including fungus kind, cure rate, cost-benefit analysis, adverse impact, medication interaction, comfortability, age, general condition, and patient medical history. Several factors that can induce medication interactions, ill effects owing to poor organ function, and teratogenic adverse effects should be considered before drug administration [17].
Azoles
Based on the amount of nitrogen atoms in the azole chain, azoles are classed as imidazole or triazole. Ketoconazole is an example of an imidazole, whereas triazoles include itraconazole, fluconazole, voriconazole, and posaconazole. Its mode of action is the inhibition of lanosterol 14-demethylase and cytochrome P450 (CYP450), which leads to the disruption of ergosterol synthesis, producing cell membrane instability and hyperpermeability, disrupting fungal growth and life sustainability. The most prevalent side effects are gastrointestinal problems, followed by liver function disorder, cardiac toxicity, hypertriglyceridemia, edema, urticaria, anaphylaxis, neuropathy, hypertension, impotence, and leukopenia. Because azole interacts with CYP3A4, combining it with medications processed by these enzymes can result in variations in the plasma concentrations of both drugs. When combined with an antacid, H2 blocker, or proton pump inhibitor, azole absorption is also impaired [17].
Allylamines
Allylamines work by inhibiting squalene epoxidase, a noncytochrome P450 enzyme, causing ergosterol production to be disrupted. This group possesses fungicidal and fungistatic properties. Fungicidal action is gained by squalene buildup, which weakens the cell membrane of fungi, whereas fungistatic activity is acquired through ergosterol shortage, which disrupts membrane function in fungal development. The most common adverse effect is gastrointestinal disorder, whereas the less common adverse effects are headache, exanthematous eruption, pustular psoriasis, chest pain, malaise, fatigue, liver disorder, and toxic epidermal necrolysis.
This class is metabolized by CYP450, so the drugs inducting or inhibiting this enzyme can disrupt the clearance of allylamines. Other interactions are also reported with the use of cyclosporine, warfarin, and drugs metabolized by CYP2D6. Terbinafine is a systemic allylamine [17].
Terbinafine
Terbinafine is absorbed in the digestive system. The preparations come in tablet and granule formats. Because it is lipophilic and keratophilic, it is found in the skin, nails, and fat. Terbinafine has a bioavailability of 40% and a half-life of 22 hours. Metabolism occurs in the liver via CYP2D6 oxidation. Urine and feces account for most of the elimination (80%). Terbinafine clearance is increased by rifampin, whereas clearance is decreased by cimetidine. Terbinafine raises the plasma concentration of a number of medications, including tricyclic antidepressants, beta blockers, selective serotonin reuptake inhibitors, and monoamine oxidase type B inhibitors [17].
Fluconazole
Fluconazole is a broad-spectrum fungistatic triazole that is notable for its great oral absorption and low influence on hepatic microsomal enzymes, making medication interactions less prevalent. Pulse oral fluconazole 150 mg twice a week for two weeks may be an effective alternative therapy, particularly in individuals with moderate or severe seborrheic dermatitis who require systemic treatment [19].
Isotretinoin
Oral isotretinoin is the most effective medicine for reducing sebaceous gland size and sebum production; however, isotretinoin also has an anti-inflammatory action, which might explain its involvement in treating seborrheic dermatitis. Especially during the second and third months of therapy, as well as the one-month follow-up [20].
Itraconazole
Itraconazole 100-mg caps, first month: 200 mg/day for 1 week, then 200 mg/day for 2 days/month up to 11 months [18]. Itraconazole is useful in the treatment of mild to severe seborrheic dermatitis. However, employing alternative doses and/or durations of Itraconazole therapy may give a basis for systemic Itraconazole usage in seborrheic dermatitis [21].
Non-pharmacological therapies
Phototherapy
Ultraviolet light has been demonstrated to limit the growth of Malassezia on the skin. UVB therapy may be explored for severe or recalcitrant SD. A previous study found that patients treated with NB-UVB three times a week for two months improved. However, recurrence occurred 2-6 weeks after medication was discontinued. Psoralen plus ultraviolet A (PUVA) research, on the other hand, is still conflicting. Furthermore, a significant risk of carcinogenic consequences in fair-skinned persons is found [17].
Conclusion
This comparative study highlights the effectiveness of different therapeutic approaches for seborrheic dermatitis. The combination therapy of antifungals and corticosteroids demonstrated the most promising results, effectively reducing symptoms such as itching, erythema, and scalp involvement. Topical corticosteroids were effective but associated with a higher risk of adverse effects, while antifungal treatment alone showed moderate efficacy. It is important to note that seborrheic dermatitis is a chronic condition, and longterm management strategies are necessary to control flare-ups and maintain remission. An integrated approach that includes proper skincare, avoidance of triggers, and stress management can greatly contribute to minimizing symptoms and preventing recurrence. While there is no definitive cure for seborrheic dermatitis, ongoing research and advancements in dermatology continue to provide insights into the underlying mechanisms of the condition. This knowledge may lead to the development of more targeted and personalized treatment approaches in the future.
Acknowledgement
None.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Luis J Borda, Tongyu C W (2015) Dermatitis S. Seborrheic Dermatitis and Dandruff: A Comprehensive Review. J Clin Investig Dermatology 3(2): 10.13188.
- Bakardzhiev Ilko (2017) New Insights into the Etiopathogenesis of Seborrheic Dermatitis. J Clin Res Dermatology 4(1): 1-5.
- Sobhan M, Gholampoor G, Firozian F, Mohammadi Y, Mehrpooya M, et al. (2019) Comparison of efficacy and safety of atorvastatin 5% lotion and betamethasone 0.1% lotion in the treatment of scalp seborrheic dermatitis. Clin Cosmet Investig Dermatol 12: 267-75.
- Mohammad M P, M Heydari, Namazi MR (2017) Successful Treatment of Chronic Scalp Seborrheic Dermatitis Using Traditional Persian Medicine: A Case Report and Literature Review. Galen Med J [Internet] 6(2): 157-159.
- Castillo DE, Gunczler I, França K, Keri J (2019) Seborrheic dermatitis. Adv Integr Dermatology: 71-88.
- Adalsteinsson JA, Kaushik S, Muzumdar S, Guttman E, Ungar J, et al. (2020) An update on the microbiology, immunology and genetics of seborrheic dermatitis. Exp Dermatol 29(5): 481-489.
- Kamamoto CSL, Nishikaku AS, Gompertz OF, Melo AS, Hassun KM, et al. (2017) Cutaneous fungal microbiome: Malassezia yeasts in seborrheic dermatitis scalp in a randomized, comparative and therapeutic trial. Dermatoendocrinol [Internet] 9(1): e1361573.
- Leung AKC, Barankin B (2015) International Journal of Pediatric Health Care & Advancements (IJPA) :4-6.
- Thomas LM, Khasraghi AH (2020) Topical treatment of seborrhoeic dermatitis and dandruff: An overview. Ann Trop Med Public Heal 23(18).
- Balighi K, Ghodsi SZ, Daneshpazhooh M, Ghale-Baghi S, Nasimi M, et al. (2017) Hydrocortisone 1% cream and sertaconazole 2% cream to treat facial seborrheic dermatitis: A double-blind, randomized clinical trial. Int J Women’s Dermatology [Internet] 3(2): 107-110.
- Del Prete S, Vullo D, Ghobril C, Hitce J, Clavaud C, et al. (2019) Cloning, purification, and characterization of a β-carbonic anhydrase from malassezia restricta, an opportunistic pathogen involved in dandruff and seborrheic dermatitis. Int J Mol Sci 20(10): 18-21.
- Salamanca-Córdoba MA, Zambrano-Pérez CA, Mejía-Arbeláez C, Motta A, Jiménez P, et al. (2020) Seborrheic dermatitis and its relationship with Malassezia spp. Infectio. 25(2): 120-129.
- Dattner AM (2018) Seborrheic Dermatitis. Integr Med Fourth Ed: 753-758.e1.
- Dall’oglio F, Nasca MR, Gerbino C, Micali G (2022) An Overview of the Diagnosis and Management of Seborrheic Dermatitis. Clin Cosmet Investig Dermatol 15: 1537-1548.
- Jackson JM, Alexis A, Zirwas M, Taylor S (2022) Unmet needs for patients with seborrheic dermatitis. J Am Acad Dermatol [Internet].
- Clark GW, Pope SM, Jaboori KA (2015) Diagnosis and treatment of seborrheic dermatitis. Am Fam Physician 91(3): 185-190.
- Widaty S, Bramono K, Listiawan M, Yosi A, Miranda E, et al. (2020) The management of seborrheic dermatitis 2020: An update. J Gen Dermatology Venereol Indones 5(1): 19-27.
- Cheong WK, Yeung CK, Torsekar RG, Suh DH, Ungpakorn R, et al. (2016) Treatment of Seborrhoeic Dermatitis in Asia: A Consensus Guide. Ski Appendage Disord 1(4): 187-196.
- Demiraj E, Vasili E, Deda L (2015) Oral fluconazole in scalp seborrheic dermatitis in Albania 2: 53-58.
- Meran A, Saeed M (2018) Efficacy and safety of low dose oral isotretinoin in comparison with oral itraconazole in the treatment of seborrheic dermatitis among patients attending Erbil dermatology teaching center in Erbil City. Zanco J Med Sci 22(3): 420-426.
- Adhikary SK, Ali ME, Uddin MJ, Akter S, Abdul Aziz MM, et al. (2021) A Study on Efficacy of Oral Itraconazole in the Treatment of Seborrheic Dermatitis. Faridpur Med Coll J 16(1): 21-24.
-
Rawan Nasser, Ana Paula Fonseca*. Seborrheic Dermatitis: Exploring the Pathogenesis, Clinical Features, And Treatment Strategies. Arch Phar & Pharmacol Res. 3(5): 2023. APPR.MS.ID.000571.
-
Seborrheic Dermatitis, Malassezia spp, Seborrheic eczema, Dandruff, Associated therapy, Corticosteroid medications
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






