 Short Communication
Short Communication
Rapid and successful response to Abatacept in non - responsive patients with morphea. Presentation of two cases
Giovanna Cuomo1*, Klodian Gjeloshi2, Francesco Masini2, Fiammetta Danzo3 and Caterina Naclerio4
1Associate Professor, Department of Precision Medicine, University of Campania, L Vanvitelli - Napoli, Italy
2Department of Advanced Medical and Surgical Sciences, University of Campania ‘‘Luigi Vanvitelli’’, Naples, Italy
3Division of Respiratory Diseases, Department Biomedical and Clinical Sciences, University of Milan, Milan Italy
4Rheumatology Unit, Scarlato-Hospital-Scafati (SA), Italy
Giovanna Cuomo, Associate Professor, Department of Precision Medicine, University of Campania, L Vanvitelli - Napoli, Italy.
Received Date:May 03, 2024; Published Date:May 08, 2024
Abstract
Objectives: The Authors described efficacy and significant response to Abatacept therapy in two patients with morphea subtypes and deep
tissue involvement.
Methods: We evaluated for this contribution two patients (one female and one male) with morphea subtypes and deep tissue involvement
characterized by non-responsive to conventional therapy with Disease modifying antirheumatic drugs (DMARDs). At baseline, the skin biopsy was
evaluated and confirmed classical deposition of dense fibrous tissue in the appropriate layer of the skin. Patients were both screened at baseline, and
we started therapy with Abatacept subcutaneous and oral prednisolone. They were reassessed at 3-6 and every six months.
Results: The patients tolerated Abatacept well and are much more likely to benefit from treatment. There were not noted severe adverse events.
Conclusion: We present two cases showing a good clinical response to Abatacept. Abatacept is considered an option for the treatment of severe
or resistant morphea, especially in patients with deep tissue involvement.
Introduction
Morphea is a rare autoimmune inflammatory fibrosing disease that has a progressive course with physical and psychological sequelae and is generally limited to the skin and subcutaneous fatty tissue, but may extend over muscular fascia, muscle tissue, tendons, joint synovia, and also bone marrow [1]. The prevalence of morphea in general based on previous epidemiologic studies is 0.4-2.7 per 100,000 population [2,3]. In systemic sclerosis, however, morphea demonstrates no solid organ involvement and even though extracutaneous manifestations are not uncommon in regard to the tipically al posto di generally to be confined within to musculoskeletal involvement, specifically there are no sclerodactyly and vascular involvement as well as Raynaud’s phenomenon and nailfold capillary changes. The acral areas are typically spared, unlike in Systemic Sclerosis. Plaques are smooth and shiny, but areas of both dermal and subcutaneous atrophy may be present particularly in chronic lesions. The morphea manifestations appear to be confined to the mesoderm, the tissue developed from the middle germinal layer.
Generally, there are two stages of the disease; an inflammatory active phase during which the skin becomes swollen, itchy, and painful and this is followed by a “burnt out” phase at which point the skin is sclerotic and hard. In this case, immunosuppressive and immunomodulatory treatments are used at early intervention in the inflammatory phase, and although some patients undergo spontaneous remission or skin softening [1], the residual damage created by previously active disease may be severe and associated with irreversible cosmetic and functional impairment [3]. Besides functional impairment, patients frequently suffer deep psychological distress due to a combination of severe pain, cosmetic disfigurement, restrictive breathing deficits, permanent deep atrophic scars, and joint contractures.
The aetiology of morphea is unknown. It is believed that inflammatory processes in the skin induce increased synthesis of collagen from fibroblasts. A possible recruitment of the effector CD4+ T-cell subpopulation Th-17 has been suggested in the pathogenesis of scleroderma [3]. At present, active superficial morphea can be treated with ultraviolet A1 (UVA1) with good results [4]. Meanwhile, there is no efficient therapy for the profound, progressive and destructive morphea variants [3]. On the basis of new insights into the key role of effector T cells, in particular Th-17, T-cell directed therapy including abatacept has been proposed to be clinically beneficial [5-7].
We describe here two patients treated with abatacept for chronic and progressive morphea profunda. Abatacept had a clinical effect on the active disease, in addition to softening old sclerotic lesions. Abatacept is a recombinant fusion protein, it works through the CD28 pathway. It has been shown to decrease memory B cells in vivo, reduce the migration of monocytes in rheumatoid arthritis, decrease the inflammatory activity of synovial macrophages in rheumatoid arthritis, and block memory CD4 T-cell activation
First Case Description
A 30-year-old male came to our observation due to a history of linear morphea of upper right arm (volar and dorsal arm and forearm), initially manifested with skin sclerosis, scleredema, tender and swollen joints, melanoderma and local hair loss. Laboratory findings showed elevated ANA (1:160 fine speckled pattern), negative anti-ENA, anti-dsDNA, anti-CCP and rheumatoid factor. Physical examination revealed absence of sclerodactyly, swollen fingers and digital scarring pitting. Nailfold video capillaroscopy excluded dilated/ aberrant capillary loops. Biopsy of the forearm skin demonstrated cutaneous and subcutaneous sclerosis, upward displacement of eccrine glands, loss of peri eccrine fat, and a predominantly infiltrated dermis of scattered lymphocytes with rare plasma cells. These findings were consistent with the diagnosis of linear morphea.
The patient was started on oral therapy with hydroxychloroquine 200 mg twice daily and methotrexate 15 mg sub-cutaneous weekly without benefit. After twelve months, he reported swelling of volar and dorsal parts of the arms and forearm and left ankle pain and worsened skin injury. Due to a significant decrease in patient’s quality of life and work (the patient was an anesthesia nurse) and concern for worsening dermal stiffness of right arm, he was started on intralesional triamcinolone acetonide injections at 10 mg/mL, oral mycophenolate mofetil, and prednisone at 20 mg daily to attempt to halt progression of the disease process. As a result, mycophenolate mofetil was initiated, but after he developed gastrointestinal intolerance, it was subsequently discontinued.
Over the course of the next year of treatment, despite intravenous (IV) solumedrol 1 g daily for 3 days, outpatient solumedrol infusions, subcutaneous methotrexate, and oral prednisone, her dermal plaque continued to expand significantly. After signing informed consent, he started injections of 125 mg subcutaneous abatacept weekly in the abdomen. After three months, he reported a significant improvement in signs of skin inflammation (decreased erythema) arm mobility, increased hair growth, and remission of arthritis. After six months, the patient was continuing a gradual taper of prednisone, currently under 5 mg a day and subcutaneous abatacept injections, with no relapse of the disease at 6 months, there was continued improvement on subcutaneous abatacept and prednisone tapering of 5 mg daily, and intralesional triamcinolone injections were discontinued.
Second Case Description
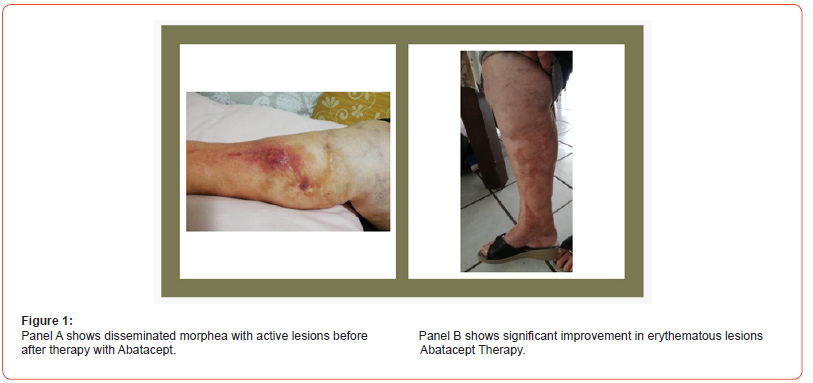
We present a case of non-responsive morphea profunda’s us of lower limbs (lateral and anterior area of legs, thighs, and buttocks) in a 60-year-old female, MIM, who initially presented for skin sclerosis, melanoderma and hair loss at level of both lower limbs, associated with tender and swollen joints in both hands and wrists. A punch biopsy showed typical histological findings of morphea. The patient had no other organ-specific manifestations. Routine blood evaluation was normal. Antinuclear antibody (ANA), rheumatic factor (RF) and anti-CCP were positive. Anti-ENAs were all negative. Previously, because of exacerbations in the disease, the patient had been treated with prednisolone, methotrexate, antimalarials and mycophenolate mofetil, in addition to physiotherapy. None of these treatments had any compelling any benefit on controlling disease activity. In February 2019, the patient had further exacerbation of the disease, with new lesions and severe pruritus. Clinically, she had disseminated morphea, mainly on the extremities, some lesions were new and active yellow-white lesions with a lilac ring; others were older, more sclerotic and atrophic with hyperkeratotic changes. Extensive post-inflammatory hyperpigmentation was also present (Figure 1; panel A). A punch biopsy from an active lesion on the left thigh confirmed the diagnosis and showed a primarily lymphocytic inflammatory infiltration around the superficial and deep blood vessels. Furthermore, inflammation at the junction between the dermis and the subcutaneous fat was observed, and the dermal collagen fibers thickened. Screening tests for hepatitis and tuberculosis performed before the treatment with abatacept, were negative; X-ray of the thorax was normal.
Following oral and written informed consent, the patient was treated with 125 mg of abatacept subcutaneous every week. During the treatment period she was treated with 15 mg prednisolone, which was carefully tapered. Furthermore, control blood tests were normal during treatment. The treatment with abatacept was well tolerated. The patient felt less itchy, and the joint motion was increased. The disease activity was reduced, both when evaluating the whole body and the single lesions. The erythema around the lesions decreased (Figure 2; panel B), and the older lesions became softer. Since the clinical response has been good and the patient has had no severe adverse events, the treatment is continuing.
Discussion
These clinical cases include two patients with morphea profunda treated with abatacept. Morphea profunda is a rare disorder and often is characterized by progressive course with sequelae and worsening quality of life. Our patients are very satisfied with the abatacept treatment. Both feel less itchy, have good response to joint movements after three months and the treatment is well tolerated. Thus, the disease activity has been improved both when evaluating the whole body and the erythema around the single lesions. Furthermore, it has been possible to taper the prednisolone treatment.
The low prevalence of the disease and the lack of valid agreed and validated treatment measures have impeded the development of an evidence-based approach to treatment. Actually, Lesions of morphea may improve with systemic glucocorticoids, methotrexate, or a combination of both treatments; in cases of morphea refractory to methotrexate, mycophenolate mofetil has been used with some efficacy [1,8] Various small-scale pilot studies and case reports have suggested some possible limited benefit from calcipotriol in combination with betamethasone diprionate [9], hydroxychloroquine, bosentan [10] (for cutaneous ulcerations), and systemic mycophenolate mofetil [8]. To assess the efficacy of mycophenolate mofetil in seven patients with localized scleroderma intolerant or resistant to previous treatment with methotrexate, the study demonstrated that the patients discontinued for adverse events (liver enzymes elevated and serious gastrointestinal effects), [8] Potential treatments awaiting further study include TNF blockers, Abatacept, thalidomide [3]. Certainly, topical treatments have no significant role to play in severe, deep, or rapidly progressive disease [3]. Stausbøl-Grøn et al. [10] reported two patients with deep morphea who responded successfully to Abatacept which encouraged us to treat patients with severe, resistant morphea subtypes and deep tissue involvement using Abatacept.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Elaine Kunzler, Stephanie Florez-Pollack, Noelle Teske, Jack O'Brien, Smriti Prasad, et al. (2019) Linear morphea: clinical characteristics, disease course, and treatment of the morphea in adults and children cohort. J Am Acad Dermatol 80(6): 1664-1670.
- Zwischenberger BA, Jacobe HT (2011) A systematic review of morphea treatments and therapeutic algorithm. J Am Acad Dermatol 65(5): 925-941.
- Fett N, Werth VP (2011) Update on morphea: part I. Epidemiology, clinical presentation, and pathogenesis. Am Acad Dermatol 64(2): 217-228.
- Alexander Kreuter, Julia Hyun, Markus Stücker, Anna Sommer, Peter Altmeyer, et al. (2006) A randomized controlled study of low-dose UVA1, medium-dose UVA1, and narrowband UVB phototherapy in the treatment of localized scleroderma. JAAD 54(3): 440-447.
- Birgitte Stausbøl-Grøn, Anne B Olesen, Bent Deleuran, Mette S Deleuran (2011) Abatacept is a promising treatment for patients with disseminated morphea profunda: presentation of two cases. Acta Derm Venereol 91(6): 686-688.
- Fage SW, Arvesen KB, Olesen AB (2018) Abatacept improves skin-score and reduces lesions in patients with localized scleroderma: a case series. Acta Derm Venereol 98(4): 465-466.
- R Lorenzetti, I Janowska, Cristian RS, Natalie F, Nadine H, et al. (2019) Abatacept modulates CD80 and CD86 expression and memory formation in human B-cells. J Autoimmun 101: 145-152.
- JS Mertens, D Marsman, Peter CM van de Kerkhof, Esther PAH Hoppenreijs, Hanneke KA Knaapen, et al. (2016) Use of mycophenolate mofetil in patients with severe localized scleroderma resistant or intolerant to methotrexate. Acta Derm Venereol 96(4): 510-513.
- A Kreuter, T Gambichler, A Avermaete, T Jansen, M Hoffmann, et al. (2001) Combined treatment with calcipotriol ointment and low-dose ultraviolet A1 phototherapy in childhood morphea. Pediatr Dermatol 18(3): 241-245.
- Zulian F (2008) New developments in localized scleroderma. Curr Opin Rheumatol 20(5): 601-607.
-
Giovanna Cuomo*, Klodian Gjeloshi, Francesco Masini, Fiammetta Danzo and Caterina Naclerio. Rapid And Successful Response to Abatacept in Non-Responsive Patients with Morphea Presentation of Two Cases. Arch Phar & Pharmacol Res. 4(3): 2024. APPR. MS.ID.000588.
-
Morphea Presentation, Abatacept, Fibrosing Disease, Psychological Sequelae, Systemic Sclerosis, Skin Softening
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






