 Opinion
Opinion
No Reliable Clinical Results Without a Proper Description of Medicinal Herbs
Anthony Cnudde1,2, Axelle Bourez3, Charaf El Khattabi1, Pierre Van Antwerpen3 and Florence Souard1*
1Department of Pharmacotherapy and Pharmaceutics (DPP), Université libre de Bruxelles (ULB), Bruxelles, Belgium
2Machine Learning Group, Université libre de Bruxelles (ULB), Bruxelles, Belgium
3RD3 - Pharmacognosy, Bioanalysis and Drug Discovery Unit & Analytical Platform of the Faculty of Pharmacy (APFP), Faculty of Pharmacy, Université libre de Bruxelles (ULB), Belgium
Florence Souard, Department of Pharmacotherapy and Pharmaceutics (DPP), Université libre de Bruxelles (ULB), Bruxelles, Belgium
Received Date:December 01, 2023; Published Date:January 04, 2024
Abstract
Complementary and Alternative Medicines (CAM), particularly herbal and natural products (phytotherapy), are gaining popularity, raising concerns about safety and interactions with conventional treatments. With potential benefits, herbs contain bioactive metabolites, necessitating careful consideration of the benefit/risk balance as with other therapeutics. This opinion paper focuses on the critical issue of insufficient herb descriptions in clinical literature, using milk thistle (Silybum marianum) as a case study. The variability in milk thistle extracts, such as Legalon® phytomedicine and other natural food supplements, is substantial, with silymarin being the presumed active extract. However, the literature lacks consistency in reporting extraction methods and metabolite identification. Analyzing clinical studies and case reports related to milk thistle over the past decade reveals significant discrepancies in product descriptions and posology, hindering the establishment of conclusive clinical evidence for its effectiveness.
This opinion article emphasizes the need for a more detailed and standardized approach in describing herbal products in clinical literature. Standardized reporting practices and collaboration between clinicians and phytochemists could be beneficial for bridging the gap between chemistry and clinics in herbal medicine research. The findings underscore the necessity of improved transparency and comprehensive reporting in clinical literature to enhance understanding, interpretation, and reliability in the assessment of herbal medicine efficacy and safety.
Keywords:Complementary And Alternative Medicines (CAM); Phytotherapy; Phytomedicine; Herbal Natural Product; Clinical Study
Abbreviations:CAM: Complementary and Alternative medicines
Introduction
Complementary and Alternative medicines (CAM) are gaining in popularity among patients, with herbs and natural products, known as phytotherapy being especially favored. If these therapies can be beneficial, particularly for minor cures and in some case preventing overuse of conventional drugs, they are often wrongly considered as inherently safe and without any adverse effects. Herbs are known to contain bioactive metabolites. When molecules are bioactive, the balance between benefits and risks must be carefully considered. This implies the potential for interactions with conventional treatments, leading to a loss of clinical effectiveness or the occurrence of toxicities. In certain instances, patients may use herbs as a substitute for conventional treatments, resulting in potentially severe consequences. Understanding the effect of herbs and their interactions with drugs is crucial for clinical practitioners. The ability to identify risks in patients and manage them is necessary to prevent any harmful adverse effects from herbs.
In our opinion, there are several critical points concerning clinical literature on herbal medicine. Since this literature often comes from clinicians rather than phytochemists/pharmacognosts, the description of the natural health product is often poorly described. It is well known that many environmental factors can influence the qualitative and quantitative composition of secondary metabolites [1, 2]. Moreover, the extraction process significantly contributes to the metabolite bioavailability in patients. For example, thyme essential oil provides access to hydrophobic volatile and fragrant metabolites, while herbal thyme tea provides access to hydrophilic metabolites. Consequently, there is a wide range of extraction methods leading to different compositions. Although the Latin scientific name of herbs is generally described in clinical literature, the extraction method is often omitted. This absence makes it challenging to interpret clinical conclusions accurately.
Herbs in literature
Logically, the main source of information regarding herbs’ effect, adverse effects, and interactions is scientific literature. The authors are classically clinicians without proper training in pharmacognosy or sometimes without all information concerning the commercial product used by patients. There are several difficulties for interpreting this phyto-clinical literature. As mentioned above, the mode of extraction is often absent (juice, herbal powder, herbal tea, decoction, maceration... with the extraction solvent and its extractive capacities), and therefore the projection of the type of metabolites is not always accessible. If the literature describes clinical data involving commercial products containing a large number of herbs (as in Asian traditional medicine) or unknown preparation methods (as often in Ayurvedic medicine), it is impossible to rationalize the pharmacological mechanism.
Mentioning only the herb name is not enough. Good redacting practice would require that any herb product mentioned in an article should be easily and undoubtedly identifiable. If for some use cases the scientific name might be sufficient, we believe that going further in herbs description is often required when describing herbs’ adverse effects. For example, the herb part used is needed. Examples in clinical literature where herb descriptions are insufficient are numerous; they are particularly present in case reports. To strengthen our proposals, we have chosen to develop the case of milk thistle.
The case of milk thistle
Hepatoprotective phytomedicines or food supplements are often used in addition to a conventional treatment for treating or preventing putative toxic liver damages [3]. Indeed, experiments on cell cultures, animals and humans have shown that natural compounds can prevent or alleviate pathological processes in the liver. For this indication, one herb frequently used is seeds of Silybum Lmarianum (L.) Gaertn, [4, 5] commonly known as milk thistle. Botanically speaking, what are commonly called seeds (Figure 1) are the fruit. However, keeping in mind evidence-based medicine standards, final conclusions regarding clinical effects and the effectiveness of this herb are still not established. Despite a large number of trials, clinical evidence remains inconclusive for the effectiveness in treating liver diseases (except in A. phaloides intoxications). In our opinion, the main bias explaining the discrepancies in activity is probably due to the different compositions of the extracts tested in these studies and the poor oral bioavailability.

Indeed, some milk thistle extracts are known to treat liver damage due to toxins, historically due Amanita phaloides poisoning (but also many other xenobiotics) and alcohol-induced damage, hepatitis, etc. [6]. In the particular case of milk thistle, there are numerous in vitro studies, animal studies, as well as human clinical studies substantiating this use. An intravenous preparations containing silybin (active metabolite – Legalon-sil®) is licensed in Europe, where it is used in emergency rooms for Amanita phaloides intoxications. The contents of the bottle correspond to 528.5 mg of C-2’,3-dihydrogenic silibinin succinate, di-sodic salt, corresponding to 315 mg of silibinin. After reconstitution with 35 ml of perfusion solution, 1 ml contains 9 mg of silibinin.
In Europe, another oral phytomedicine is available - Legalon®. It is a dry extract of Silybum marianum (L.) Gaertn, fruit obtained with a drug extraction (DER) of 36-44:1 equivalent to 140 mg of silymarin (dosed by DNPH or Brady reactive), or equivalent to 108.2 mg silymarin (assayed by LC-UV) calculated on silibinin. It is an ethyl acetate extract with 173.0 - 186.7 mg of dry extract per capsule. There are an innumerable number of other products sold on the market under the status of food supplements with very variable compositions. These products generally contain herb powders for extemporaneous extraction (tea) or direct ingestion, liquid or dry extracts, or purified secondary metabolites isolated from fruits named silymarin.
Milk thistle – chemistry
Silymarin, the supposed “active” extract of this herb, is a standardized extract consisting of approximately 70 to 80 % silymarin flavonolignans generally assayed by LC-UV or LC-MS [7–11]. In numerous bioactive studies, silybin (synonyms: silibinin) is used as standard, as key metabolite. Pharmacologically speaking, it seems to be the most active flavonolignan compound in silymarin.
Silymarin is constituted by:
a. Flavones: silybin, silybin, isosilybin and isosilybin A & B (depending
on the configuration in C-10 and C-11; C25H22O12); silychristin
A & B (C25H22O10); silymonin (C25H22O9); silhermin and
neosilhermin A & B (C25H22O9)
b. Flavanols: silandrin, cisilandrin, isolandrin, isocislandrin A &
B, (C25H22O9); silydianin and isosilychristin A & B (C25H22O10).
c. Flavonoids (taxifolin= dihydro-quercetin C15H12O7 and quercetin
C15H10O7), and the remaining 20-30 % consisting of a chemically
undefined fraction (Figure 2) [12].
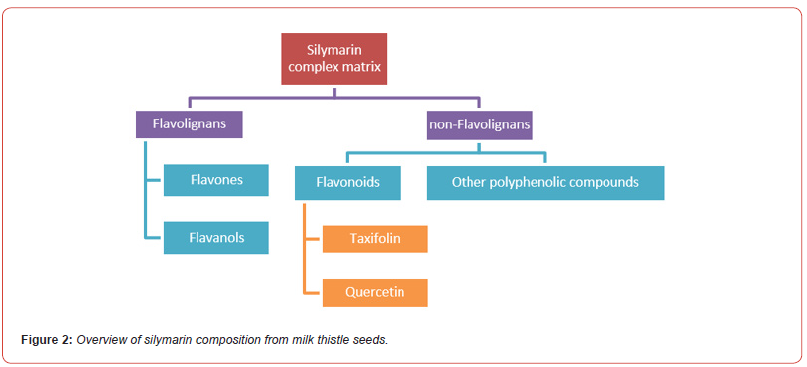
The seeds contain about 3 to 6 % of these silymarin extract.
As silybin is the main component of silymarin in quantity, the literature
mainly focuses on this compound, often ignoring all other
components [9]. To our knowledge, 27 compounds have been
identified, including all the diastereomers, without considering the
“other phenolic compounds” (blue rectangular in Figure 2) [9]. The
main component in quantity of silymarin is indeed silybin [13] (in
fact a quasi-equimolar mixture of A and B diastereoisomers), and
due to this fact biological activity of the whole complex extract is
often assigned to both compounds. There are also flavonoids as taxifolin
and quercetin (green rectangular in Figure 2). The fruit also
contains up to 30 % herbal oil rich in linoleic acid and phytosterols.
Supposed active ingredients are not water-soluble, and the preparation
of herbal tea and others water solutions seems to make no
sense a priori. Low hydro-alcoholic tinctures also appear obsolete.
Surprisingly, the EMA in its monograph [14] describes traditional
use of different extracts including herbal tea, plant powder or hydroalcoholic
tincture with a hepatotropic indication. Effectively,
traditional use registration (Article 16a (1) of Directive 2001/83/
EC) is described by:
a) No clinical tests and trials on safety and efficacy are required
as long as sufficient safety data and plausible efficacy are
demonstrated.
b) Involves assessment of mostly bibliographic safety and efficacy
data.
c) Must have been used for at least 30 years, including at least 15
years within the EU.
d) Are intended to be used without the supervision of a medical
practitioner and are not administered by injection.
To our knowledge, plethoric herbal extracts are sold as herbal food supplements. In the EU, food supplements are regulated as foods and are concentrated sources of nutrients or other substances with a nutritional or physiological effect that are marketed in “dose” form (e.g., pills, tablets, capsules, liquids in measured doses). A wide range of nutrients and other ingredients might be present in food supplements, including, but not limited to, vitamins, minerals, etc. and various plants and herbal extracts. They are generally standardized organic extracts with silymarin contents of 65 to 80 %, and dosage is determined by the content of silymarin and not by the total volume of extract. Bioactive silymarins are definitely lipophilic and poorly soluble in water, so only about 20–50 % is absorbed from the gastrointestinal tract after ingestion [15, 16]. We believe that one reason why silybin is so popular is because of its very easy method of isolation from silymarin: by precipitation with ethanol from acetone silymarin extract [13].
To support our proposal and our demonstration, we analyzed the phytomedicine Legalon®, an herbal tea that we prepared from fruits, and two herbal food supplements. by LC-MS (procedure will be published soon). The first supplement is a capsule of herbal powder from Arkopharma-France and the second is a hydro-alcoholic (EtOH) extract for NaturActive-France (Figure 3). According to the brand website for the first herbal supplement sold in EU, one capsule contains 390 mg of plant powder with 9 mg of silymarin. It is recommended to take 3 capsules per day at mealtime with a large glass of water, totaling 1170 mg of plant powder or 27 mg of silymarin.
For the second product, the brand website describes that the ethanolic extract contains a “concentrated extract which is the result of a process aimed at extracting the useful components of the plant in order to increase its virtues tenfold”. The recommended dosage is 2 capsules per day, in the evening at mealtimes, with a large glass of water, i.e., 84 mg of concentrated dry extract of milk thistle fruits. The drug/extract ratio presented is 46 to 70 kg of dried fruits are necessary to obtain 1 kg of concentrated dry extract of organic milk thistle. The exact quantity of silymarin is not specified.
In (Figure 3A), on the left, it is immediately noticeable that the distribution of the different metabolites is completely different according to the commercial products. In phytomedicine (first chromatogram, dry ethyl acetate extract), there are almost as many silychristin A as “silychristin B + silydianin” (peaks around 11 min). On the other hand, in the herbal food supplement (second chromatogram, ethanolic extract), there is three times more silychristin A than “silychristin B + silydianin”. In the herbal food supplement based on plant powder (third chromatogram, water at room temperature), the ratio considered is almost respected but the isosylibin A and B are not detectable (peaks around 18 min). Finally, in herbal tea (last chromatogram, water infusion), it is isosilychristin B + silydianin that are absent.
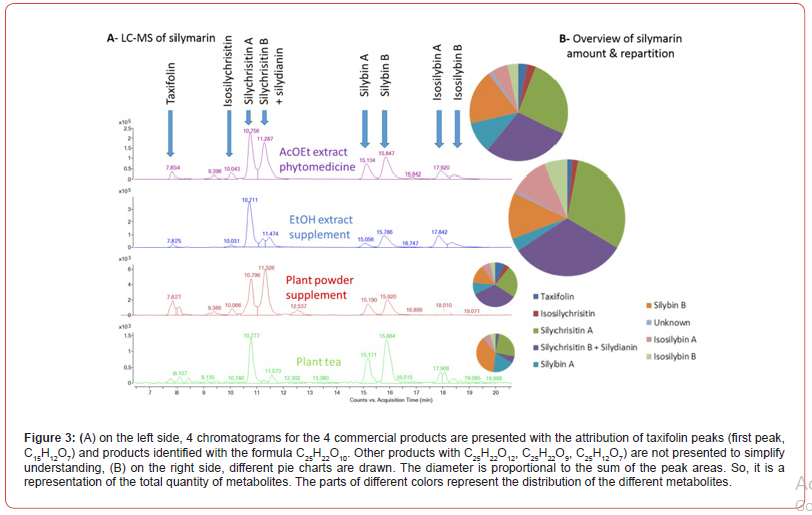
But the most striking aspect is in the right part of (Figure 3B). We drew pie charts with the areas under the curve to approach the relative quantities of the different products. The product that provides access to the greatest amount of silymarins is not phytomedicine. Contrary to what one might think intuitively, it is the herbal food supplement obtained with the EtOH extract. The major metabolites in our study are silychristins in 3 of the products and not silybin A & B. In the case of herbal tea, silybin B is the most present.
Milk thistle – clinical data
In these circumstances, it is now clear that clinical evidence of human activity cannot be robust. Especially since the legislation and controls for food supplements are not as strict as for drugs. We are not certain that we would have obtained the same results by analyzing other batches. The typical oral adult dose of silymarin is 240-800 mg/day in 2 or 3 divided doses [17]. Silymarin, the complex matrix, possesses diverse pharmacological activities, including hepatoprotective. Although clinical trials have shown silymarin is safe at high doses (>1500 mg/day) in humans, the pharmacokinetic (PK) studies of silymarin revealed a poor but rapid absorption (T max = 2-4 h) from oral formulation and its half-life is approximately 6 h. After gastrointestinal absorption, silibinin is rapidly metabolized by phase I and phase II biotransformation in the liver. Around 80% of silibinin is excreted as glucuronide and sulfate conjugates with bile. Around 20–40 % of bile silybin is recovered, whereas the remaining part is excreted via feces. Less than 10 % of orally administrated silybin is excreted in an unchanged form in the urine [18]. At a supratherapeutic concentration (1 μmol/l), silybin had negligible inhibition of the CYP450 enzymes 1A2, 2A6, 2B6, 2C8, 2C9 and 2E1, minor (< 20 %) inhibition of CYP 3A4 and moderate (< 40 %) inhibition of CYP 2C19 and 2D6. Since the therapeutic concentration of silybin is ~ 0.2 μmol/l, authors concluded that silymarin is unlikely to cause hepatic herbal-drug interaction at therapeutic doses [19]. Clinical trials suggest that milk thistle does not affect CYP 1A2, 2C9, 2D6, 2E1, 3A4 or 3A5 [20,21]. In two multiple-dose PK studies, silymarin (160–450 mg every 8 h) did not reduce levels of the CYP 3A4 substrate midazolam, indinavir, rifampin and clarithromycin [22,23]. Although the interaction mechanisms of silymarin via CYP 450 are well studied, there are still many unknowns concerning the pharmacokinetics and impact on CYP. Flavolignans are studied, but the effect of the matrix remains poorly understood, keeping in mind that commercially products have various chemical extracts. In phytotherapy, a totum is used, what impact do the “other” metabolites than silymarin have on the absorption of flavolignans? What is the digestion impact? For liver disease, clinical trials on milk thistle studied in an old Cochrane review in 2007 assessed 13 randomized trial (915 patients) have demonstrated safety and efficacy of silymarin (at doses of 1,200-1,500 mg/day) for alcoholic and/or hepatitis B or C diseases [6]. Silymarin have also demonstrated an antidiabetic type-2 effect on patients, reducing levels of glycated hemoglobin, fasting blood glucose, total cholesterol, LDL, triglyceride, SGOT and SCPT compared to placebo in a 4-month treatment in a randomized double-bind controlled trial [24].
To have an overview of how the milk thistle in described in literature, we performed a rapid search on PubMed in November 2023 using the query “milk thistle AND (hepat* OR liver)” and we used the “clinical trial”, “randomized clinical trial “, and “case report” filters. We thus limited the search to the 10 previous years (2013-2023). This query resulted in 20 results. Among these articles, we decided to discard 2 studies that focused on horses, 1 was discarded due to being related to a specific case of exposure during professional activity, and we were unable to access 1 other article due to paywall. In the 16 remaining articles, the products were described as follows in (Table 1). As show in these results, only one of the articles presents [36] detailed compositions of the extract used while all others used simpler definition ranging from the name of a supplement (Legalon® in most cases) to the vernacular name ‘milk thistle’. Phytomedicine such as Legalon® are likely to always provide the same constituents. Quality must be maintained. One can therefore think that in 8 cases out of 16, the same product is certainly studied. For the other products, it is difficult to draw any conclusion. Posology is consistent through all articles, with daily doses ranging from 140 to 700 mg. The only inconsistencies occur in a case report where doses are lacking, and a metabolic study where a single intake was tested. These differences can be explained by the nature of the article itself.
Table 1:Key information in clinical studies and case reports involving milk thistle products (if found in articles). Information about the product and posology refers to the most precise description we were able to find in the articles.
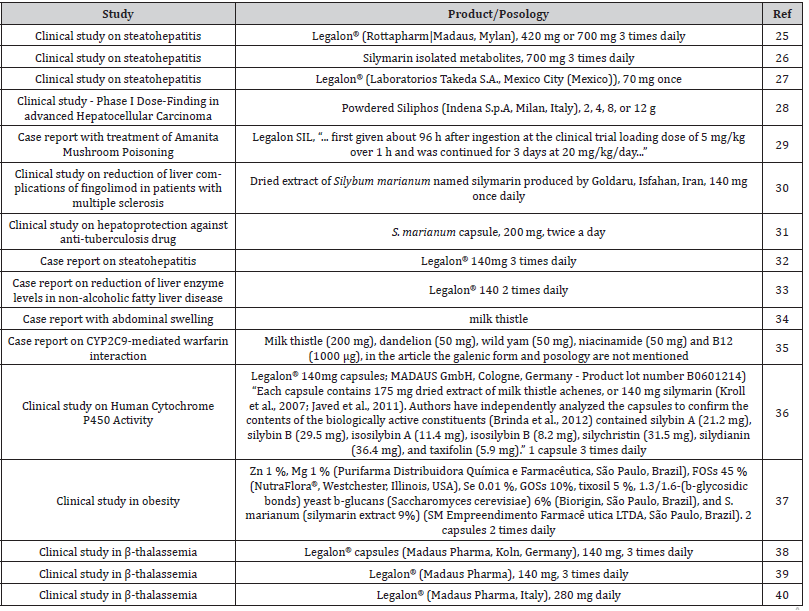
As a result, (Table 1) describes commercial products that might actually be different from each other in terms of composition while being associated to the same herb. In our opinion, final conclusions regarding clinical effects and effectiveness of this herb, considering the diversity of products sold, cannot be made. We think that the main bias explaining the discrepancies in activity is probably due to the different compositions of the extracts tested in these studies. These discrepant data reinforce the skepticism by the medical profession. If there is a lack of striking effects on the hepatic disease, it can be said with certainty that some extracts of milk thistle have hepatoprotective and hepatoregenerative effects while also increasing the intracellular concentration of glutathione in liver, and consequently the redox state [41].
Conclusion
In the example of milk thistle, we have shown that many commercial products exist with quite variable compositions. We have analyzed in our lab different commercial products of the different classes available in Europe and demonstrated qualitative and quantitative variations. By analysis of the clinical literature of the last 10 years, we observe that the products change that the posology are heterogeneous. This literature is therefore not directly interpretable. The name of the herb is largely insufficient. In our opinion, we outlined here and integrated existing and emerging transdisciplinary knowledge for a better description of natural products from chemistry to clinic.
Acknowledgement
This study was supported by the Belgian Fund for Scientific Research (FRS-FNRS) and the Université libre de Bruxelles (ULB). The Analytical Platform of the Faculty of Pharmacy (APFP) was also supported by both the FRS-FNRS and ULB. P.V.A. would like to thank MA for her constant support and making his life easier. FLS thanks Todo for doing so much and LMS for his shared laughter.
Conflict of Interest
No conflict of interest exists.
References
- Yang L, Wen KS, Ruan X, Zhao YX, Wei F, et al. (2018) Response of Plant Secondary Metabolites to Environmental Factors. Molecules 23(4): 762.
- Li Y, Kong D, Fu Y, Sussman MR, Wu H, et al. (2020) The Effect of Developmental and Environmental Factors on Secondary Metabolites in Medicinal Plants. Plant Physiol. Biochem 148: 80-89.
- Ramos-Tovar E, Muriel P (2019) Chapter 9 - Phytotherapy for the Liver∗. In Dietary Interventions in Liver Disease; Watson, R. R., Preedy, V. R., Eds.; Academic Press pp: 101-121.
- Porwal O, Ameen MSM, Anwer ET, Uthirapathy S, Ahamad J, et al. (2019) (Milk Thistle): Review on Its Chemistry, Morphology, Ethno Medical Uses, Phytochemistry and Pharmacological Activities. J. Drug Deliv. Ther 9(5): 199-206.
- Jiang G, Sun C, Wang X, Mei J, Li C, et al. (2022) Hepatoprotective Mechanism of Silybum Marianum on Nonalcoholic Fatty Liver Disease Based on Network Pharmacology and Experimental Verification. Bioengineered 13(3): 5216-5235.
- Rambaldi A, Jacobs BP, Gluud C (2007) Milk Thistle for Alcoholic and/or Hepatitis B or C Virus Liver Diseases. Cochrane Database Syst. Rev 4: CD003620.
- Kuki Á, Nagy L, Deák G, Nagy M, Zsuga M, et al. (2012) Identification of Silymarin Constituents: An Improved HPLC–MS Method. Chromatographia 75(3): 175-180.
- Kvasnička F, Bı́ba B, Ševčı́k R, Voldřich M, Krátká J, et al. (2003) Analysis of the Active Components of Silymarin. J. Chromatogr. A 990(1): 239-245.
- Chambers CS, Holečková V, Petrásková L, Biedermann D, Valentová K, et al. (2017) The Silymarin Composition… and Why Does It Matter??? Food Res. Int 100(Pt 3): 339-353.
- Wallace S, Carrier DJ, Beitle RR, Clausen EC, Griffis CL, et al. (2003) HPLC-UV and LC-MS-MS Characterization of Silymarin in Milk Thistle Seeds and Corresponding Products. J. Nutraceuticals Funct. Med. Foods 4(2): 37-48.
- Gilabadi S, Stanyon H, DeCeita D, Pendry BA, Galante E, et al. (2023) Simple and Effective Method for the Extraction of Silymarin from Silybum Marianum (L.) Gaertner Seeds. J. Herb. Med. 37: 100619.
- Sunil C, Xu B (2019) An Insight into the Health-Promoting Effects of Taxifolin (Dihydroquercetin). Phytochemistry 166: 112066.
- Biedermann D, Vavříková E, Cvak L, Křen V (2014) Chemistry of Silybin. Nat. Prod. Rep 31(9): 1138-1157.
- Silybi mariani fructus. European Medicines Agency.
- Gillessen A, Schmidt HHJ (2020) Silymarin as Supportive Treatment in Liver Diseases: A Narrative Review. Adv. Ther 37(4): 1279-1301.
- Zhu HJ, Brinda BJ, Chavin KD, Bernstein HJ, Patrick KS, et al. (2013) An Assessment of Pharmacokinetics and Antioxidant Activity of Free Silymarin Flavonolignans in Healthy Volunteers: A Dose Escalation Study. Drug Metab. Dispos. 41(9): 1679-1685.
- Dixit N, Baboota S, Kohli K, Ahmad S, Ali J, et al. (2007) Silymarin: A Review of Pharmacological Aspects and Bioavailability Enhancement Approaches. Indian J. Pharmacol. 39(4): 172-179.
- Kim Y, Kim E, Lee E, Kim J, Jang S, et al. (2003) Comparative Bioavailability of Silibinin in Healthy Male Volunteers. Int. J. Clin. Pharmacol. Ther 41(12): 593-596.
- Gurley BJ, Barone GW, Williams DK, Carrier J, Breen P, et al. (2006) Effect of Milk Thistle (Silybum Marianum) and Black Cohosh (Cimicifuga Racemosa) Supplementation on Digoxin Pharmacokinetics in Humans. Drug Metab. Dispos. Biol. Fate Chem. 34(1): 69-74.
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, et al. (2004) In Vivo Assessment of Botanical Supplementation on Human Cytochrome P450 Phenotypes: Citrus Aurantium, Echinacea Purpurea, Milk Thistle, and Saw Palmetto. Clin. Pharmacol. Ther 76(5): 428-440.
- Piscitelli SC, Formentini E, Burstein AH, Alfaro R, Jagannatha S, et al. (2002) Effect of Milk Thistle on the Pharmacokinetics of Indinavir in Healthy Volunteers. Pharmacother. J. Hum. Pharmacol. Drug Ther 22(5): 551-556.
- Gurley BJ, Swain A, Hubbard MA, Williams DK, Barone G, et al. (2008) Clinical Assessment of CYP2D6-Mediated Herb-Drug Interactions in Humans: Effects of Milk Thistle, Black Cohosh, Goldenseal, Kava Kava, St. John’s Wort, and Echinacea. Mol. Nutr. Food Res 52(7): 755-763.
- Huseini HF, Larijani B, Heshmat R, Fakhrzadeh H, Radjabipour B, et al. (2006) The Efficacy of Silybum Marianum (L.) Gaertn. (Silymarin) in the Treatment of Type II Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Clinical Trial. Phytother. Res 20(12): 1036-1039.
- Navarro VJ, Belle SH, D’Amato M, Adfhal N, Brunt EM, et al. (2019) Group, on behalf of the S. in N. and C. H. (SyNCH) S. Silymarin in Non-Cirrhotics with Non-Alcoholic Steatohepatitis: A Randomized, Double-Blind, Placebo Controlled Trial. PLOS ONE 14(9): e0221683.
- Kheong CW, Mustapha NRN, Mahadeva S (2017) A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol 15(12): 1940-1949.e8.
- Méndez-Sánchez N, Dibildox-Martinez M, Sosa-Noguera J, Sánchez-Medal R, Flores-Murrieta FJ, et al. (2019) Superior Silybin Bioavailability of Silybin–Phosphatidylcholine Complex in Oily-Medium Soft-Gel Capsules versus Conventional Silymarin Tablets in Healthy Volunteers*. BMC Pharmacol. Toxicol 20(1): 5.
- Siegel AB, Narayan R, Rodriguez R, Goyal A, Jacobson D, et al. (2014) A Phase I Dose-Finding Study of Silybin Phosphatidylcholine (Milk Thistle) in Patients with Advanced Hepatocellular Carcinoma. Integr. Cancer Ther 13(1): 46-53.
- Gores KM, Hamieh TS, Schmidt GA (2014) Survival Following Investigational Treatment of Amanita Mushroom Poisoning: Thistle or Shamrock? CHEST 146(4): e126-e129.
- Ghiasian M, Nafisi H, Ranjbar A, Mohammadi Y, Ataei S, et al. (2021) Antioxidative Effects of Silymarin on the Reduction of Liver Complications of Fingolimod in Patients with Relapsing–Remitting Multiple Sclerosis: A Clinical Trial Study. J. Biochem. Mol. Toxicol 35(8): e22800.
- Zhang S, Pan H, Peng X, Lu H, Fan H, et al. (2016) Preventive Use of a Hepatoprotectant against Anti-Tuberculosis Drug-Induced Liver Injury: A Randomized Controlled Trial. J. Gastroenterol. Hepatol 31(2): 409-416.
- Hashem A (2023) Silymarin and Management of Liver Function in Nonalcoholic Steatohepatitis: A Case Report. Drugs Context 12: 1-5.
- Chantarojanasiri T (2023) Silymarin Treatment and Reduction of Liver Enzyme Levels in Non-Alcoholic Fatty Liver Disease: A Case Report. Drugs Context 12: 1-4.
- McNeal PM, Bush JS, Reeves CF, Connors NJ (2016) Man with Abdominal Swelling. Ann. Emerg. Med 68(2): 163.
- Lash DB, Ward S (2020) CYP2C9-Mediated Warfarin and Milk Thistle Interaction. J. Clin. Pharm. Ther 45(2): 368-369.
- Kawaguchi-Suzuki M, Frye RF, Zhu HJ, Brinda BJ, Chavin KD, et al. (2014) The Effects of Milk Thistle (Silybum Marianum) on Human Cytochrome P450 Activity. Drug Metab. Dispos 42(10): 1611-1616.
- Nehmi-Filho V, Santamarina AB, de Freitas JA, Trarbach EB, de Oliveira DR, et al. (2022) Novel Nutraceutical Supplements with Yeast β-Glucan, Prebiotics, Minerals, and Silybum Marianum (Silymarin) Ameliorate Obesity-Related Metabolic and Clinical Parameters: A Double-Blind Randomized Trial. Front. Endocrinol 13: 1089938.
- Moayedi B, Gharagozloo M, Esmaeil N, Maracy MR, Hoorfar H, et al. (2013) A Randomized Double-Blind, Placebo-Controlled Study of Therapeutic Effects of Silymarin in β-Thalassemia Major Patients Receiving Desferrioxamine. Eur. J. Haematol 90(3): 202-209.
- Reisi N, Esmaeil N, Gharagozloo M, Moayedi B (2022) Therapeutic Potential of Silymarin as a Natural Iron-Chelating Agent in β-Thalassemia Intermedia. Clin. Case Rep 10(1): e05293.
- Gharagozloo M, Karimi M, Amirghofran Z (2013) Immunomodulatory Effects of Silymarin in Patients with β-Thalassemia Major. Int. Immunopharmacol 16(2): 243-247.
- Valenzuela A, Aspillaga M, Vial S, Guerra R (1989) Selectivity of Silymarin on the Increase of the Glutathione Content in Different Tissues of the Rat. Planta Med 55(5): 420-422.
- Egresi A, Süle K, Szentmihályi K, Blázovics A, Fehér E, et al. (2020) Impact of Milk Thistle (Silybum Marianum) on the Mycotoxin Caused Redox-Homeostasis Imbalance of Ducks Liver. Toxicon Off. J. Int. Soc. Toxinology 187: 181-187.
-
Anthony Cnudde, Axelle Bourez, Charaf El Khattabi, Pierre Van Antwerpen and Florence Souard*. No Reliable Clinical Results Without a Proper Description of Medicinal Herbs. Arch Phar & Pharmacol Res. 4(1): 2024. APPR.MS.ID.000577.
-
Vaccinated people, Virus, Viral vectors, DNA molecules, SARS-CoV-2 virus, COVID-19
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






