 Short Communication
Short Communication
Natural Transformation of Gram-Positive Streptococcus Pneumoniae
Sunil Palchaudhuri*
Wayne State University, Detroit, USA and Atlanta Health and Welfare Centre for Women, Kolkata, India
Sunil Palchaudhuri, Wayne State University, Detroit, USA and Atlanta Health and Welfare Centre for Women, Kolkata, India
Received Date:May 01, 2024; Published Date:May 06, 2024
Abstract
Natural transformation is the growth curve of Gram-positive pathogen Streptococcus pneumoniae and its avirulent derivatives. This bacterium grows in three phases: pre-competent, competent and post-competent but uptake of exogenous DNA seems to be unimportant. In 1944 Avery et al of Rockefeller Institute (NY) delayed the progress of Gram-positive bacterial genetics by his ignorance about DNA bio-macromolecule, their TCA insoluble precipitate were just few nucleotides before the discovery of the double helix DNA of Watson and Crick (1953).
Difference between Natural and Artificial transformation
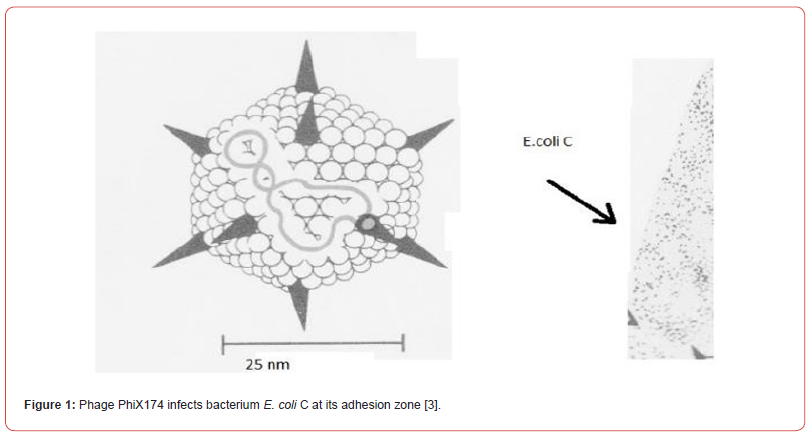
The artificial transformation with recombinant DNA differs considerably from the natural transformation! Because of his ignorance Dr Stanley Cohen of Stanford University has never mentioned that his recombinant DNA is responsible for the antibiotic resistance crisis by the spread of transposons (Tn4, Tn10), not only in his research laboratory of Stanford University but also all over the world. Why did he inspire his research associateAnnie Chang to initiate an in-vitro gene cloning experiments with a multicopy cloning vector pBR322 with two transposons Tn4 and Tn10? These transposons are not just antibiotic resistance traits but recognized as mobile DNA elements. In this context we should also introduce the two laboratory strains of E. coli, E. coli K-12 and E. coli C, which differ by their sensitivity to the bacteriophage phiX174. Significantly enough that this phage genome (single stranded covalently closed circular DNA) penetrates into the adhesion zone of E. coli C (Figure 1) - where its two membranes and the cell wall are fused but the E. coli K-12 is lacking such a zone. Between the period 1944-2004 many investigators have mixed upNatural transformation with the artificial transformation because of global publicity of gene cloning experiments with the recombinant DNA in Gram-negative E. coli K-12 Briefly, the recombinant DNA, formed by ligating a DNA fragment of choice to a multicopy cloning vector pBR322 and its entry into the Gram-negative competent E. coli K-12 not the E. coli C. How is the E. coli K-12 made competent? E. coli K-12 is made competent after its growth to an early log phase in a rich broth containing calcium chloride (0.01M). The competent E. coli K-12 cells (pellet) are mixed with the recombinant DNA cooled in an ice bucket and then applied thermal shock at 420 C I (SP) dare to speculate that the two membranes (both inner and outer) are melted to some extent but the adhesion zones where the two membranes remain attached to the same site of the cell wall facilitating the attachment of recombinant DNA if supercoiled (unpublished data) (Figure 1) This was done with Gram-negative E. coli K-12 as an internal control [1-3].
Some years later our knowledge of molecular biology and DNA sequencing has shown that the single chromosomes of bacterial strains E. coli K-12 and E. coli C are almost the same except the xylitol operon of E. coli C shows the location of Xylitol operon in E. coli K-12 genetic linkage map. How such operon evolved remains to be studied. In Antibiotics Conference, October 11(2018) held in Edinburgh, Scotland I have presented data showing the growth patterns of these three bacterial strains E. coli K-12, E. coli C(Gramnegative) and the Streptococcus pneumoniae (Gram-positive). Morphologically the S.pneumoniae and E. coli C don’t show much difference but it is worth mentioning that the S.pneumniae has also xylitol loci. E. coli K-12 is lacking xylitol operon but also lacking normal growth pattern (Figure 2A and Figure 2B).
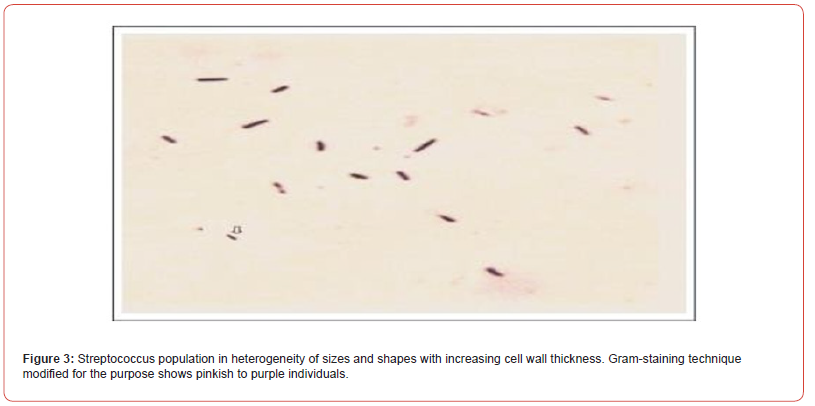
In 2013, I have described the natural transformation of Streptococcus pneumoniae that grows in three phases precompetent, competent and post- competent (spheroplast) but without requiring the uptake of any exogenous double stranded DNA fragments (linear). Our data confirms that Avery et al (1944) abused the word DNA without the knowledge of double helix DNA as bio-macromolecule (Watson & Crick in 1953 Nobel Prize winners). The spheroplasts of S. pneumoniae are really carrying their progeny in heterogeneity of shapes and sizes with increasing optical density but without increasing the colony forming unit (cfu). My optical micrograph demonstrates the growth of Streptococcus population in heterogeneity of shapes and sizes (Figure 3). Their colour changes (pinkish to deep purple).
However, Avery et al made an attempt to understand Dr Griffith’s observation without the academic preparation required [4,5]. They mixed up the difference between TCA insoluble precipitate and the DNA bio-macromolecule. Double helix DNA was discovered in 1953 by Watson & Crick. They were awarded the Nobel Prize because they defined the bio- macromolecule which is not just TCA insoluble precipitate of Avery et al [6]. Of course, Dr Avery was a faculty of Rockefeller Institute, New York City but lacking the background to appreciate that his TCA insoluble precipitate was just a few nucleotides long but was never DNA biomacromolecule (3). At least Dr Griffith knew that this is a serious pathogen (Streptococcus pneumoniae) responsible for lobar pneumonia and therefore he decided not to go for Gram staining technique. Many years later (1971) I decided to reproduce Dr. Griffith’s observation and impressed by the reproducibility and accuracy of his observation. After Gram stained with crystal violet. I (SP) have made an effort to see this pathogen under an optical microscope without washing off with acetone-alcohol mix. It appears to be morphologically diplococcic but never the two (7). When its spheroplast is ruptured by applying a shearing force the heterogeneity of population was observed (0.1um to 1um) but they were not colony forming unit(cfu). Are they contaminants? The answer is “No” because my data has confirmed that they really grow in heterogeneity of shapes and sizes -round to oval and then oval to diplococcic (8). Dr Stanley Cohen, a medical faculty of Stanford University, has never mentioned that his recombinant DNA is responsible for the antibiotic resistance crisis by the spread of transposons (Tn4, Tn10), not only in his research laboratory of Stanford University but also all over the world (9). It seems to me that the cloning vector pBR322 is apparently responsible for antibiotic resistance crisis(globally) but I am preparing another manuscript with a solution to such antibiotic resistance crisis.
Conclusion
Mitis group Streptococcus progeny is not released from the spheroplast(mother) when the overnight culture is grown without shaking and avoiding the shearing force. If released by using shearing force, their varying sizes and shapes confirm the growth in heterogeneity- round to oval, oval to diplococcic (Fig 3). We must remember the diplococcic individual does never means the two. Gram-staining technique (modified) to confirm their growth showed the heterogeneity of such a population in three different phases: pre-competent, competent and post-competent. The post - competent phase is the spheroplast carrying the progeny. If ruptured, then we see the entire population of the progeny growing in heterogeneity of different sizes and shapes. What is more, their cell wall thickness (peptidoglycan layers) thickness varies.
Acknowledgement
Financial support of Atlanta Health and Welfare Centre for Women (India) and Professor SP for paying Tripti bhattacharya as an academic assistant.
Conflict of Interest
No conflict of Interest.
References
- Arthur Kornberg (1974) DNA Replication. W.H. FREEMAN AND COMPANY, San Francisco.
- SN Cohen, AC Chang, HW Boyer, RB Helling (1973) Construction of biologically functional bacterial plasmids In vitro. Proc. Natl. Acad. Sci, USA 70(11): 3240-3244.
- SR Palchaudhuri, AJ Mazaitis, WK Maas, AK Kleinschmidt (1972) Characterization by electron microscopy of fused F-prime factors in Escherichia coli. Proc. Natl. Acad. Sci. USA 69(7): 1873-1876
- Griffith F (1928) The significance of pneumococcal types. J. Hyg 27(2): 113-159.
- Avery OT, CM MacLeod, M McCarty (1944) Studies on the chemical nature of the substance inducing transformation of pneumococcal types. J Exp.Med 79(2): 137-158.
- Watson JD, Crick FH (1953) Molecular structure of nucleic acids: a structure for deoxyribose nucleic acid. Nature 171(4356): 737-738.
- Palchaudhuri S (2013) Growth curve of Mitis group streptococcus - (unpublished data).
- Palchaudhuri S (2021) Growth curve of S. pneumoniae-a serious pathogen. Med Case Rep 1(1): 1-3
- Cohen SN (2013) DNA cloning: a personal view after 40 years. Proc Natl Acad Sci USA 110(39): 15521-15529.
-
Sunil Palchaudhuri*. Natural Transformation of Gram-Positive Streptococcus Pneumoniae. Arch Phar & Pharmacol Res. 4(3): 2024. APPR.MS.ID.000587.
-
Streptococcus Pneumoniae, Gram-Positive, Natural Transformation, Artificial Transformation, E. Coli K-12
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






