 Short Communication
Short Communication
Innovative Approaches with Talquetamab in Recurrent Multiple Myeloma
Dhruv Kumawat1, Vartika Jain1*, Vijaya Mishra1, Pallavi2 and Pragya Sharma2*
1School of Pharmaceutical Sciences, Jaipur National University, Jaipur, Rajasthan, 302017, India
2Amity Institute of Pharmacy, Amity University Rajasthan, Jaipur, Rajasthan, 303002, India
Vartika Jain, School of Pharmaceutical Sciences, Jaipur National University, Jaipur, Rajasthan, 302017, India and Pragya Sharma, Amity Institute of Pharmacy, Amity University Rajasthan, Jaipur, Rajasthan- 303002, India
Received Date:February 14, 2025; Published Date:February 26, 2025
Abstract
Plasma cell is affected from variety of leukocytes and is a type of carcinoma called multiple myeloma. The damage to the bone is increased by cancerous plasma cells patient suffering from multiple myeloma don’t have many plasma cells in bone marrow. Bispecific antibody is a type of antibody which is prepared in vitro and Talquetamab is a kind of drug. At 405 μg/kg weekly there were reduced 60% erythrocyte levels, 40% leukocytes, 67% neutrophils, 40% lymphocytes along with 37% decreased platelet count.
Keywords:Relapsed Refractory/Multiple Myeloma; B Cell Maturation Antigen; Cytokine Release Syndrome; Multiple Myeloma
Abbreviations:RR/MM: Relapsed refractory/multiple myeloma; BCMA: B Cell Maturation Antigen; GPRC5D: G Protein-coupled Receptor class C group 5 member D; CRS: Cytokine Release Syndrome; MM: Multiple Myeloma; Ig G: Immunoglobulin G; BCG: Bacillus Calmette-Guérin
Introduction
Talquetamab is a sort of medication, and bispecific antibodies are an in vitro-prepared form of antibody. This antibody may bind to two different receptors at the same time. One protein, Talquetamab, is also found on the surface of CD3 T cells and the multiple myeloma cell epithelium protein GPRC5D. The US FDA also refers to Talquetamab as an orphan medicine, which is used to treat patients with recurrent multiple myeloma who were receiving treatment with four previous medications [1].
BCMA: Activation in MM and up regulation is preferred on plasma cell epithelium in immune cancer therapy by BCMA for novel purpose. A short and soluble form is converted from 184 amino acid membrane which is expressed on BCMA. In MM the authenticated target is BCMA which decrease efficacy and decrease binding of agents like Belantamab that affect the pharmacokinetics of significant control variable. BCMA targeting agents would majorly change the efficacy but is indefinite [2].
GPRC5D: On the epithelium of multiple myeloma cells there is large amounts of receptors found of GPRC5D. There is an effect produced on the surface of receptor when specific drug binds to receptor. On the surface of CD3 T cells there is highly expressed on plasma cells GPRC5D receptor [3]. Plasma cell is affected from variety of leukocytes and is a type of carcinoma called multiple myeloma. The damage to the bone is increased by cancerous plasma cells patient suffering from multiple myeloma don’t have many plasma cells in bone marrow. Multiple myeloma has signs and symptoms the main like generalized weakness, myalgia, renal disorder, hypercalcemia, reduced RBC level and fractures with bone or back ache. Patient suffering from multiple myeloma have chances to get infections than patients not suffering more seriously [4].
Mechanism of Action
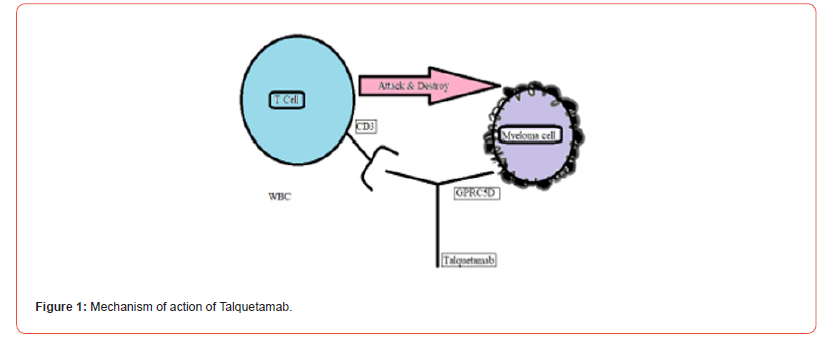
Talquetamab fuses to two GPRC5D along with CD3 to start slaying GPRC5D indicating MM cells through method of T-cell demand and triggering illustrated in Figure 1 [3].
Pharmacokinetics
Plasma time concentration graph revealed decreased varying and extra continuous system subsequently hypodermic giving than later iv dispense, with lower peak to trough fraction. Both quantity that were proposed for phase 2 survey had homogenous pharmacokinetic outline at stable attitude, accompanied by antibody exhibiting maintained above 90% most functional concentration in former living organism noxious test, 14 reports which on assuming support for two dosage slots. This therapy maximised stimulate T cell and produce cytokines, with corresponding stimulation at two dosages for phase 2 study [3].
Side Effects
Haematological
• At 405μg/kg weekly there were reduced 60% erythrocyte
levels, 40% leukocytes, 67% neutrophils, 40% lymphocytes along
with 37% decreased platelet count.
• At 800 μg/kg every other week there were reduced
43% erythrocyte levels, 18% leukocytes, 36% neutrophils, 39%
lymphocytes along with 23% decreased platelet count.
Common Symptoms
1. Reduced Erythrocyte: fatigue, asthenia, arrhythmia,
dyspnoea
2. Reduced Leukocytes: contagion, rigor, pyrexia more than
100.4 F, mouth ulcer
3. Reduced Neutrophils: Chronic and relapsed infection,
tiredness, mouth ulcers
4. Reduced Lymphocytes: splenomegaly,
immunosuppression, lymphadenopathy, alteration in skin
5. Decreased Platelet: ecchymosis, menometrorrhagia [4]
Neurological Effects: At dose of 405 μg/kg there were mild
side effects whereas at 800 μg/kg there were relieving response
who took Talquetamab.
• Common Symptoms: delirium, anosmia, neuroplasticity,
aphasia
Others: Weight loss- About a 33% of patients who received Talquetamab experienced cachexia. Of the patients who underwent cachexia, all except one lost less than 20% of their starting weight [4].
Dosage
• 2mg/ml (1.5-ml single-dose vial)
• 40mg/ml (single- dose vial)
• Ready to use SC solution [5-10].
Adverse Events
CRS- Major adverse event of Talquetamab. It happens when immune system is activated and becomes hyperactive. This gives rise to huge eruption of small protein called as cytokines that are allowed to leave in blood stream. This can take place when T cells, which are main part of immunity that are triggered by therapy such as Talquetamab. Hyperthermia, travel sickness, lethargy, hypotension, hypoxemia and body pain are manifestations of CRS [4].
Use in Specific Population
Pregnancy: Pregnancy-related risks associated with talquetamab are not well understood, nor are there any data on the drug’s use in expecting women or animals. It is known that after the first trimester of pregnancy, human IgG penetrates the placenta. As a result, talquetamab may be transferred from the mother to the growing fetus. It is uncertain how talquetamab affects the growing fetus. It is not advised for women who are pregnant or who are not planning to become pregnant to take talquetamab. It is possible that talquetamab during pregnancy will cause a weakened neonatal immune system. As a result, live immunizations for newborns, such the BCG vaccine, should not be administered until four weeks after birth.
Breast-feeding: The excretion of Talquetamab in human milk is unknown. Patients should not breastfeed during Talquetamab treatment or for at least three months following the final dosage because to the unknown risk of major adverse events in breastfed newborns.
Administration
Injectable solution for subcutaneous [10].
Storage
In refrigerators with temperatures between 2 and 8°C, as well as between 15 and 30°C, up to 24 hours of chemical and physical in-use stability have been proven. Avoid freezing. If kept at room temperature for longer than 24 hours or longer than 24 hours in a refrigerator, discard. To keep it safe from light, store it in its original packaging [10].
Conclusion
Talquetamab treatment moreover directed to reactions in patients who took prior T-cell–redirecting treatments aiming BCMA. These outcomes propose that an operative immune response can remain attached in contradiction of a diverse antigen even later former exposure and sickness evolution by T-cell therapy. The safety outline of talquetamab remained categorised through ADR related T-cell activation (e.g., CRS), by dermal noxious effects, parageusia, in addition nail noxious properties. These properties remained mostly of grade 1 or else 2, achieved by supportive carefulness, then intermittently dosage alterations, also irregularly directed towards management termination.
Declarations
• Ethics approval and consent to participate
Not Applicable
• Availability of data and material
The review work has been carried out by us and we assure you that it can be provide to you whenever required.
• Funding Not applicable
Authors’ contributions
DK analyzed various articles that were previously and reviewed them one by one and contributed in writing the review. VJ assisted with the writing as well as selecting an appropriate title.VM, Pallavi, PS contributed in the review of literature and helped in the review of literature and final corrections. We have assured that “all authors have read and approved the manuscript”. All the authors have equal contribution and participation in this research work
Acknowledgment
The Authors are thankful to VC Prof Amit Jain, Amity University Rajasthan, India and VC Prof. R.L Raina, Jaipur National University, Rajasthan, India for providing necessary facilities for completing this study.
Conflict of Interest
No conflict of interest.
References
- Landgren O, Nadeem O (2023) Bispecific Monoclonal Antibodies in Multiple Myeloma: Data from ASH 2022: A Podcast. Adv Ther 40(8): 3291-3303.
- Girgis S, Wang Lin SX, Pillarisetti K, Verona R, Vieyra D, et al. (2023) Effects of teclistamab and talquetamab on soluble BCMA levels in patients with relapsed/refractory multiple myeloma. Blood Adv 7(4): 644-648.
- Ajai Chari, Monique C Minnema, Jesus G Berdeja, Albert Oriol, Niels W C J van de Donk, et al. (2022) Talquetamab, a T-Cell-Redirecting GPRC5D Bispecific Antibody for Multiple Myeloma. The New England journal of medicine 387(24): 2232-2244.
- Ajai Chari, Elham Askari, Jo Caers, Luciano J Costa, Brandi W Hilder, et al. (2023) Plain language summary of the MonumenTAL-1 study of talquetamab in people with relapsed or refractory multiple myeloma. Future Oncol 19(27): 1823-1840.
- Narayan N, Williams B, Lipe B, De Benedetto A (2022) Onychomadesis and palmoplantar keratoderma associated with talquetamab therapy for relapsed and refractory multiple myeloma. JAAD Case Rep 31: 66-68.
- Zhao J, Ren Q, Liu X, Guo X, Yongping S (2023) Bispecific antibodies targeting BCMA, GPRC5D, and FcRH5 for multiple myeloma therapy: latest updates from ASCO 2023 Annual Meeting. J HematolOncol 16(1): 92.
- Christie P M Verkleij, Marloes E C Broekmans, Mark van Duin, Kristine A Frerichs, Rowan Kuiper, et al. (2021) Preclinical activity and determinants of response of the GPRC5DxCD3 bispecific antibody talquetamab in multiple myeloma. Blood Adv 5(8): 2196-2215.
- Kaplon H, Crescioli S, Chenoweth A, Visweswaraiah J, Janice M Reichert. (2023) Antibodies to watch in 2023. Mabs 15(1): 2153410.
- (2023) Europa Eu (N.d.) from https://www.ema.europa.eu/en/documents/product-information/talvey-epar-product-information_en.pdf
- (2023) Talvey (talquetamab) dosing, indications, interactions, adverse effects, and more.
-
Dhruv Kumawat, Vartika Jain*, Vijaya Mishra, Pallavi and Pragya Sharma*. Innovative Approaches with Talquetamab in Recurrent Multiple Myeloma. Arch Phar & Pharmacol Res. 4(5): 2024. APPR.MS.ID.000597.
-
Talquetamab, Leukocytes, Multiple Myeloma, B Cell Maturation Antigen, Cytokine Release Syndrome
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






