 Research Article
Research Article
Ideal Body Weight-Based Dosing of Rabbit Anti- Thymocyte Globulin in Obese Kidney Transplant Recipients: Cutting Costs Without Adding Risk
Hayley P Harrington1, Amy H White2, Emily G Morgan3, Allison N Wells4 and Darby A Derringer5*
1Department of Pharmacy, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA
2Department of Pharmacy, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA
3Department of Pharmacy, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA
4Department of Transplant, Biostatistics, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA
5Department of Pharmacy, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA
Darby Derringer, Department of Pharmacy, University of Arkansas for Medical Sciences, Little Rock, Arkansas, USA
Received Date:August 08, 2024; Published Date:October 02, 2024
Abstract
Obesity among kidney transplant recipients is becoming increasingly prevalent. It is important to ensure the same quality of immunosuppression in this patient population. Rabbit anti- thymocyte globulin (rATG) is utilized to reduce acute rejection rates at the time of kidney transplantation by depleting lymphocytes. Optimal dosing to limit cost and toxicity and maximize immunosuppression is not well defined. The aim of this study was to compare the rejection rates in obese and non-obese kidney transplant recipients receiving rATG dosed based on IBW. This study was a single center, retrospective chart review of adult kidney transplant recipients who received rATG induction therapy between September 2016 and December 2019. The primary outcome was a composite of the incidence of biopsy proven rejection and presumed rejection post-transplant in patients with a body mass index (BMI) <30 kg/m2 compared to those with a BMI ≥30 kg/m2. A total of 86 kidney transplant recipients were included in this study. Rejection was present in 11 of 42 patients with a BMI <30 kg/m2 and 9 of 44 patients with a BMI ≥30 kg/m2 (p = 0.529). These results suggest that dosing rATG on IBW in obese patients does not increase rates of rejection compared to non-obese patients.
Keywords:Kidney Transplantation, Thymoglobulin, Obesity, Graft Rejection
Abbreviations:BMI: Body Mass Index, CIT: Cold Ischemia Time, CMV: Cytomegalovirus, DCD: Donation After Cardiac Death, DGF: Delayed Graft Function, DRG: Diagnosis Related Group, DSA: Donor Specific Antibody, eGFR: Estimated Glomerular Filtration Rate, ESRD: End-Stage Renal Disease, HLA: Human Leukocyte Antigen, IBW: Ideal Body Weight, IV: Intravenous, KDPI: Kidney Donor Profile Index, MFI: Mean Fluorescence Intensity, PCR: Polymerase Chain Reaction, PJP: Pneumocystis Jiroveci, PRA: Panel Reactive Antibody, rATG: Rabbit Anti-Thymocyte Globulin, TBW: Total Body Weight
Introduction
Induction therapy is utilized to reduce acute rejection rates at the time of organ transplantation by either depleting or modulating lymphocyte response [1]. Rabbit anti-thymocyte globulin (rATG), the most commonly used agent for induction immunosuppression in kidney transplant, works by depleting T lymphocytes [2,3]. The 2009 KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients recommends using lymphocyte-depleting agents, like rATG, in kidney transplant recipients with high immunologic risk [1]. Induction therapy is known to be costly, and lymphocyte depleting agents are known to be associated with increased risk of infection and malignancy [4]. The recommended dosing of rATG for the prophylaxis of acute rejection is 1.5 mg/ kg/day intravenously (IV) for 4 to 7 days [5]. The package insert specifies weight-based dosing, however, does not state which type of weight to use [5]. Due to the cost and risks associated with rATG, clinicians have studied several dosing methods to limit cost and toxicity and maximize immunosuppression.
Studies have shown that lower doses of rATG used for kidney transplant induction therapy results in cost savings without increasing the risk of rejection in the general population [4,6]. A study published in 1996 by Bunn and colleagues demonstrated that rATG does not distribute largely into adipose tissue [7]. Following this discovery, researchers began investigating utilization of ideal body weight (IBW) to calculate rATG dose instead of total body weight (TBW) [2]. One study by Vacha and colleagues evaluated the safety and efficacy of using IBW versus TBW when dosing rATG [2]. The rates of biopsy proven rejection and the safety profiles, including viral and fungal infections, were similar during the evaluation periods for both cohorts. Mean BMI was not compared between groups, however the mean TBW was 86.4 kg and 75.7 kg in the IBW and TBW groups respectively. This study also found that the median cost of dosing patients using IBW was lower, though this difference was not statistically significant. A second study by Bubik and colleagues looked at rejection rates when comparing rATG dosing based on IBW versus TBW [4]. There was no statistically significant difference between the two dosing methods and the IBW dosing saved approximately $2,500 per course. The results from these studies support the use of IBW for dosing rATG in non-obese patients, but there is currently not adequate data to support using IBW, TBW, or adjusted body weight based rATG dosing in obese patients. In 2016, our institution started dosing rATG based on IBW in all kidney transplant recipients. With obesity becoming more prevalent among those patients awaiting a kidney transplantation, there is concern that obese patients may experience an increased rate of rejection due to lower total exposure to rATG. The aim of this study is to compare the rate of rejection in obese and non-obese patients receiving rATG dosed using IBW.
Materials and Methods
Study Design
This study was a single center, retrospective chart review of adult patients who received a kidney transplant between September 2016 and December 2019 at an academic medical center. To be included in this study, patients must have received a rATG for induction and be 18 years of age or older. Patients were excluded if they underwent transplant surgery at an outside facility, received a multiorgan transplant, or their cumulative rATG dose was less than 5 mg/kg based on IBW. This study was approved by the Institutional Review Board.
Protocol induction immunosuppression at our institution during the study period was rATG 1.5 mg/kg daily for 4 days for high-risk recipients (panel reactive antibody (PRA) >80%, cold ischemia time (CIT) >36 hours, donation after cardiac death (DCD), Black patients <60 years old, and/or donor specific antibody (DSA) with mean fluorescence intensity (MFI) >1,000) and basiliximab for low-risk recipients (PRA <80% not meeting criteria for high risk). Starting in October 2018, alemtuzumab was used in place of rATG for high-risk recipients for cost savings if available. Starting in April 2019, total rATG doses were decreased in a patient specific manner based on provider discretion. Patients receiving <5 mg/ kg of rATG were excluded as providers decreased total doses for patients they considered to be at a lower risk of rejection. Protocol initial maintenance immunosuppression for all patients was tacrolimus, mycophenolate mofetil, and prednisone. Maintenance immunosuppression was then tailored to patient specific needs based on provider discretion. All patients received 1 year of pneumocystis jiroveci (PJP) prophylaxis with sulfamethoxazoletrimethoprim 400/80 mg oral daily (unless allergy or intolerance; alternative agents included atovaquone, dapsone, and inhaled pentamidine).
Cytomegalovirus (CMV) prophylaxis was stratified based on donor and recipient risk, with high-risk patients (D+/R-) receiving 6 months and intermediate risk (D-/R+, D+/R+) receiving 3 months of renally dosed valganciclovir. Low risk patients (D-/R-) received 3 months of renally dosed acyclovir. All patients received fungal prophylaxis with 10 days of nystatin. Patients were monitored monthly post-transplant for BK viruria. A positive value would trigger BK viremia monitoring. CMV was not routinely monitored, and polymerase chain reaction (PCR) testing was only checked with clinical suspicion for CMV infection or disease.
Outcome Measures
The primary outcome was a composite of the incidence of biopsy proven rejection and presumed rejection at 1-, 3-, 6-, and 12-months post-transplant. A patient was considered to have experienced rejection if they received treatment for acute rejection (presumed rejection) or had an allograft biopsy diagnostic for rejection. Biopsy results documented as borderline were also considered rejection. Rejection was graded by a pathologist using the most up to date Banff Criteria at the time of the allograft biopsy [8]. Secondary outcomes included mortality rates at 6- and 12-months posttransplant, estimated glomerular filtration rate (eGFR) calculated using CKD-EPI at 6- and 12-months post-transplant, incidence of polyomavirus (BK virus), CMV, and urinary tract infections at 3, 6, and 12 months post-transplant, leukopenia and thrombocytopenia at post-operative day 4, and the incidence of biopsy proven rejection in patients with a BMI <25 kg/m2 versus those a BMI ≥35 kg/m2 at 1, 3, 6, and 12 months post- transplant. BK virus and CMV infection were defined as any detectable value. The lower limit of detection for BK plasma was 39 copies/mL, BK urine was 500 copies/mL, and CMV was 300 IU/mL. Leukopenia was defined as white blood cell count less than 3 K/μL. Thrombocytopenia was defined as platelets less than 75 K/μL. Thrombocytopenia and leukopenia thresholds were chosen based on dose adjustment recommendations provided by the rATG package insert [5]. The outcomes of patients receiving rATG with a BMI <30 kg/m2 (non-obese) were compared to those of patients with a BMI ≥30 kg/m2 (obese) for most outcomes. Cost savings were evaluated. The average wholesale price was compared between rATG actual dose based on IBW and the expected dose based on TBW to calculate a percent cost reduction.
Data Collection
Patients were identified using a registry of kidney transplant recipients maintained by the kidney transplant department at our institution. Data collected for baseline characteristics included age at time of transplant, sex, race, height, weight, BMI, cause of end-stage renal disease (ESRD), cumulative dose of rATG (mg), donor organ status, re-transplant status, CIT, human leukocyte antigen (HLA) mismatch, presence of donor-specific antibodies (DSA) at time of transplant, kidney donor profile index (KDPI), panel reactive antibody (PRA), occurrence of delayed graft function (DGF), donor/recipient CMV risk status, and planned maintenance immunosuppressants. DGF was defined using the United Network for Organ Sharing definition of need for at least one dialysis treatment in the first week after kidney transplant [9].
Additional information collected that was needed to evaluate the primary and secondary outcomes included the date of organ transplant, date of death, serum creatinine at 6- and 12- months post-transplant, and biopsy results. This data was collected manually via chart review of electronic medical records and entered stored in the University of Arkansas for Medical Sciences secure REDCap database (Research Electronic Data Capture, Version 12.0.12) [10].
Statistical Analysis
All data was tested for normality. When comparing the demographic and clinical information among BMI groups, a Pearson’s Chi-square test or Fisher’s exact test were utilized to analyze categorical variables. Normally distributed continuous data were compared using an independent t-test. Non-normally distributed continuous data were compared using a Mann- Whitney U test. For the primary outcome, a time to event analysis was performed. The event of interest being 12-month rejection status and time being days from transplant. Patients were censored after 12 months and those who experienced rejection before the cutoff were counted as events. The Kaplan-Meier method was used to produce two curves, one for obese patients and one for non-obese patients in order to compare the probability of rejection based on this status. A log rank test was conducted to determine if the curves differed significantly. A two- sided P value of <0.05 was considered statistically significant. Two statistical software were utilized for analysis - IBM® SPSS® Statistics version 28 (Armonk, NY) and R Core Team version 4.1.0 (Vienna, Austria) [11, 12].
Results
Baseline Characteristics
A total of 335 patients received a kidney transplant at the investigational site between September 1, 2016, and December 31, 2019. Of these patients, 147 were excluded for not receiving rATG for induction immunosuppression and 102 were excluded for receiving <5 mg/kg of rATG dosed based on IBW (Figure 1). Eighty-six patients met the inclusion criteria. Forty-four patients had a BMI ≥30 kg/m2 and were included in the obese patient group. The baseline characteristics for the two groups were similar except age at time of transplant, CIT, and DGF (Table 1). The majority of patients were Black, developed ESRD secondary to diabetes, and had tacrolimus, mycophenolate, and prednisone as their planned maintenance immunosuppression. As expected, weight and BMI were higher and average dose of rATG by TBW was lower in the obese group.
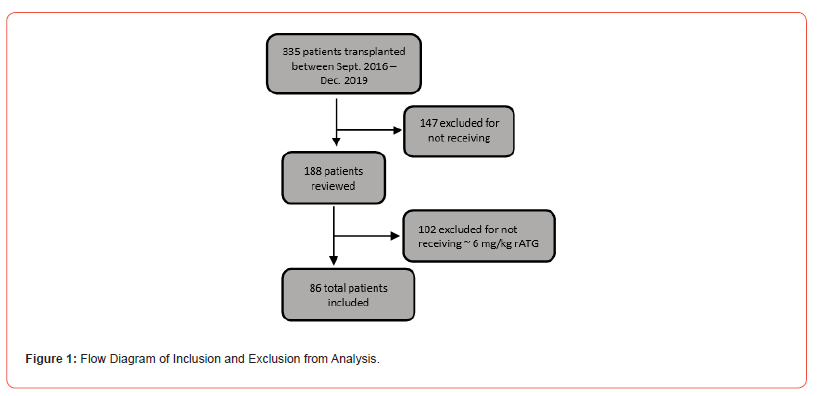
Table 1:Baseline Characteristics
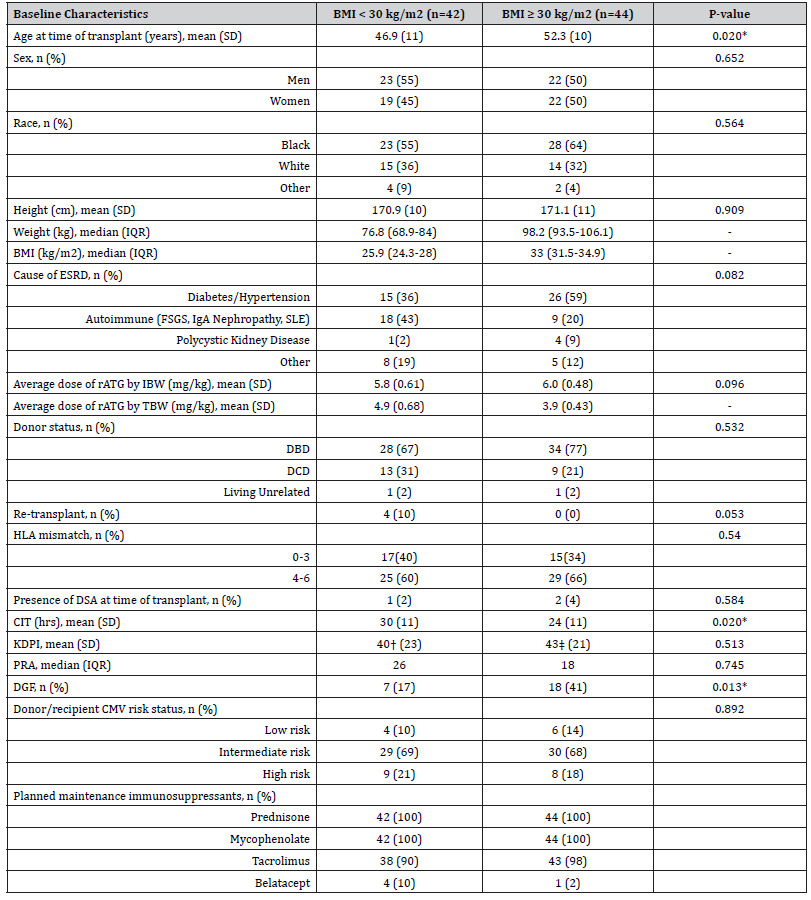
*Denotes Clinical Significance
†n=41 (excludes 1 living unrelated donor)
‡n=43 (excludes 1 living unrelated donor)
Abbreviations: SD - Standard Deviation | IQR - Interquartile Range | BMI - Body Mass Index | ESRD - End Stage Renal Disease | rATG - Rabbit
Anti-Thymocyte Globulin | IBW - Ideal Body Weight | TBW - Total Body Weight | DBD - Donation After Brain Death | DCD – Donation After Cardiac
Death | HLA – Human Leukocyte Antigen | DSA - Donor Specific Antibody | CIT - Cold Ischemia Time | KDPI - Kidney Donor Profile Index | PRA -
Panel Reactive Antibody | DGF - Delayed Graft Function | CMV – Cytomegalovirus
Outcomes
There was no difference in the probability of rejection at any time point between groups as shown in Figure 2 (p=0.84). An initial rejection episode at 12 months occurred in six patients in the non-obese group compared to seven in the obese group (14% vs 16%, p=0.834; Table 2). All rejection episodes were biopsy proven. Four rejection episodes in each group were classified by the pathologist as borderline according to Banff criteria. Although numerically higher, there was no statistical difference in mortality at 12 months between the two groups as shown in Table 3 (5% non-obese vs 0% obese, p=0.143). There was no difference in rate of thrombocytopenia or leukopenia on post-operative day 4 between groups (Table 3). When comparing rates of infection, the occurrence was similar at all time intervals for CMV, BK, and urinary tract infections (Table 3). There was a small improvement in eGFR between 6- and 12-months post- transplant, and this improvement was similar between groups (Table 3). Lastly, there was no difference in rejection rates in patients with a BMI <25 kg/ m2 or ≥35 kg/m2 when compared to the remainder of the patient population. Over the study period, by dosing rATG based on IBW instead of TBW, 474 vials of rATG were saved leading to a 26% reduction in cost associated with rATG.
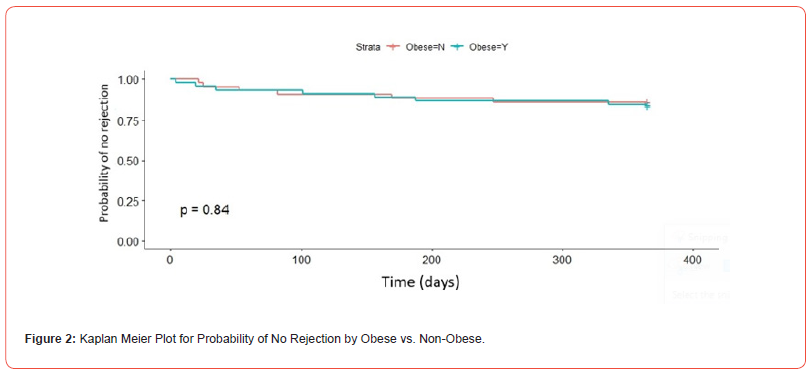
Table 2:Primary Outcome Results
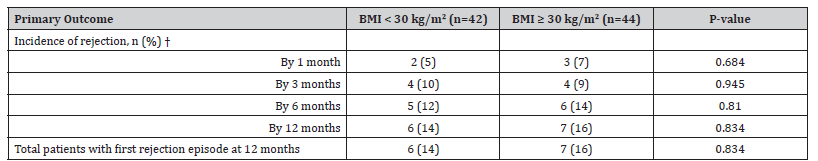
†Rejection episodes are additive over time (previous rejections were counted in next time frame)
Abbreviations: BMI - Body Mass Index
Table 3:Results for Secondary Outcomes
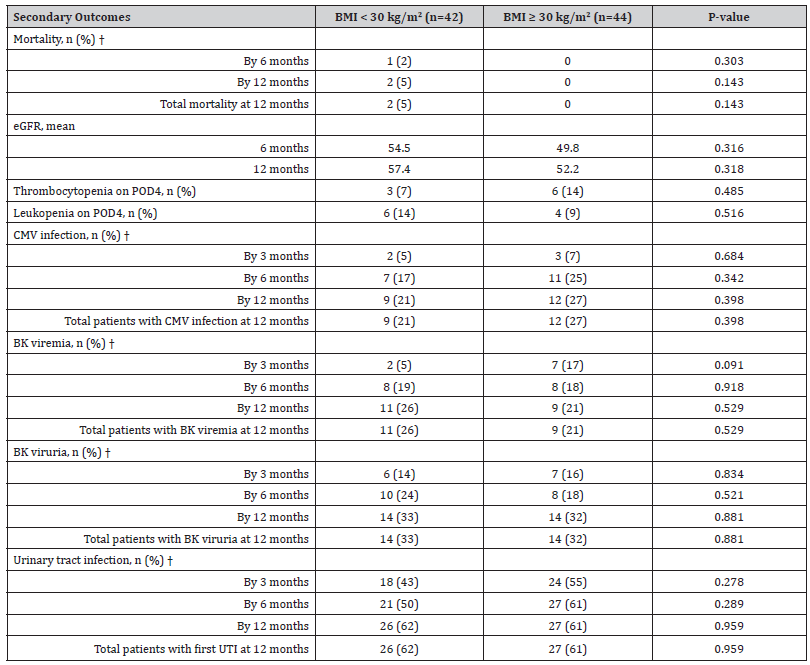
Abbreviations: eGFR - Estimated Glomerular Filtration Rate | CMV - Cytomegalovirus | UTI - Urinary Tract Infection †Mortality, CMV infection, BK viremia, BK viruria, and UTI are additive over time (previous episodes were counted in next time frame).
Discussion
To our knowledge, this study was the first to investigate whether dosing rATG based on IBW in obese patients increases risk of rejection. Overall, the two study groups had similar baseline characteristics. Mean age was statistically higher in the obese group; however, this is not likely to be clinically significant. CIT, a known predictor of DGF, was higher in the non-obese group, but rates of DGF were significantly higher in the obese group [13]. Obese patients have higher baseline levels of inflammatory markers IL-6 and TNF-alpha [14]. This increased tendency toward inflammation could predispose obese patients to an increased risk of DGF compared to their non-obese counterparts, although, more studies are needed to verify this finding. As expected, the average dose of rATG based on TBW for the obese group was significantly lower than the non-obese group. Despite this difference, obese patients did not have an increased rate of rejection compared to non-obese patients at any time point. These results suggest that a lower total exposure to rATG does not increase rejection risk in obese kidney transplant recipients. The majority of rejection episodes in both groups were graded as borderline by the pathologist.
Despite the non-obese group receiving higher dose of rATG per kilogram, there was no difference in the rate of thrombocytopenia or leukopenia at post-operative day 4. Thrombocytopenia and leukopenia are common reasons to decrease or hold rATG doses leading to prolong hospital stays. Given these rates were similar between groups, it is likely that there was no difference in the rate of prolonged admission due to delayed rATG administration from myelosuppression. Infection commonly leads to reduction in immunosuppression, potentially increasing risk of rejection. Given infection rates were similar between groups, this variable did not impact the results of the primary outcome. eGFR was also similar between obese and non-obese patients. This suggests that long term graft function is preserved despite increased rates of DGF in the obese population. Two patients died during the study period, both in the non-obese group. The cause of death was identified as cryptococcal meningitis in one patient and was unknown in the second. Cryptococcal meningitis is an opportunistic infection commonly associated with immunosuppression, however mortality rates between the two groups were not statistically significant. When compared to other studies investigating use of IBW to dose rATG in kidney transplant recipients, our primary and secondary outcome results appear to align with previous findings.
Cost savings is a priority given that healthcare institutions are reimbursed for hospitalizations based on diagnosis related groups (DRG). Any money spent to care for a patient above the rate of reimbursement for the DRG is cost to the healthcare institution. Minimizing care costs allows for less overall cost to the facility and potentially leading to increased revenue. A reduction in rATG related cost of 26% is relevant given the high overall cost of the medication. However, it is difficult to quantify exact cost savings due to differences in purchase price based on patient location within the hospital and purchase prices differing between institutions.
A study in 2011 by Patel and colleagues compared the long-term effects of low dose rATG versus basiliximab in obese and non-obese patients [15]. Low dose rATG was defined as a total dose of 3-5 mg/ kg IV (based on TBW). The authors concluded that for non-obese patients, there was a statistically significant difference between basiliximab and rATG, with fewer rejections in the rATG group. For obese patients, the number of rejections was lower with the rATG group than the basiliximab group, but this did not reach statistical significance. Similar to our study, Patel and colleagues did not find a significant difference of infection rates or renal function between the groups.
Our study is limited by the non-randomized, retrospective, single-center design. The small sample size and low overall rate of rejection make it difficult to draw conclusions from this data. Specifically, the secondary outcomes looking at patients with a BMI <25 kg/m2 and ≥35 kg/m2, had a small number of patients with an uneven distribution between groups. This study also did not include dosing of or changes to maintenance immunosuppression agents, including therapeutic drug monitoring results or adherence rates. Given this study was retrospective in nature, the reliability of documentation of these changes is low and there is no way to determine whether therapeutic drug levels were accurate as drawn. The limited reliability of data evaluating therapeutic drug levels could skew conclusions drawn from the results and was subsequently not collected or analyzed. In summary, our study suggests that utilizing IBW to dose rATG in obese kidney transplant recipients does not increase rates of rejection compared to non-obese patients. These results further expand upon those seen in previously published articles. Further prospective studies, with larger sample sizes and monitoring of maintenance immunosuppression are needed to confirm the results of this study.
Author Contributions:
Hayley Harrington - Participated in research design, data
collection, data analysis, writing of the paper, and editing of the
paper
Amy White - Participated in research design, writing of the
paper, and editing of the paper
Emily Morgan - Participated in writing and editing of the paper.
Allison Wells - Participated in data analysis, writing of the
paper, and editing of the paper.
Darby Derringer - Participated in research design, data
collection, data analysis, writing of the paper, and editing of the
paper.
Funding
None.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group (2009) KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9(suppl 3): S1-155.
- Vacha M, Gommer J, Rege A, Sanoff S, Sudan D, et al. (2016) Effects of ideal versus total body weight dosage of rabbit antithymocyte globulin on outcomes of kidney transplant patients with high immunologic risk. Exp Clin Transplant 14(5): 511-517.
- Rajil B Mehta, Kristen Shimko, Xingyu Zhang, Chethan Puttarajappa, Christine Wu, et al. (2022) Rabbit antithymocyte globulin dose and early subclinical and clinical rejections in kidney transplantation. Clin Transplant 36(4): e14582.
- Bubik RJ, Peterson KT, Myhre LJ, Bernard SA, Dean P, et al. (2021) Ideal body weight-based dosing of rabbit antithymocyte globulin for cost minimization in kidney transplantation. Prog Transplant 31(2): 184-189.
- (2020) Thymoglobulin. Prescribing information. Genzyme Corporation.
- Singh N, Rossi AP, Savic M, Rubocki RJ, Parker MG, et al. (2018) Tailored rabbit antithymocyte globulin induction dosing for kidney transplantation. Transplant Direct 4(2): e343.
- Bunn D, Lea CK, Bevan DJ, Higgins RM, Hendry BM (1996) The pharmacokinetics of anti- thymocyte globulin (ATG) following intravenous infusion in man. Clin Nephrol 45(1): 29-32.
- Loupy A, Mengel M, Haas M (2022) Thirty years of the International Banff Classification for Allograft Pathology: the past, present, and future of kidney transplant diagnostics. Kidney Int 101(4): 678-691.
- Lim MA, Bloom RD (2020) Medical therapies to reduce delayed graft function and improve long-term survival: Are we making progress? Clin J Am Soc Nephrol 15(1): 13-15.
- (2022) REDCap. Version 12.0.12. Research Electronic Data Capture.
- (2022) SPSS. Version 28. International Business Machines Corporation.
- (2022) R Core Team. Version 4.1.0. R Foundation for Statistical Computing.
- Kawakita S, Beaumont JL, Jucaud V, Everly MJ (2020) Personalized prediction of delayed graft function for recipients of deceased donor kidney transplants with machine learning. Sci Rep 10(1): 18409.
- Ellulu MS, Patimah I, Khaza’ai H, Rahmat A, Abed Y (2017) Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci 13(4): 851-863.
- S Patel, O Pankewycz, R Kohli, M Said, M Alnimri, et al. (2011) Obesity in renal transplantation: the role of induction therapy on long-term outcomes. Transplant Proc 43(2): 469-471.
-
Hayley P Harrington, Amy H White, Emily G Morgan, Allison N Wells and Darby A Derringer*. Ideal Body Weight-Based Dosing of Rabbit Anti- Thymocyte Globulin in Obese Kidney Transplant Recipients: Cutting Costs Without Adding Risk. Arch Phar & Pharmacol Res. 4(4): 2024. APPR.MS.ID.000591.
-
Thymoglobulin, Obesity, Kidney Transplantation, Graft Rejection, Induction therapy, Rabbit anti-thymocyte globulin (rATG), Cytomegalovirus (CMV)
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






