 Review Article
Review Article
F-652 (Recombinant Human Interleukin-22) For Schizophrenia
Adonis Sfera1*, Nyla Jafri2 and Leah Rahman3
1Department of Psychiatry, Patton State Hospital, USA
2Department of Ecology and Evolutionary Biology, USA
3Department of Neuroscience, University of Oregon, USA
Adonis Sfera, Department of Psychiatry, Patton State Hospital, USA.
Received Date:March 15, 2023; Published Date:March 24, 2023
Introduction
Emil Kraepelin believed that dementia praecox, the disorder we now call schizophrenia, was the result of brain poisoning with toxins generated in other parts of the body, especially the mouth, intestine, or genitals [1]. In this regard, Kraepelin hinted at the mi crobiome and believed that microbial molecules could drive the pathogenesis of severe psychiatric illness. However, the infectious model of schizophrenia drew limited attention until the launching of Human Microbiome Project and the discovery of innate lymphoid cells (ILCs).
The gut and schizophrenia
Several recent studies have connected schizophrenia with microbial translocation (MT) from the gastrointestinal (GI) tract into the host systemic circulation, eventually reaching the brain [2-5]. MT refers to the migration of intestinal microorganisms or toxins into host tissues by passing through the intestinal barrier and lamina propria to reach mesenteric lymph nodes and the systemic circulation. In the central nervous system (CNS), bacteria or their components can trigger psychosis by several mechanisms, including inflammation, impaired autophagy, premature cellular senescence, and aberrant microglial activation [6-9].
The evidence of MT in schizophrenia
1. Patients with schizophrenia have a high prevalence of inflam
matory bowel diseases (IBD), such as ulcerative colitis and
Chron’s disease, conditions associated with increased gut barrier
permeability and MT [10-12].
2. Patients with schizophrenia present with elevated bacterial
translocation markers, including soluble CD14 (sCD14) and
lipopolysaccharide binding protein (LBP), emphasizing microbial
migration from the immune tolerant GI tract into the
bacteria-intolerant systemic circulation [13-15].
3. Patients with schizophrenia exhibit increased blood brain barrier
(BBB) permeability, enabling bacteria and/or toxins to ingress
the CNS [16-19].
4. The 2011 outbreak of Escherichia coli (E. coli) in Germany has
been associated with the new onset psychosis, connecting this
pathogen to neuropsychiatric illnesses [20-21]. In addition, E.
coli has been implicated in urinary tract infection (UTI), conditions
often associated with new onset psychosis or schizophrenia
exacerbation, linking this bacterium to psychopathology
[22-23].
Hypothesis
We hypothesize that F-652, a recombinant human interleukin- 22 (IL-22) can alleviate psychotic symptoms by blocking MT. In addition, IL-22/ F-652 share several properties with antipsychotic drugs as summarized in Table [1].
Table 1:IL-22/ F-652 comparison with antipsychotic drugs..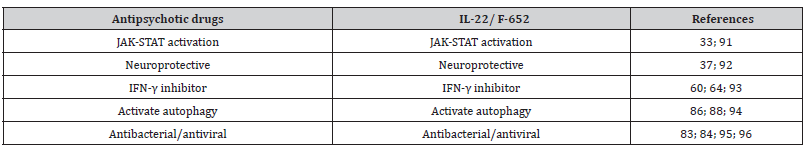
Interleukin-22, the “guardian” of gut barrier.
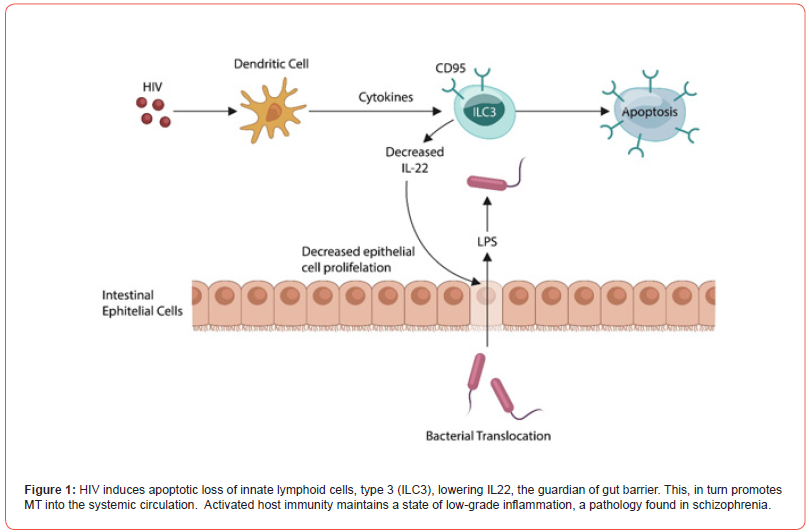
During the human immunodeficiency virus (HIV) epidemic in 1980s, MT drew the attention of researchers and clinicians as this virus depleted interleukin-22 (IL-22), enabling lipopolysaccharide (LPS) migration into host tissues [22-23] [Figure 1]. Indeed, patients with HIV are more likely to develop new onset psychosis compared to the general population, suggesting that dysfunctional gut barrier may play a role in pathology [24-29]. Discovered in 2000, IL-22 is a member of IL-10 family generated by several lymphocyte types, including T helper (Th) 17 cells, γδ T cells, NKCs, and innate lymphoid cells (ILCs) [30]. IL-22 controls several intestinal epithelial cells (IEC) functions, including mucus formation, permeability, synthesis of both complement and antimicrobial peptides (AMPs), indicating that this cytokine functions as the master regulator of gut barrier permeability [31].
The crosstalk between IL-22 and its receptor (IL-22R), a dimeric protein comprised of IL-22R1 and IL-10R2, activates the JAK/ STAT pathway, a key antibacterial and antiviral system, protecting against pathogens [32-33]. As IL22R contains IL-10R2, it can be cross-activated by IL-10, a cytokine previously connected to schizophrenia [34-36]. In addition, several studies have shown that IL-22 possesses neuroprotective properties, and its disruption has been associated with schizophrenia [37-39]. Moreover, aside from safeguarding neuronal cells, IL-22 protects against COVID-19, influenza, and IBD, indicating that supplementation with this cytokine via F-652 may ameliorate or totally alleviate these pathologies [39-42].
IL-22 and innate lymphoid cells (ILCs)
Novel studies have reported that a subgroup of NKCs produce IL-22, enhancing the gut barrier thus, averting MT [43-45]. Indeed, research over the past two decades has connected defective NKCs with both IBD and schizophrenia [46-49]. Moreover, in the CNS, NKCs participate in adult neurogenesis, cognition, and cellular senescence, suggesting that under pathological circumstances, these processes may be disrupted [50-53].
NKCs belong to innate lymphoid cells (ILCs), mucosa-anchored non-T, non-B lymphocytes, which also include ILC-1, ILC-2, and ILC- 3 [52] [Figure 2]. These systems play a pivotal role in gut barrier homeostasis, nutrient transport, and immune tolerance of both food proteins and gut commensals [52-53]. The ILCs in CNS have been implicated in neuropathology, including schizophrenia, major depressive disorder (MDD) and neurodegeneration [55-57].
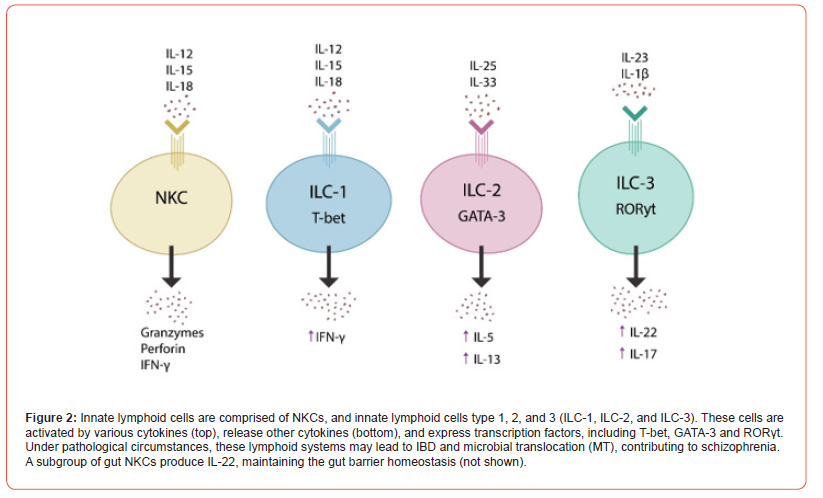
For example, aberrant activation of NKCs by IL-12, IL-15, and IL- 18 was reported in IBD and schizophrenia, linking ILCs to both conditions [58-59]. In addition, NKCs-generated interferon-γ (IFN-γ) has been linked to schizophrenia, emphasizing the key role of ILCs in neuropathology. On the other hand, conventional antipsychotic drugs have been shown to suppress IFN-γ, suggesting that at least in part, IL-22 may contribute to the pathogenesis of schizophrenia [60-62]. Indeed, dysfunctional IFN-γ was associated with the loss of dopaminergic neurons in Parkinson’s disease (PD), connecting ILCs to dopamine pathology [63]. Like antipsychotic agents, IL-22 is an IFN-γ inhibitor, suggesting a mechanism through which F-652 may ameliorate the schizophrenia symptoms [64]. Taken together, dysfunctional ILCs, including NKCs, connect IBD with schizophrenia. In contrast, IL-22 (and F-652) likely exhibit antipsychotic properties not only by lowering MT, restoring the integrity of intestinal barrier, but also by suppressing IFN-γ.
Psychosis and gut microbes
The BBB regulates CNS entry of peripheral molecules, including gut microbes and toxins, by being comprised of specialized endothelial cells kept together by tight junctions. Interestingly, experimental colitis has been shown to increase BBB permeability, indicating a close crosstalk between the gut barrier and BBB that under pathological circumstances may enable intestinal microbes to reach the brain [65].
The link between gut bacteria and schizophrenia has been documented for several decades. For example, antibodies against various E. coli proteins were detected in patients with schizophrenia but not in healthy controls [66]. In addition, schizophrenia and colibactin, an E. coli toxin, were demonstrated to trigger premature cellular senescence and thymic dysfunction [67-70]. On the other hand, IL-22 drives the regeneration of the thymus, restoring the ho meostasis of senescent cells and, according to one study induces rejuvenation [71-73].
Several gut and urinary tract microbes, including E. coli, were associated with schizophrenia, while IL-22 was shown to neutralize this pathogen [74-78]. Other gut microbes, including Hafnei alvei, Pseudomonas aeruginosa, Pseudomonas putida, and Klebsiella pneumoniae have been associated with schizophrenia, further substantiating the infectious paradigm in this disorder [79-80].
Taken together, intestinal inflammation can alter the BBB, enabling microbes, including E. coli, to enter the CNS. Antibodies against microbial antigens (which often mimic human proteins) trigger pathology in distant organs, including the brain. IL-22 opposes MT by lowering gut barrier permeability and by enhancing thymic function to strengthen host immune defenses.
IL-22 safety, pharmacokinetic and pharmacodynamic data
F-652 is a recombinant human IL-22 currently in Phase II clinical trials for the treatment of COVID-19, COVID-19 pneumonia, acute pancreatitis, chronic acute liver failure, alcoholic hepatitis, and graft versus host disease (GVHD)(NCT02406651). Several studies have evaluated the safety, pharmacokinetics, pharmacodynamics, and tolerability of F-652, demonstrating that this is a safe compound with favorable pharmacological parameters [81-82].
Aside from functioning as the guardian of intestinal barrier, IL-22 possesses antibacterial and antiviral properties probably by enhancing autophagy [83-85]. Impaired autophagy has been documented in both schizophrenia and IBD, while IL-22 (or F-652) enhances autophagy, a property of many antipsychotic drugs, including clozapine [85-89]. Moreover, like antipsychotics, F-652 lowers INF-γ and protects intestinal barrier against IBD and MT [89-94].
Conclusions
Recombinant Human IL-22 is an antibacterial and antiviral molecule that promotes tissue repair and autophagy. Like antipsychotic drugs, F-652 suppresses INF-γ, promoting neuroprotection. In addition, F-652 drives thymic regeneration, opposing premature cellular and immune senescence. In clinical trials, F-652 decreased serum triglycerides, correcting dyslipidemia (often found in patients with schizophrenia), and presented with anti-inflammatory properties, warranting further evaluation for schizophrenia.
Acknowledgement
None.
Conflict of Interest
None.
References
- Richard Noll (2007) Kraepelin's 'lost biological psychiatry'? Autointoxication, organotherapy and surgery for dementia praecox. Hist Psychiatry 18(71 Pt 3): 301-320.
- Chong W, T Zhang, Lei He, Ji-Yong Fu, Hong-Xin D, et al. (2021) Bacterial Translocation Associates with Aggression in Schizophrenia Inpatients. Front Syst Neurosci 15: 704069.
- Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, et al. (2013) Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res 148(1-3): 130-137.
- Feng Zhu, Yanmei Ju, Wei Wang, Qi Wang, Ruijin Guo, et al. (2020) Metagenome-wide association of gut microbiome features for schizophrenia. Nat Commun 11: 1612.
- Dickerson F, Severance E, Yolken R (2017) The microbiome, immunity, and schizophrenia and bipolar disorder. Brain Behav Immun 62: 46-52.
- Sibony M, Abdullah M, Greenfield L, Raju D, Wu T, et al. (2015) Microbial Disruption of Autophagy Alters Expression of the RISC Component AGO2, a Critical Regulator of the miRNA Silencing Pathway. Inflamm Bowel Dis (12): 2778-2786.
- Secher T, Samba-Louaka A, Oswald E, Nougayrède JP (2013) Escherichia coli producing colibactin triggers premature and transmissible senescence in mammalian cells. PLoS One 8(10): e77157.
- Franchi L, Muñoz-Planillo R, Núñez G (2012) Sensing and reacting to microbes through the inflammasomes. Nat Immunol 13(4): 325-332.
- Fernández-Arjona MDM, Grondona JM, Fernández-Llebrez P, López-Ávalos MD (2019) Microglial activation by microbial neuraminidase through TLR2 and TLR4 receptors. J Neuroinflammation 16(1): 245.
- Sung KY, Zhang B, Wang HE, Bai YM, Tsai SJ, et al. (2022) Schizophrenia and risk of new-onset inflammatory bowel disease: a nationwide longitudinal study. Aliment Pharmacol Ther 55(9): 1192-1201.
- Bernstein CN, Hitchon CA, Walld R, Bolton JM, Sareen J, et al. (2019) CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Increased Burden of Psychiatric Disorders in Inflammatory Bowel Disease. Inflamm Bowel Dis 25(2): 360-368.
- Marrie RA, Walld R, Bolton JM, Sareen J, Walker JR, et al. (2017) CIHR Team in Defining the Burden and Managing the Effects of Psychiatric Comorbidity in Chronic Immunoinflammatory Disease. Increased incidence of psychiatric disorders in immune-mediated inflammatory disease. J Psychosom Res 101: 17-23.
- Szeligowski T, Yun AL, Lennox BR, Burnet PWJ (2020) The Gut Microbiome and Schizophrenia: The Current State of the Field and Clinical Applications. Front Psychiatry 11: 156.
- Najjar S, Pahlajani S, De Sanctis V, Stern JNH, Najjar A, et al. (2017) Neurovascular Unit Dysfunction and Blood-Brain Barrier Hyperpermeability Contribute to Schizophrenia Neurobiology: A Theoretical Integration of Clinical and Experimental Evidence. Front Psychiatry 8: 83.
- S Puvogel, A Alsema, L Kracht, MJ Webster, CS Weickert, et al. (2022) Single-nucleus RNA sequencing of midbrain blood-brain barrier cells in schizophrenia reveals subtle transcriptional changes with overall preservation of cellular proportions and phenotypes. Mol Psychiatry 27: 4731-4740.
- Greene C, Hanley N, Campbell M (2020) Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry 10(1): 373.
- Cheng Y, Wang T, Zhang T, Yi S, Zhao S, et al. (2022) Increased Blood-Brain Barrier Permeability of the Thalamus Correlated with Symptom Severity and Brain Volume Alterations in Patients with Schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging 7(10): 1025-1034.
- Kleimann A, Toto S, Eberlein CK, Kielstein JT, Bleich S, et al. (2014) Psychiatric symptoms in patients with Shiga toxin-producing E. coli O104:H4 induced haemolytic-uraemic syndrome. PLoS One 9(7): e101839.
- Wiwanitkit V (2012) Psychosis and E. coli Infection: A Forgotten Issue. Indian J Psychol Med 34(4): 407-408.
- Graham KL, Carson CM, Ezeoke A, Buckley PF, Miller BJ, et al. (2014) Urinary tract infections in acute psychosis. J Clin Psychiatry 75(4): 379-85.
- Buchholz U, Bernard H, Werber D, Böhmer MM, Remschmidt C, et al. German outbreak of Escherichia coli O104:H4 associated with sprouts. N Engl J Med 365(19): 1763-1770.
- Sandler NG, Douek DC (2012) Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol 10(9): 655-666.
- Kim CJ, Nazli A, Rojas OL, Chege D, Alidina Z, et al. (2012) A role for mucosal IL-22 production and Th22 cells in HIV-associated mucosal immunopathogenesis. Mucosal Immunol (6): 670-680.
- Harris MJ, Jeste DV, Gleghorn A, Sewell DD (1991) New-onset psychosis in HIV-infected patients. J Clin Psychiatry 52(9): 369-376.
- de Ronchi D, Faranca I, Forti P, Ravaglia G, Borderi M, et al. (2000) Development of acute psychotic disorders and HIV-1 infection. Int J Psychiatry Med 30(2): 173-183.
- Alciati A, Fusi A, D'Arminio Monforte A, Coen M, Ferri A, et al. (2001) New-onset delusions and hallucinations in patients infected with HIV. J Psychiatry Neurosci 26(3): 229-234.
- Sewell DD (1996) Schizophrenia and HIV. Schizophr Bull 22(3): 465-473.
- Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, et al. (2012) Interleukin-22 drives endogenous thymic regeneration in mice. Science 336(6077): 91-95.
- Keir M, Yi Y, Lu T, Ghilardi N (2020) The role of IL-22 in intestinal health and disease. J Exp Med 217(3): e20192195.
- Arshad T, Mansur F, Palek R, Manzoor S, Liska V, et al. (2020) A Double Edged Sword Role of Interleukin-22 in Wound Healing and Tissue Regeneration. Front Immunol 11: 2148.
- Ezeonwumelu IJ, Garcia-Vidal E, Ballana E (2021) JAK-STAT Pathway: A Novel Target to Tackle Viral Infections. Viruses 13(12): 2379.
- Perusina Lanfranca M, Lin Y, Fang J, Zou W, Frankel T, et al. (2016) Biological and pathological activities of interleukin-22. J Mol Med (Berl) 94(5): 523-534.
- Fu G, Zhang W, Dai J, Liu J, Li F, et al. (2019) Increased Peripheral Interleukin 10 Relate to White Matter Integrity in Schizophrenia. Front Neurosci 13: 52.
- Kapelski P, Skibinska M, Maciukiewicz M, Pawlak J, Zaremba D, et al. (2016) Family-based association study of interleukin 10 (IL10) and interleukin 10 receptor alpha (IL10RA) functional polymorphisms in schizophrenia in Polish population. J Neuroimmunol 297: 92-7.
- Mattapallil MJ, Kielczewski JL, Zárate-Bladés CR, St Leger AJ, Raychaudhuri K, et al. (2019) Interleukin 22 ameliorates neuropathology and protects from central nervous system autoimmunity. J Autoimmun 102: 65-76.
- Rachel Caspi, Mary Mattapallil, Rachael Rigden, Carlos Zarate-Blades, Phyllis Silver, et al. (2015) Neuroprotective effects of IL-22 during CNS inflammation (CCR4P.203). J Immunol 194 (1_Supplement): 118.3.
- Subbanna M, Shivakumar V, Talukdar PM, Narayanaswamy JC, Venugopal D, et al. (2018) Role of IL-6/RORC/IL-22 axis in driving Th17 pathway mediated immunopathogenesis of schizophrenia. Cytokine 111: 112-118.
- Barthelemy A, Sencio V, Soulard D, Deruyter L, Faveeuw C, et al. (2018) Interleukin-22 Immunotherapy during Severe Influenza Enhances Lung Tissue Integrity and Reduces Secondary Bacterial Systemic Invasion. Infect Immun 86(7): e00706-17.
- Albayrak N, Orte Cano C, Karimi S, Dogahe D, Van Praet A, et al. (2022) Distinct Expression Patterns of Interleukin-22 Receptor 1 on Blood Hematopoietic Cells in SARS-CoV-2 Infection. Front Immunol 13: 769839.
- Li LJ, Gong C, Zhao MH, Feng BS (2014) Role of interleukin-22 in inflammatory bowel disease. World J Gastroenterol 20(48): 18177-18188.
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, et al. (2009) A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity 457(7230): 722-725.
- Colonna M (2009) Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity 31(1): 15-23.
- Kumar P, Thakar MS, Ouyang W, Malarkannan S (2013) IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol 6(1): 69-82.
- Zaiatz Bittencourt V, Jones F, Tosetto M, Doherty GA, Ryan EJ, et al. (2021) Dysregulation of Metabolic Pathways in Circulating Natural Killer Cells Isolated from Inflammatory Bowel Disease Patients. J Crohns Colitis 15(8): 1316-1325.
- Fernandez-Egea E, Vértes PE, Flint SM, Turner L, Mustafa S, et al. (2016) Peripheral Immune Cell Populations Associated with Cognitive Deficits and Negative Symptoms of Treatment-Resistant Schizophrenia. PLoS One 11(5): e0155631.
- N Tarantino, Leboyer M, Bouleau A, Hamdani N, Richard JR, et al. (2021) Natural killer cells in first-episode psychosis: an innate immune signature? Mol Psychiatry 26(9): 5297-5306.
- Steel AW, Mela CM, Lindsay JO, Gazzard BG, Goodier MR, et al. (2011) Increased proportion of CD16(+) NK cells in the colonic lamina propria of inflammatory bowel disease patients, but not after azathioprine treatment. Aliment Pharmacol Ther 33(1):115-126.
- Sedgwick AJ, Ghazanfari N, Constantinescu P, Mantamadiotis T, Barrow AD, et al. (2020) The Role of NK Cells and Innate Lymphoid Cells in Brain Cancer. Front Immunol 11: 1549.
- Jin WN, Shi K, He W, Sun JH, Van Kaer L, et al. (2021) Neuroblast senescence in the aged brain augments natural killer cell cytotoxicity leading to impaired neurogenesis and cognition. Nat Neurosci 24(1): 61-73.
- Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, et al. (2018) Innate Lymphoid Cells: 10 Years On. Cell 174(5): 1054-1066.
- Artis D, Spits H (2015) The biology of innate lymphoid cells. Nature 517(7534): 293-301.
- Fan H, Wang A, Wang Y, Sun Y, Han J, et al. (2019) Innate Lymphoid Cells: Regulators of Gut Barrier Function and Immune Homeostasis. J Immunol Res: 2525984.
- Borovcanin MM, Minic Janicijevic S, Jovanovic IP, Gajovic NM, Jurisevic MM, et al. (2020) Type 17 Immune Response Facilitates Progression of Inflammation and Correlates with Cognition in Stable Schizophrenia. Diagnostics (Basel) 10(11): 926.
- Barichello T (2022) The role of innate lymphoid cells (ILCs) in mental health. Discov Ment Health 2(1): 2.
- Yeung SSH, Ho YS, Chang RCC (2021) The role of meningeal populations of type II innate lymphoid cells in modulating neuroinflammation in neurodegenerative diseases. Exp Mol Med 53(9): 1251-1267.
- K Uchiyama, T Takagi, K Mizushima, M Kajiwara-Kubota, S Kashiwagi, et al. (2021) Increased mucosal IL-12 expression is associated with relapse of ulcerative colitis. BMC Gastroenterol 21: 122.
- Langer V, Vivi E, Regensburger D, Winkler TH, Waldner MJ, et al. (2019) IFN-γ drives inflammatory bowel disease pathogenesis through VE-cadherin-directed vascular barrier disruption. J Clin Invest 129(11): 4691-4707.
- Kato T, Monji A, Hashioka S, Kanba S (2007) Risperidone significantly inhibits interferon-gamma-induced microglial activation in vitro. Schizophr Res 92(1-3): 108-115.
- Wilson KE, Demyanovich H, Rubin LH, Wehring HJ, Kilday C, et al. (2018) Relationship of Interferon-γ to Cognitive Function in Midlife Women with Schizophrenia. Psychiatr Q 89(4): 937-946.
- Arolt V, Weitzsch C, Wilke I, Nolte A, Pinnow M, et al. (1997) Production of interferon-gamma in families with multiple occurrences of schizophrenia. Psychiatry Res 66(2-3): 145-152.
- Mount MP, Lira A, Grimes D, Smith PD, Faucher S, et al. (2007) Involvement of interferon-gamma in microglial-mediated loss of dopaminergic neurons. J Neurosci 27(12): 3328-3337.
- Pennino D, Bhavsar PK, Effner R, Avitabile S, Venn P, et al. (2013) IL-22 suppresses IFN-γ-mediated lung inflammation in asthmatic patients. J Allergy Clin Immunol 131(2): 562-570.
- Natah SS, Mouihate A, Pittman QJ, Sharkey KA (2005) Disruption of the blood-brain barrier during TNBS colitis. Neurogastroenterol Motil 17(3): 433-446.
- Chen BY, Hsu CC, Chen YZ, Lin JJ, Tseng HH, et al. (2022) Profiling antibody signature of schizophrenia by Escherichia coli proteome microarrays. Brain Behav Immun 106: 11-20.
- Secher T, Samba-Louaka A, Oswald E, Nougayrède JP (2013) Escherichia coli producing colibactin triggers premature and transmissible senescence in mammalian cells. PLoS One 8(10): e77157.
- Papanastasiou E, Gaughran F, Smith S (2011) Schizophrenia as segmental progeria. J R Soc Med 104(11): 475-484.
- Mizuno Y, Muraoka M, Shimabukuro K, Toda K, Miyagawa K, et al. (1990) Inflammatory bowel diseases and thymus disorder: reactivity of thymocytes with monoclonal antibodies. Bull Tokyo Dent Coll 31(2): 137-141.
- Watanabe M, Funahashi T, Suzuki T, Nomura S, Nakazawa T, et al. (1982) Antithymic antibodies in schizophrenic sera. Biol Psychiatry 17(6): 699-710.
- Pan B, Wang D, Li L, Shang L, Xia F, et al. (2019) IL-22 Accelerates Thymus Regeneration via Stat3/Mcl-1 and Decreases Chronic Graft-versus-Host Disease in Mice after Allotransplants. Biol Blood Marrow Transplant 25(10): 1911-1919.
- Falk W (2006) A ticket to the gut for thymic T cells. Gut 55(7): 910-2.
- Shang L, Duah M, Xu Y, Liang Y, Wang D, et al. (2021) Dynamic of plasma IL-22 level is an indicator of thymic output after allogeneic hematopoietic cell transplantation. Life Sci 265: 118849.
- Li Y, Wang J, Li Y, Wu H, Zhao S, et al. (2019) Protecting intestinal epithelial cells against deoxynivalenol and E. coli damage by recombinant porcine IL-22. Vet Microbiol 231: 154-159.
- Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, et al. (2008) IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 14(3):275-281.
- Le PT, Pearce MM, Zhang S, Campbell EM, Fok CS, et al. (2014) IL22 regulates human urothelial cell sensory and innate functions through modulation of the acetylcholine response, immunoregulatory cytokines and antimicrobial peptides: assessment of an in vitro model. PLoS One 9(10): e111375.
- Ingersoll MA, Starkey MR (2020) Interleukin-22 in urinary tract disease - new experimental directions. Clin Transl Immunology 9(6): e1143.
- Ronald A (2002) The etiology of urinary tract infection: traditional and emerging pathogens. Am J Med 113 Suppl 1A: 14S-19S.
- Rudzki L, Szulc A (2018) "Immune Gate" of Psychopathology-The Role of Gut Derived Immune Activation in Major Psychiatric Disorders. Front Psychiatry 9: 205.
- Maes M, Vojdani A, Geffard M, Moreira EG, Barbosa DS, et al. (2019) Schizophrenia phenomenology comprises a bifactorial general severity and a single-group factor, which are differently associated with neurotoxic immune and immune-regulatory pathways. Biomol Concepts 10(1): 209-225.
- Tang KY, Lickliter J, Huang ZH, Xian ZS, Chen HY, et al. (2019) Safety, pharmacokinetics, and biomarkers of F-652, a recombinant human interleukin-22 dimer, in healthy subjects. Cell Mol Immunol 16(5): 473-482.
- Arab JP, Sehrawat TS, Simonetto DA, Verma VK, Feng D, et al. (2020) An Open-Label, Dose-Escalation Study to Assess the Safety and Efficacy of IL-22 Agonist F-652 in Patients with Alcohol-associated Hepatitis. Hepatology 72(2): 441-453.
- Dempsey L (2017) Antimicrobial IL-22. Nat Immunol 18: 373.
- Das S, St Croix C, Good M, Chen J, Zhao J, et al. (2020) Interleukin-22 Inhibits Respiratory Syncytial Virus Production by Blocking Virus-Mediated Subversion of Cellular Autophagy. iScience 23(7): 101256.
- L Shao, X Xiong, Y Zhang, H Miao, Y Ren, et al. (2020) IL-22 ameliorates LPS-induced acute liver injury by autophagy activation through ATF4-ATG7 signaling. Cell Death Dis 11: 970.
- A Merenlender-Wagner, A Malishkevich, Z Shemer, M Udawela, A Gibbons et al. (2015) Autophagy has a key role in the pathophysiology of schizophrenia. Mol Psychiatry 20(1): 126-32.
- Iida T, Onodera K, Nakase H (2017) Role of autophagy in the pathogenesis of inflammatory bowel disease. World J Gastroenterol 23(11): 1944-1953.
- Mo R, Lai R, Lu J, Zhuang Y, Zhou T, et al. (2018) Enhanced autophagy contributes to protective effects of IL-22 against acetaminophen-induced liver injury. Theranostics 8(15): 4170-4180.
- Kim SH, Park S, Yu HS, Ko KH, Park HG, et al. (2018) The antipsychotic agent clozapine induces autophagy via the AMPK-ULK1-Beclin1 signaling pathway in the rat frontal cortex. Prog Neuropsychopharmacol Biol Psychiatry 81: 96-104.
- Kim YK, Suh IB, Kim H, Han CS, Lim CS, et al. (2002) The plasma levels of interleukin-12 in schizophrenia, major depression, and bipolar mania: effects of psychotropic drugs. Mol Psychiatry 7(10): 1107-1114.
- Singh RK, Dai Y, Staudinger JL, Muma NA (2009) Activation of the JAK-STAT pathway is necessary for desensitization of 5-HT2A receptor-stimulated phospholipase C signalling by olanzapine, clozapine and MDL 100907. Int J Neuropsychopharmacol 12(5): 651-665.
- He J, Kong J, Tan QR, Li XM (2009) Neuroprotective effect of atypical antipsychotics in cognitive and non-cognitive behavioral impairment in animal models. Cell Adh Migr 3(1): 129-137.
- Kato T, Mizoguchi Y, Monji A, Horikawa H, Suzuki SO, et al. (2008) Inhibitory effects of aripiprazole on interferon-gamma-induced microglial activation via intracellular Ca2+ regulation in vitro. J Neurochem 106(2): 815-25.
- Vucicevic L, Misirkic-Marjanovic M, Harhaji-Trajkovic L, Maric N, Trajkovic V, et al. (2018) Mechanisms and therapeutic significance of autophagy modulation by antipsychotic drugs. Cell Stress 2(11): 282-291.
- Girgis RR, Lieberman JA (2021) Anti-viral properties of antipsychotic medications in the time of COVID-19. Psychiatry Res 295: 113626.
- Nehme H, Saulnier P, Ramadan AA, Cassisa V, Guillet C, et al. (2018) Antibacterial activity of antipsychotic agents, their association with lipid nanocapsules and its impact on the properties of the nanocarriers and on antibacterial activity. PLoS One 13(1): e0189950.
-
Adonis Sfera*, Nyla Jafri and Leah Rahman. F-652 (Recombinant Human Interleukin-22) For Schizophrenia. Arch Phar & Pharmacol Res. 3(3): 2023. APPR.MS.ID.000564.
-
Schizophrenia, Central nervous system (CNS), Gastrointestinal (GI), Psychopathology, Enabling lipopolysaccharide (LPS), COVID-19, F-652
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
- Introduction
- The gut and schizophrenia
- The evidence of MT in schizophrenia
- Hypothesis
- Interleukin-22, the “guardian” of gut barrier.
- IL-22 and innate lymphoid cells (ILCs)
- Psychosis and gut microbes
- IL-22 safety, pharmacokinetic and pharmacodynamic data
- Conclusions
- Acknowledgement
- Conflict of Interest
- References






